Abstract
The development of the nervous system is influenced by environmental factors. Among all environmental factors, temperature belongs to a unique category. Besides activating some specific sensory pathways, it exerts nonspecific, pervasive effects directly on the entire nervous system, especially in exothermic species. This study uses mutants to genetically discover how temperature affects nerve terminal arborization at larval neuromuscular junctions of Drosophila. It is known that hyperexcitability in K+ channel mutants leads to enhanced ramification of larval nerve terminals. Elevated cAMP levels in dunce mutants with reduced phosphodiesterase activity also cause enhanced arborization. These genetic alterations are thought to perturb mechanisms relevant to activity-dependent neural plasticity, in which neuronal activity activates the cAMP pathway, and consequently affect nerve terminal arborization by regulating expression of adhesion molecules. Here we demonstrate the robust influence of rearing temperature on motor nerve terminal arborization. Analysis of ion channel and cAMP pathway mutants indicates that this temperature-dependent plasticity is mediated via neuronal activity changes linked to mechanisms controlled by the rutabaga-encoded adenylyl cyclase.
Keywords: environment-dependent plasticity, cAMP, mutants, Drosophila, excitability, adenylyl cyclase
Introduction
Environmental influences on the development of the nervous system have been well documented. Most mechanistic studies of environmental effects have focused on well defined sensory inputs, such as visual (Hubel et al., 1977; Constantine-Paton et al., 1990; Crair et al., 1998) and mechanical (Simons and Land, 1987; Lendvai et al., 2000) stimuli, because sensory activities are required for activity-dependent refinement of sensory connections in the CNS. However, temperature, a different category of environmental factors, is more difficult to study. Temperature not only activates some specific sensory pathways (Spray, 1986; Caterina et al., 1997; Reichling and Levine, 2000) but also exerts nonspecific, pervasive effects, such as changing ion channel kinetics, enzymatic activities, and gene expression, etc., directly on the entire nervous system, especially in exothermic species. This study examines how temperature affects nerve terminal arborization at larval neuromuscular junctions of Drosophila and how to dissect the cellular and molecular bases of such effects with mutational analysis.
Nerve terminal arborization at larval neuromuscular junctions of Drosophila is activity dependent. Hyperexcitability resulting from mutations of K+ channel subunits, as in the double mutants ether à go-go (eag) Shaker (Sh) and Hyperkinetic (Hk) eag, or from overexpression of Na+ channels, as in duplication of paralytic (para), leads to enhanced ramification of larval nerve terminals (Budnik et al., 1990). The eag, Sh, and Hk genes encode different subunits of K+ channels (Kamb et al., 1987; Papazian et al., 1987; Warmke et al., 1991; Chouinard et al., 1995), and para encodes an Na+ channel subunit (Loughney et al., 1989). This activity-dependent enhancement has been suggested to be mediated by elevated cAMP levels in response to hyperneural activities, because dunce (dnc) mutants with reduced phosphodiesterase activity (Byers et al., 1981), and hence higher cAMP levels, also cause enhanced arborization (Zhong et al., 1992; Renger et al., 2000). It remains to be determined how increased neural activity leads to activation of the cAMP pathway, which might be achieved via activation of one or multiple forms of adenylyl cyclase or by inhibition of phosphodiesterase activity. cAMP is thought to regulate expression of cell adhesion molecules, such as Fasciclin II (Fas II) (Schuster et al., 1996a,b), and consequently modify nerve terminal arborization, as shown by enhanced ramification in the adhesion molecule mutants Fas I (Zhong and Shanley, 1995) and Fas II (Schuster et al., 1996b).
We demonstrated that increasing the rearing temperature enhances motor nerve terminal arborization, indicating that nerve terminal plasticity observed in this model preparation is not merely an abnormal phenotype that is only seen in mutants but a natural mechanism for adaptation to environmental changes. Analysis of ion channel and cAMP pathway mutants reveals that this temperature-dependent plasticity is mediated by neuronal activity changes. There is an optimal level of neuronal activity at which nerve terminals will show the highest degree of ramification. This neuronal activity change was linked to nerve terminal arborization via mechanisms controlled by the rutabaga (rut)-encoded adenylyl cyclase.
Materials and Methods
The fly stocks were reared at room temperature (RT) (19-22°C), 25°C, and 30°C as specified in the experiments. To raise the larvae at 25 or 30°C, the parents were allowed to lay eggs at room temperature for an ∼10 hr period. After the parents were cleared, the vials containing eggs were then incubated at specified temperatures. The stock rut2080;UAS-rut+ was provided by Dr. Troy Zars (University of Missouri, Columbia, MO) (Zars et al., 2000). All other mutants and GAL4 lines used to drive UAS-rut+ expression have been described previously, as indicated throughout.
Immunohistochemistry. The larval neuromuscular preparation and anti-HRP staining protocol have been described in detail previously by Budnik et al. (1990). The dissected body-wall neuromuscular preparations of the third instar larvae were fixed in nonalcoholic Bouin's solution (25 ml of formalin, 5 ml of glacial acetic acid, and 75 ml of saturated picric acid) for 1-2 hr. The samples were then treated in sequence with 1:200 anti-HRP (Sigma, St. Louis, MO) and then 1:20 goat anti-rabbit HRP-conjugated IgG (Cappel, Cochranville, PA). Staining was revealed by diaminobenzidine reaction.
