Short abstract
The UN Food and Agriculture Organisation's global rinderpest eradication programme (GREP)—the first and only attempt to eradicate an animal pathogen—provides several learning points from the veterinary perspective
Rinderpest is (was is possibly more accurate) an ancient disease of cattle, believed to have been the origin of human measles,1 caused by an epitheliotropic and lymphotropic morbillivirus. Characterised by high fever, ocular and nasal discharges, dysentery, and dehydration it can cause death in up to 100% of cattle, water buffaloes, and yaks. Many wild ungulates are also highly susceptible, but not humans. Not surprisingly, it was the dread of farmers throughout the European, Asian, and African continents for centuries, even millennia. Sweeping west, east, and south out of central Asia, this devastating disease changed the course of history, following in the wake of marauding armies bringing death and devastation that contributed to the fall of the Roman empire, the conquest of Europe by Charlemagne, the French revolution, the impoverishment of Russia, and the colonisation of Africa.2 Having been defeated in Europe by 1928, it was the subject of intensive eradication effort in Africa and Asia for most of the last century, yet not until 1993 was a programme mounted by the Food and Agriculture Organisation of the United Nations to bring about global eradication. The eradication programme aimed to provide coordination of autonomous, regional campaigns rather than being a centrally managed campaign. Twelve years later, in 2005, we are conceivably very close to the goal, with growing confidence that almost the whole world is now free; suspicion persists only for some pastoralist communities of the Horn of Africa, even though the virus has not been detected for four years.3
Herd immunity
Control, elimination, or eradication of rinderpest were long considered to depend almost solely on mass, pulsed vaccination campaigns, assuming that herd immunity would rise sufficiently to extinguish transmission of the virus. If it had been possible to maintain a sufficient proportion of the population immune for a sufficient length of time (taking into account ephemeral maintenance of virus by wildlife over which we had no control) one could surely have expected rapid success. But what is a sufficient proportion? How could high herd immunity be achieved? How long must it be sustained? Only lately have we started to gain epidemiological insights into answering these questions.4
West Africa as an example?
Annual campaigns targeted the most susceptible domestic species to achieve a desired seroprevalence between 80% and 90%, until the disease disappeared. Initially no time limit was set, but latterly the duration was set at three years, essentially for pragmatic reasons. This approach worked for West Africa, making it an often cited example: twice it proved relatively easy to free that region (or virtually all of it) early in campaigns that ran from the 1960s to the 1970s and 1986 to 1998. However, a more careful appraisal finds that mass rinderpest vaccination alone might not have been responsible for the perceived success. We know from field experience that high vaccination coverage, and even immunity, can be achieved in vaccination campaigns where the efficacy of immunisation is monitored and remedial action (revaccination) taken if needed.5 However, most vaccination programmes fell far short of achieving the magical 90%, or even 80%, immunity figure. In fact, the figure rarely even reached 65% per cent, yet convincing evidence shows that the virus was eliminated from West Africa in 1988 after three years of mass vaccination. Perhaps the answer lies in the discovery that peste des petits ruminants (PPR), prevalent in West Africa and much of Asia, infects cattle, subclinically inducing immunity against rinderpest in up to 50% of cattle.6 PPR immunity in the population summated with that of the rinderpest vaccine to give high herd immunity to rinderpest, explaining how elimination of rinderpest occurred despite suboptimal vaccination coverage, not only in West Africa but also in India.7 It is fortunate that PPR fortified immunity conferred by vaccination because field studies and mathematical modelling indicate that moderate herd immunity actually helps to sustain viral transmission networks.4
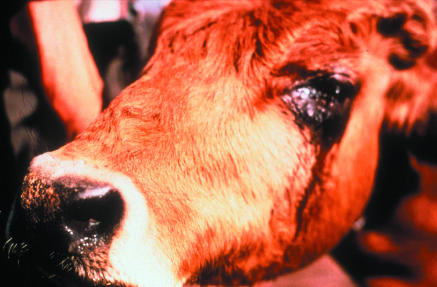
Figure 1.
A case of rinderpest, showing ocular discharge
Credit: VEIN
Vaccination not necessary
Combined with the understanding that it was difficult to achieve an adequately high immune population by annual, pulsed vaccination across the whole population at risk, we started to appreciate that it was actually not necessary and was wasteful of resources. In Ethiopia in the early 1990s, we realised that the areas of the country where rinderpest outbreaks were most evident were in fact “indicators” of spread from persisting, endemic reservoirs in remote, extensive, pastoral communities marginalised from services and surveillance. Eliminating these residual reservoirs of infection became the core issue in eradication. Mass vaccination was therefore relegated to the start of campaigns to reduce the frequency of outbreaks and disclose sustained virus transmission networks. Targeted vaccination programmes were mounted from 1993, using innovative, community based delivery systems, and this cleared rinderpest from the 35 million Ethiopian cattle herd within three years, something that 30 years of institutionalised, mass vaccination programmes had failed to do. A similarly focused approach achieved success in the sedentary village populations of southern India at about the same time.8 The last handful of Asian and African endemic foci (except for the Somali ecosystem) were cleared by 2000. The most important lesson we learnt, perhaps rather slowly, was that a sound epidemiological understanding must precede, and be applied to guiding and focusing, any disease control programme.