The anti-HRP immunoreactive varicosities have been classified into four subtypes: type Ib (big boutons), type Is (small boutons), type II, and type III, on the basis of immunoreactivity, bouton size, types of synaptic vesicles contained, and electrophysiological responses (Johansen et al., 1989; Budnik et al., 1990; Kurdyak et al., 1994; Renger et al., 2000). These subtypes were not distinguished in this study. Data from both muscles 12 and 13 were pooled to obtain the total numbers of branches and varicosities for quantitative analysis of the terminal morphology. Data were obtained from the right third abdominal hemisegment of each larva (or from the left hemisegment if the right side was damaged).
Results
To investigate the temperature effect on nerve terminal arborization, the flies were allowed to deposit eggs in vials at room temperature (19-22°C) for 10-20 hr. The parents were then removed, and the collected eggs were grown in incubators at different temperatures until reaching the late third instar stage. The larvae were dissected for immunohistostaining of the body-wall muscles, which were arranged in a regular pattern (Crossley, 1978; Johansen et al., 1989). The terminal projections of motoneurons innervating these muscles were revealed by anti-HRP staining (Johansen et al., 1989; Budnik et al., 1990), which stains neurons in specific insect preparations (Jan and Jan, 1982). Our study focused on muscle fibers 12 and 13 (Crossley, 1978) in abdominal segment 3. Activity-dependent plasticity at these motor nerve terminals has been examined extensively (Budnik et al., 1990; Zhong et al., 1992; Atwood et al., 1993; Broadie and Bate, 1993; Jia et al., 1993; Jarecki and Keshishian, 1995; Schuster et al., 1996a,b; Renger et al., 2000).
Rearing temperature-induced enhancement in motor terminal arborization
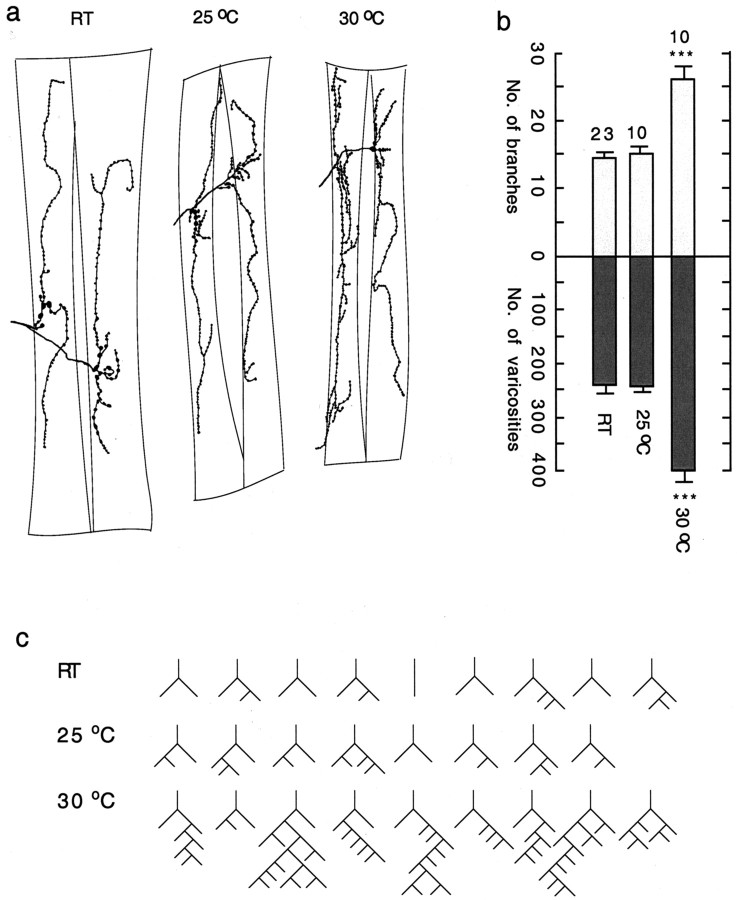
Figure 1a is an example of camera lucida drawings of motor terminals and varicosities. These varicosities are thought be the synaptic site for transmitter release (Johansen et al., 1989; Atwood et al., 1993; Jia et al., 1993; Renger et al., 2000). As shown in Figure 1b, the numbers of branches and varicosities in wild-type larvae were enhanced greatly at 30°C compared with those reared at room temperature or 25°C. There were no significant differences between larvae reared at room temperature and those reared at 25°C. More extensive arborization (i.e., increases in higher-order branching) at 30°C is evident in isomorphic representations of the branching patterns (Fig. 1c). This isomorphic representation depicts a branching pattern of the single primary process that shows the highest number of branches in either muscle 12 or muscle 13 in the larva (Zhong et al., 1992).
Figure 1.
Temperature-dependent enhancement of motor nerve terminal arborization. a, Camera lucida tracings of anti-HRP staining of motor axon terminals on muscle fibers 12 and 13 of abdominal segment 3 in third instar larvae of Drosophila reared at different temperatures. b, Histogram of the numbers of terminal branches and varicosities in muscles 12 and 13. A branch is defined as a terminal process containing at least two varicosities. For this and the following figures, the numbers indicate the total varicosities and branches on both muscle fibers 12 and 13 in one hemisegment. The mean and SEM in each genotype are presented for the number of larvae indicated. Statistical significance of the difference (t test; *p < 0.05; **p < 0.01; ***p < 0.001) in this figure represents a comparison of the indicated data with normal data obtained at room temperature. Temperatures at which larvae were reared are shown. c, Isomorphic representations of branching pattern. For this and the following figures, each representation illustrates the branching pattern of the single primary process that showed the highest number of branching in either muscle fiber 12 or muscle fiber 13. For this and the following figures, RT ranges from 19 to 22°C.
Alteration of temperature-induced enhancement in excitability mutants
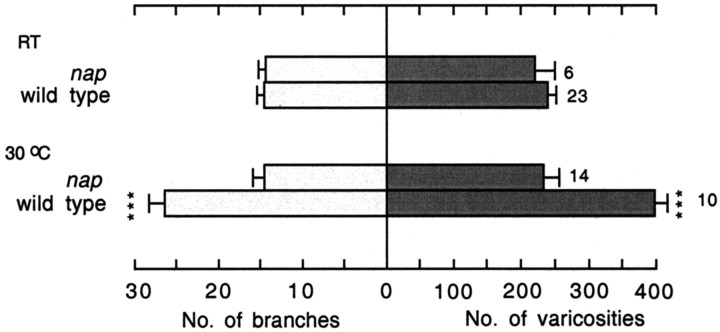
To determine whether temperature-dependent plasticity depends on neural activity, we examined the temperature-dependent no action potential (napts) mutant. It is known that the number of Na+ channels in the napts mutant is reduced, lowered excitability and lengthened refractory periods at room temperature, and blocked action potential at temperatures above 37°C (Wu et al., 1978; Wu and Ganetzky, 1980; Jackson et al., 1984; Kernan et al., 1991). In this study, we found that increasing the temperature to 30°C failed to induce nerve terminal overgrowth at napts neuromuscular junctions. The numbers of branches and varicosities were not significantly different between napts larvae reared at room temperature and those reared at 30°C (Fig. 2). This observation shows that with a weakened neuronal excitability, an increase in rearing temperature will fail to enhance nerve terminal arborization. Therefore, it leads to the notion that higher temperatures increase neural activity, which in turn enhances ramification in nerve terminal arborization.
Figure 2.
Suppression of temperature-induced enhancement of arborization in napts mutants. As in Figure 1b, the histogram of the numbers of terminal branches and varicosities in muscles 12 and 13 is presented. Comparisons are made between wild-type larvae and napts mutants. The number of larvae and temperatures at which larvae were reared are shown for each genotype.
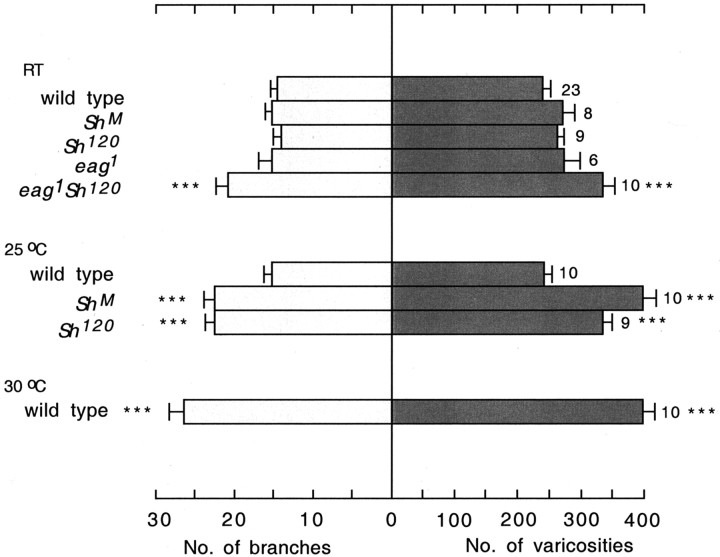
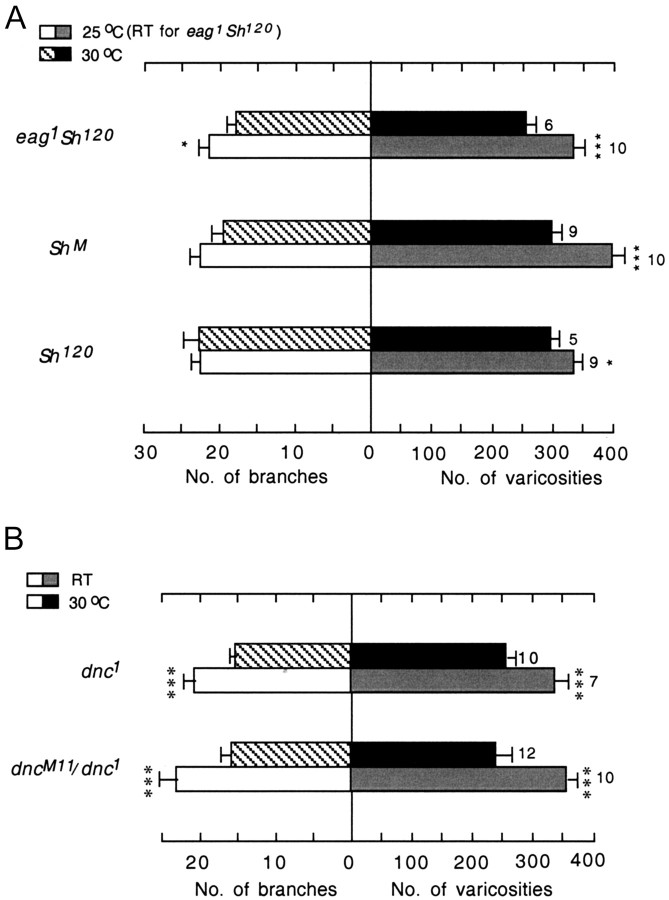
This idea is also supported by observations from hyperexcitable K+ channel mutants, including Sh and eag. A transient K+ current is eliminated in ShM muscles but only reduced in Sh120 muscles (Haugland and Wu, 1990), and multiple K+ currents are reduced in eag1 mutant muscles (Wu et al., 1983; Zhong and Wu, 1991). Enhanced excitability in these single mutants (Ganetzky and Wu, 1982) is insufficient to increase nerve terminal arborization at room temperature (Budnik et al., 1990). As demonstrated in Figure 3, when reared at room temperature, none of the Sh single mutants showed significant differences in the numbers of varicosities and branches from wild type. In contrast, eag1 Sh120 double mutants show significant enhancement in the numbers of varicosities and branches, indicating that a threshold level of excitability is required to induce nerve terminal overgrowth (Budnik et al., 1990; Zhong et al., 1992). We reasoned that at an intermediate temperature (25°C), Sh alleles but not wild-type larvae might show enhanced arborization because of a concomitant increase in neuronal excitability activity and temperature, albeit individually subthreshold. Indeed, our observations confirmed that the motor terminals of wild-type larvae remained the same, whereas the numbers of branches and varicosities were significantly increased in both Sh120 and ShM at 25°C (Fig. 3). From the samples collected, the number of branches between Sh120 and ShM were almost identical, but the number of varicosities was significantly greater in ShM (Fig. 3). The length of individual branches appeared to be longer in ShM (Fig. 4), which is consistent with the more extreme defect in the excitability in ShM.
Figure 3.
Similar effects on arborization of hyperexcitability mutations and temperature increments. The histogram of the numbers of terminal branches and varicosities in muscles 12 and 13 is presented. Comparisons are made with wild-type data obtained at room temperature. The number of larvae included is indicated along with the temperatures at which larvae were reared for each genotype.
Figure 4.
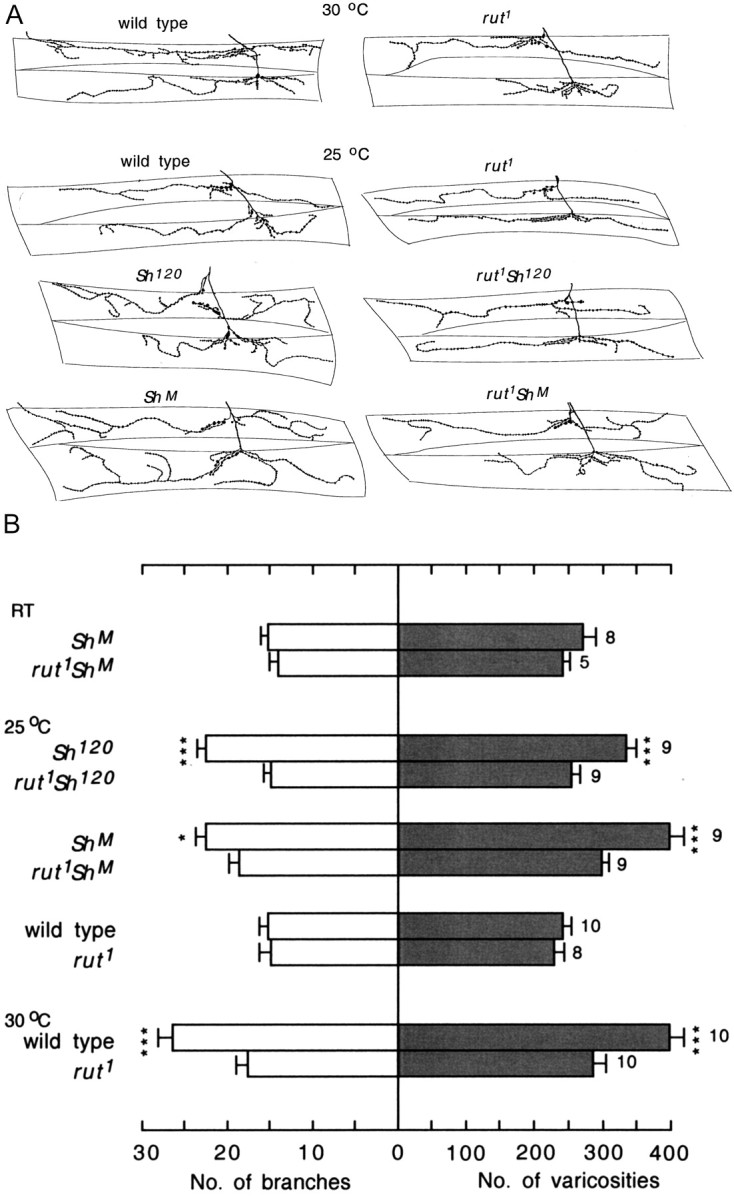
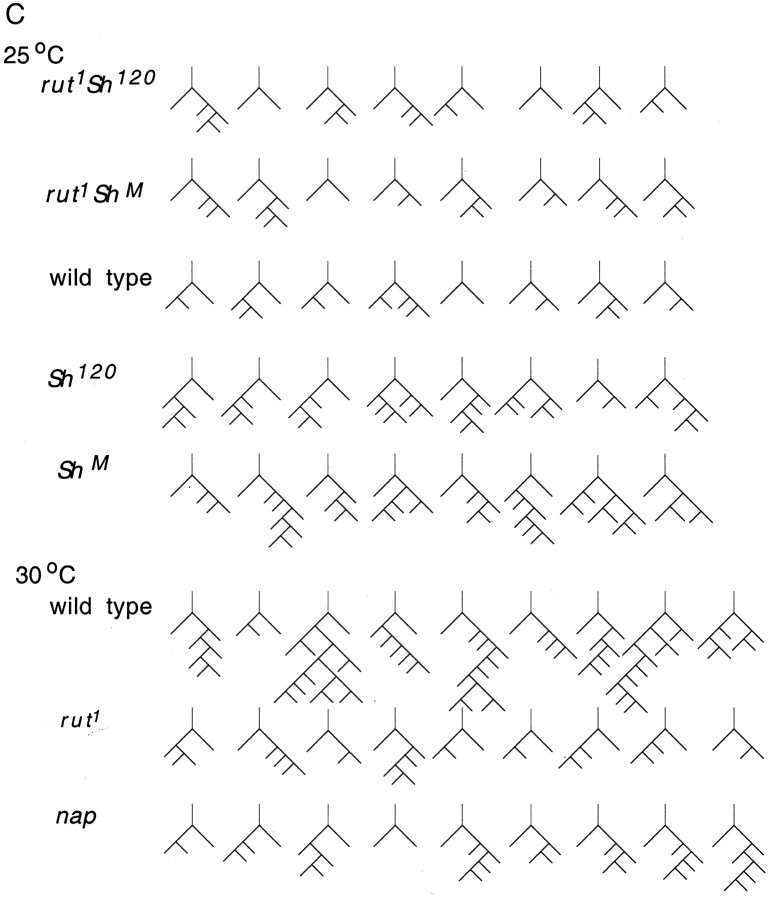
Suppression of hyperexcitability and temperature-induced enhancement of nerve terminal arborization by the rut mutation. A, Camera lucida tracings of anti-HRP staining of larval motor nerve terminals on muscle fibers 12 and 13. All samples were obtained from right hemisegment 3. Temperatures and genotypes are indicated. B, Histogram of the numbers of terminal branches and varicosities in muscles 12 and 13 is shown for segregated pairs of controlled experiments for the effect of the rut mutation. C, Isomorphic representations of branching pattern showing the effects of the rut1 and napts mutations. The first eight (or nine) larvae in the samples are presented.
Abolishing temperature- and hyperexcitability-induced enhancement by rut mutations
We then examined the involvement of the cAMP pathway. It has been suggested that the cAMP pathway mediates activity-dependent arborization at these nerve terminals. As mentioned above, the elevated cAMP levels in dnc mutants enhance motor terminal arborization (Byers et al., 1981; Chen et al., 1986; Zhong et al., 1992). cAMP synthesis by rut-encoded adenylyl cyclase (Livingstone et al., 1984; Levin et al., 1992) is activated by G-protein-dependent mechanisms (Levin et al., 1992; Guo et al., 2000) as well as by Ca2+ (Livingstone et al., 1984; Levin et al., 1992), which accumulates during neuronal activity. However, a genetic study of the role of rut has been hampered by difficulties in constructing rut eag Sh triple mutants (no visible markers are available between the closely located rut and eag for recognizing their recombinants). Temperature as well as Sh single mutant-induced arborization (at 25°C) enabled an examination to establish the role of rut in activity-dependent neural plasticity. At room temperature as well as at 25°C, there were no significant differences in the numbers of branches and varicosities between wild-type and rut1 mutant larvae (Fig. 4A,B). However, when reared at 30°C, the terminal projection in wild type was much more ramified compared with that in rut1 (Fig. 4). This indicates that temperature-induced expansion in terminal projection was strongly suppressed by the rut1 mutation. Similarly, the expansion of terminal projection induced by Sh mutations at 25°C was also suppressed by the rut1 mutation, as revealed in the double mutants rut1 Sh120 and rut1 ShM (Fig. 4). The isomorphic representation also indicates that higher-order branches were reduced by the rut mutation (Fig. 4C) (compare rut1 Sh120 and rut1 ShM with Sh120 and ShM at 25°C; also compare the similar effects on napts and rut1 with wild type at 30°C). The suppression appeared to be incomplete in some cases. The numbers of branches and varicosities in rut1 larvae at 30°C and in rut1 ShM at 25°C (Fig. 4B) were both significantly higher than that in rut1 larvae at 25°C (t test; p < 0.02). Our results suggest that there is an important link between increased neural activity and enhanced nerve terminal arborization in activation of rut-encoded adenylyl cyclase. Because rut1 is a functionally null allele (Livingstone et al., 1984; Levin et al., 1992), the incomplete suppression of terminal ramification indicated involvement of other mechanisms.
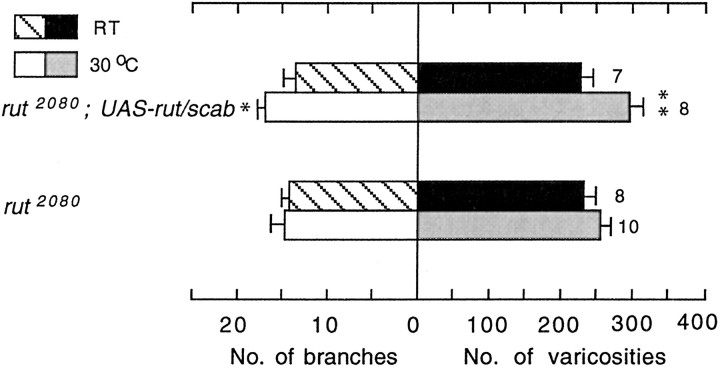
It is known that rut mutations cause defined phenotypes in both neurons (Zhao and Wu, 1997) and muscle cells (Zhong and Wu, 1993). To determine the presynaptic and postsynaptic actions of rut-encoded adenylyl cyclase, we examined how the observed rut mutant phenotype was modified by expressing a wild-type rut transgene in neurons (presynaptic) versus in muscle cells (postsynaptic). Expression of UAS-rut+ driven by a scab-Gal4 promoter for restricted expression in neurons (Rohrbough et al., 2000) appeared to rescue the rut defect in rut2080/Y;UAS-rut+/scab-Gal4 larvae (Fig. 5). In contrast, activity-dependent plasticity remained defective, with UAS-rut+ expression restricted to muscle fibers (Fig. 5), in rut2080/Y;UAS-rut+/BG487-Gal4 larvae [for muscle-specific driver BG487, see the study by Koh et al. (2002)]. It is worth noting that the enhancement of arborization in rut2080/Y;UAS-rut+/scab-Gal4 larvae in response to a temperature increase was still not as strong as that seen in wild-type larvae, suggesting that the rescue is incomplete (compare Figs. 1, 5). This discrepancy may result from nonoptimal levels of rut+ expression. Together, our results suggest that presynaptic rut-encoded adenylyl cyclase activity is required to regulate the synaptic plasticity described here. This is consistent with the observation that reduced expression of Na+ channels, which are expressed only in presynaptic neurons and not in postsynaptic muscle cells (Singh and Wu, 1999), was capable of blocking activity-induced enhancement of arborization in napts larval mutants (Fig. 2).
Figure 5.
Effects of expressing a wild-type rut+ transgene in neurons or in muscle cells. In the case of rut+ expression in neurons, UAS-rut+ is driven by the scab-Gal4 promoter (Rohrbough et al., 2000). For rut+ expression in muscle cells, UAS-rut+ is driven by BG478-Gal4 (Koh et al., 2002). Comparisons are made within the same genotype at different temperatures.
Reducing arborization by excessively high activity level
A question could be raised as to whether an even more extreme terminal ramification could be induced in Sh or eag Sh alleles by increasing temperature further. Results summarized in Figure 6A indicate that the phenotype of enhanced arborization observed in eag Sh double mutants reared at RT and in Sh reared at 25°C was in fact suppressed when reared at 30°C. The resultant numbers of varicosities in Sh120 and ShM at 30°C were significantly lower than those at 25°C, although the numbers of branches were not statistically different. Most strikingly, eag1 Sh120 double mutants showed a dramatic reduction at 30°C in the numbers of both varicosities and branches compared with those at RT. Moreover, the double-mutant terminal arbors demonstrated the least amount of outgrowth at 30°C among all of the genotypes examined, including wild type and the Sh single mutant.
Figure 6.
Suppression of nerve terminal arborization at a high temperature in hyperexcitability and dnc mutants. A, Histogram of numbers of terminal branches and varicosities in muscles 12 and 13 of hyperexcitability mutants. Comparisons are made for each genotype between different rearing temperatures. Note that there is very little difference between Sh120 at 25 and 30°C in the numbers of branches and varicosities. This is consistent with the interpretation that excitability in the other two mutants is increased beyond the optimal level for axon outgrowth at 30°C. Among the Sh alleles, the increase in excitability is weakest in Sh120 (Haugland and Wu, 1990). B, Histogram of the numbers of terminal branches and varicosities in muscles 12 and 13 of dnc mutants. The 30°C data (mean ± SEM) for varicosities and branches are 256 ± 39 and 15.7 ± 2.9 in dnc1 and 242 ± 73 and 15.8 ± 4.9 in dnc1/dncM11, respectively. Arborization data for dnc mutants at room temperature have been described previously (Zhong et al., 1992). All data sets for the dnc mutants presented here were collected at the same time.
We also examined the temperature effect on dnc mutants in which nerve terminal arborization is known to be enhanced at RT (Zhong et al., 1992), presumably as a result of accumulation of cAMP attributable to reduced degradation by phosphodiesterase (Byers et al., 1981; Chen et al., 1986). We found that there was a striking reduction in both varicosity and branch numbers in homozygous dnc1 and heterozygous dnc1/dncM11 larvae raised at 30°C compared with those raised at RT (Fig. 6B). This observation paralleled the above results of eag1 Sh120 larvae, in which enhanced nerve terminal outgrowth seen at RT was suppressed at 30°C. These observations suggest that an optimal level of neuronal activity stimulates the cAMP pathway to a corresponding optimal level, which in turn promotes maximal nerve outgrowth.
Discussion
From our observations, four conclusions can be drawn. First, developmental temperature is a robust environmental factor that influences neuronal outgrowth in larval neuromuscular junctions of Drosophila. Second, critical temperature-dependent neuronal growth is mediated by neural activity, although temperature may exert nonspecific pervasive effects on cellular or molecular activities. Third, nerve terminal arborization increases with activity level but becomes suppressed beyond an optimal activity level. Fourth, Rut-regulated cAMP pathways play an essential role in mediating activity-dependent nerve terminal arborization. Our result suggests that presynaptic Rut activity is critical.
It is conceivable that neuronal activity may be generally increased at higher rearing temperatures in flies. For instance, transient K+ currents inactivate faster at increased temperatures, which should allow higher-frequency firing of action potentials. It is also noted that a wings-down phenotype presumably resulting from extreme hyperexcitability (Engel and Wu, 1992; Wang et al., 2000) is observed only in eag Sh double mutants but not in the corresponding single mutants at room temperature. However, this phenotype could be found among a fraction of eag or Sh single mutants reared at 30°C (our unpublished observation). Thus, it is logical to speculate that increased neural activity at higher rearing temperatures leads to modification of nerve terminal arborization. The present study provides several lines of evidence in support of this idea, as summarized below. It appears that temperature increase and hyperexcitability mutations exert similar influences on nerve terminal arborization. The effect of a small increase in temperature (from RT to 25°C) is equivalent to that of single eag or Sh mutations, whereas a large increase (from RT to 30°C) affects arborization similar to that of the double mutants (Fig. 3). More conclusive evidence comes from the observation that temperature-induced enhancement of arborization can be suppressed by the nap mutation (Fig. 2), in which neuronal activity is lowered because of a reduced number of Na+ channels. Moreover, both activity- and temperature-dependent arborization are linked to the cAMP pathway. Both Sh (at 25°C)- and temperature (at 30°C)-induced enhancement in arborization are suppressed by the rut mutation (Fig. 4).
The cAMP pathway has been suggested to be a necessary component in visual experience-dependent cortical plasticity of ocular dominance (Imamura et al., 1999; Beaver et al., 2001) and has been shown to be a critical signal transduction pathway in mediating synaptic reorganization during long-term memory formation in Aplysia (Bailey et al., 1996). Previous studies have indicated that elevated cAMP levels in dnc mutants lead to enhancement of arborization at the larval neuromuscular junction, and this enhanced ramification in dnc mutants can be suppressed by the rut mutation, as shown in dnc rut double mutants (Zhong et al., 1992). This establishes that cAMP is able to influence arborization, but its role in mediating this activity-dependent arborization has not been resolved previously. In this study, it is clearly demonstrated in rut and rut Sh double mutants that arborization is not enhanced (even at high temperatures or in hyperexcitability mutants) if rut-encoded adenylyl cyclase activity is removed (Fig. 4). In contrast, dnc-encoded cAMP-specific phosphodiesterase is not a component directly mediating activity-dependent plasticity. Arborization in dnc mutants still varies with temperature in a striking manner (Fig. 5), whereas hyperexcitability and temperature are unable to alter arborization in rut mutants.
It is interesting to note that motor nerve terminal arborization is reduced in dnc, Sh, and eag Sh mutants reared at 30°C (Fig. 6A). This observation has prompted the proposal that there is an optimal level of activity, hence of cAMP, for promoting axon outgrowth and arborization (Figs. 3, 6). In other words, there is a bell-shaped relationship curve between neuronal activity and ramification of arbors: motor nerve terminal arborization is enhanced with an increase in activity and will become suppressed with additional increases in activity. In fact, a similar relationship has been suggested between intracellular calcium concentrations and growth cone formation and neurite outgrowth in cultured neurons (Kater et al., 1988). In summary, the results presented demonstrate that developmental temperature is a robust environmental factor that influences neuronal outgrowth, and that temperature-dependent neuronal growth is mediated by neural activity. The effect of rut and dnc at different developmental temperatures and their interaction with channel mutations demonstrate an essential role of the Rut-regulated cAMP pathway in developmental neural plasticity in response to environmental changes.
Footnotes
This work was supported by National Institutes of Health Grants NS26528 to C.-F.W. and NS34779 to Y.Z. We thank Dr. T. Zars for providing the UAS-rut+ stock.
Correspondence should be addressed to Yi Zhong, Cold Spring Harbor Laboratory, P.O. Box 100, Cold Spring Harbor, NY 11724. E-mail: zhongyi@cshl.org.
Copyright © 2004 Society for Neuroscience 0270-6474/04/241439-07$15.00/0
References
- Atwood HL, Govind CK, Wu C-F (1993) Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J Neurobiol 24: 1008-1024. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Bartsch D, Kandel ER (1996) Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA 93: 13445-13452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver CJ, Ji Q, Fischer QS, Daw NW (2001) Cyclic AMP-dependent protein kinase mediates ocular dominance shifts in cat visual cortex. Nat Neurosci 4: 159-163. [DOI] [PubMed] [Google Scholar]
- Broadie K, Bate M (1993) Activity-dependent development of the neuromuscular synapse during Drosophila embryogenesis. Neuron 11: 607-619. [DOI] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, Wu C-F (1990) Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci 10: 3754-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D, Davis RL, Kiger JA (1981) Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster Nature 289: 79-81. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816-824. [DOI] [PubMed] [Google Scholar]
- Chen CN, Denome S, Davis RL (1986) Molecular analysis of cDNA clones and the corresponding genomic coding sequences of the Drosophila dunce+ gene, the structural gene for cAMP phosphodiesterase. Proc Natl Acad Sci USA 83: 9313-9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard SW, Wilson GF, Schlimgen AK, Ganetzky B (1995) A potassium channel beta subunit related to the aldo-keto reductase superfamily is encoded by the Drosophila hyperkinetic locus. Proc Natl Acad Sci USA 92: 6763-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT, Debski E (1990) Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci 13: 129-154. [DOI] [PubMed] [Google Scholar]
- Crair MC, Gillespie DC, Stryker MP (1998) The role of visual experience in the development of columns in cat visual cortex. Science 279: 566-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley CA (1978) The morphology and development of the Drosophila muscular system. In: The genetics and biology of Drosophila Vol IIb (Ashburner M, Wright TRF, eds), pp 499-560. New York: Academic. [Google Scholar]
- Engel JE, Wu CF (1992) Interactions of membrane excitability mutations affecting potassium and sodium currents in the flight and giant fiber escape systems of Drosophila J Comp Physiol [A] 171: 93-104. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF (1982) Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J Neurophysiol 47: 501-514. [DOI] [PubMed] [Google Scholar]
- Guo H-F, Tong J, Hannan F, Luo L, Zhong Y (2000) A neurofibromatosis-1-regulated pathway is required for learning in Drosophila Nature 403: 895-898. [DOI] [PubMed] [Google Scholar]
- Haugland FN, Wu C-F (1990) A voltage-clamp analysis of gene dosage effects of the Shaker locus on larval muscle potassium currents in Drosophila J Neurosci 10: 1357-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S (1977) Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci 278: 377-409. [DOI] [PubMed] [Google Scholar]
- Imamura K, Kasamatsu T, Shirokawa T, Ohashi T (1999) Restoration of ocular dominance plasticity mediated by adenosine 3′,5′-monophosphate in adult visual cortex. Proc R Soc Lond B Biol Sci 266: 1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson FR, Wilson SD, Strichartz GR, Hall LM (1984) Two types of mutants affecting voltage-sensitive sodium channels in Drosophila melanogaster Nature 308: 189-191. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN (1982) Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and grasshopper embryos. Proc Natl Acad Sci USA 72: 2700-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarecki J, Keshishian H (1995) Role of neural activity during synaptogenesis in Drosophila J Neurosci 15: 8177-8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X-X, Gorczyca M, Budnik V (1993) Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J Neurobiol 24: 1025-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J, Halpern ME, Johansen KM, Keshishian H (1989) Stereotypic morphology of glutamatergic synapses on identified muscle cells of Drosophila larvae. J Neurosci 9: 710-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamb A, Iverson LE, Tanouye MA (1987) Molecular characterization of Shaker, a Drosophila gene that encodes a potassium channel. Cell 50: 405-413. [DOI] [PubMed] [Google Scholar]
- Kater SB, Mattson MP, Cohan C, Connor J (1988) Calcium regulation of the neuronal growth cone. Trends Neurosci 11: 315-321. [DOI] [PubMed] [Google Scholar]
- Kernan MJ, Kuroda MI, Kreber R, Baker BS, Ganetzky B (1991) napts, a mutation affecting sodium channel activity in Drosophila, is an allele of mle, a regulator of X chromosome transcription. Cell 66: 949-959. [DOI] [PubMed] [Google Scholar]
- Koh Y-H, Ruiz-Canada C, Gorczyca M, Budnik V (2002) The Ras1-mitogen-activated protein kinase signal transduction pathway regulates synaptic plasticity through fasciclin II-mediated cell adhesion. J Neurosci 22: 2496-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdyak P, Atwood HL, Stewart BA, Wu C-F (1994) Differential physiology and morphology of motor axons to ventral longitudinal muscles in larval Drosophila J Comp Neurol 350: 463-472. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Stern E, Chen B, Svoboda K (2000) Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo Nature 404: 876-881. [DOI] [PubMed] [Google Scholar]
- Levin LR, Han PL, Hwang PM, Feinstein PG, Davis RL, Reed RR (1992) The Drosophila learning and memory gene rutabaga encodes a Ca2+/calmodulin-responsive adenylyl cyclase. Cell 68: 479-489. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Sziber PP, Quinn WG (1984) Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell 37: 205-215. [DOI] [PubMed] [Google Scholar]
- Loughney K, Kreber R, Ganetzky B (1989) Molecular analysis of the para locus, a sodium channel gene in Drosophila Cell 58: 1143-1154. [DOI] [PubMed] [Google Scholar]
- Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY (1987) Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila Science 237: 749-753. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Levine JD (2000) In hot pursuit of the elusive heat transducers. Neuron 26: 555-558. [DOI] [PubMed] [Google Scholar]
- Renger JJ, Ueda A, Atwood HL, Govind CK, Wu C-F (2000) Role of cAMP cascade in synaptic stability and plasticity: ultrastructural and physiological analyses of individual synaptic boutons in Drosophila memory mutants. J Neurosci 20: 3980-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbough J, Grotewiel MS, Davis RL, Broadie K (2000) Integrin-mediated regulation of synaptic morphology, transmission, and plasticity. J Neurosci 20: 6868-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS (1996a) Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17: 641-654. [DOI] [PubMed] [Google Scholar]
- Schuster CM, Davis GW, Fetter RD, Goodman CS (1996b) Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron 17: 655-667. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Land PW (1987) Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature 326: 694-697. [DOI] [PubMed] [Google Scholar]
- Singh S, Wu CF (1999) Ionic currents in larval muscles of Drosophila Int Rev Neurobiol 43: 191-220. [DOI] [PubMed] [Google Scholar]
- Spray DC (1986) Cutaneous temperature receptors. Annu Rev Physiol 48: 625-638. [DOI] [PubMed] [Google Scholar]
- Wang JW, Humphreys JM, Phillips JP, Hilliker AJ, Wu CF (2000) A novel leg-shaking Drosophila mutant defective in a voltage-gated K+ current and hypersensitive to reactive oxygen species. J Neurosci 20: 5958-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke J, Drysdale R, Ganetzky BA (1991) A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science 252: 1560-1562. [DOI] [PubMed] [Google Scholar]
- Wu C-F, Ganetzky B (1980) Genetic alteration of nerve membrane excitability in temperature-sensitive paralytic mutants of Drosophila melanogaster Nature 286: 814-816. [DOI] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B, Jan LY, Jan YN (1978) A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc Natl Acad Sci USA 75: 4047-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B, Haugland FN, Liu AX (1983) Potassium currents in Drosophila: different components affected by mutations of two genes. Science 220: 1076-1078. [DOI] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M (2000) Localization of a short-term memory in Drosophila Science 288: 672-675. [DOI] [PubMed] [Google Scholar]
- Zhao M-L, Wu C-F (1997) Alterations in frequency coding and activity dependence of excitability in cultured neurons of Drosophila memory mutants. J Neurosci 17: 2187-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Shanley J (1995) Altered nerve terminal arborization and synaptic transmission in Drosophila mutants of cell adhesion molecule fasciclin I. J Neurosci 15: 6679-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Wu CF (1991) Alteration of four identified K+ currents in Drosophila muscle by mutations in eag Science 252: 1562-1564. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wu CF (1993) Differential modulation of potassium currents by cAMP and its long-term and short-term effects: dunce and rutabaga mutants of Drosophila J Neurogenet 9: 15-27. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Budnik V, Wu CF (1992) Synaptic plasticity in Drosophila memory and hyperexcitable mutants: role of cAMP cascade. J Neurosci 12: 644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]