GREP was designed with a deadline for fully accredited rinderpest freedom of 2010, and this has proved to be a wise move to provide a timetable to guide activities and focus minds.
The foregoing are the most important issues but other factors favouring the success of the programme deserve mention. These include:
Active, adaptively managed, global coordination.
A robust, efficacious, safe, and affordable vaccine9 with a thermostable formulation10 to avoid cold chain restrictions.
An independent vaccine quality assurance service and production guidelines.
Robust, affordable diagnostic tests; the ability to discriminate between wild infection and vaccination would have greatly facilitated surveillance as would penside tests if they had been available earlier.
An international accreditation mechanism operated by the Office International des Epizooties.
Guidelines for surveillance combined with performance indicators—the World Health Organization's pioneering work was taken as a model.11,12
Molecular characterisation of viruses had a seminal effect on both epidemiological understanding and the conduct of eradication programmes. Designation of a world reference laboratory, hosted by the UK Institute for Animal Health, was invaluable.
Conclusions
Much has been learnt since the start of GREP that merits consideration when mounting control or eradication efforts for human or animal diseases. Whether other diseases will follow for eradication or be singled out for progressive control in geographically defined areas, as are foot and mouth disease and classical swine fever in Latin America, depends largely on the outcome of the global rinderpest eradication programme and the attitude of the international community towards funding such endeavours. There is no shortage of candidates, and new ones constantly arise. Medical and veterinary epidemic disease control is becoming a single continuum. The recent zoonotic Rift Valley fever, severe acute respiratory syndrome (SARS), Hendra virus or Nipah virus, and avian influenza indicate that we need to move on from separate human and veterinary scenarios.
Competing interests: None declared.
References
- 1.Diamond J. Evolution, consequences and future of plant and animal domestication. Nature 2002;418: 700-7. [DOI] [PubMed] [Google Scholar]
- 2.Scott GR, Provost A. Global eradication of rinderpest. Background paper prepared for the FAO expert consultation on the strategy for global rinderpest eradication. Rome: UN Food and Agriculture Organisation, 1992: 109.
- 3.Mariner J, Roeder PL. The use of participatory epidemiology in studies of the persistence of lineage 2 rinderpest virus in East Africa. Vet Rec 2003; 152, 641-7. [DOI] [PubMed]
- 4.Mariner JC, McDermott J, Heesterbeek JAP, Catley A, Roeder P. A model of lineage-1 and lineage-2 rinderpest virus transmission in pastoral areas of East Africa. Prev Vet Med 2004;69: 245-63. [DOI] [PubMed] [Google Scholar]
- 5.Taylor WP, Roeder PL, Rweyemamu MM, Melewas JN, Majuva P, Kimaro RT, et al. The control of rinderpest in Tanzania between 1997 and 1998. Trop Animal Health Prod 2002;34: 471-87. [DOI] [PubMed] [Google Scholar]
- 6.Anderson J, McKay JA. The detection of antibodies against peste des petits ruminants virus in cattle, sheep and goats and the possible implications for rinderpest control programmes. Epidemiol Infect 1994;112: 225-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor WP, Roeder PL, Rweyemamu MM. Use of rinderpest vaccine in international programmes for the control and eradication of rinderpest. In: Pastoret PP, Barrett T, eds. Elsevier biology of animal infections. Volume 2. Rinderpest and peste des petits ruminants. Oxford: Elsevier (in press).
- 8.Roeder PL, Taylor WP, Rweyemamu MMR. Rinderpest in the 20th and 21st centuries. In: Pastoret PP, Barrett T, eds. Biology of animal infections. Volume 2. Rinderpest and peste des petits ruminants. Oxford: Elsevier (in press).
- 9.Plowright W, Ferris RD. Studies with rinderpest virus in tissue culture. The use of attenuated culture virus as a vaccine for cattle. Res Veterinary Sci 1962; 3, 172-82.
- 10.Mariner JC, House JA, Mebus CA, Sollod A, Stem C. Production of a thermostable VERO cell-adapted rinderpest vaccine. J Tissue Culture Methods 1991;13: 253-6. [Google Scholar]
- 11.Birmingham M. Surveillance for achieving global polio eradication. In: Animal production and health paper 129: the world without rinderpest. Proceedings of the FAO Technical Consultation on the Global Rinderpest Eradication Programme, Rome, Italy, 22 to 24 July, 1996: 133-40. www.fao.org/docrep/003/w3246e/W3246E10.htm#ch3.7.5 (accessed 12 Nov 2005).
- 12.Mariner JC, Jeggo MH, van't Klooster GGM, Geiger R, Roeder PL. Rinderpest surveillance performance monitoring using quantifiable indicators. OIE Sci Technical Rev 2003;22: 837-47. [DOI] [PubMed] [Google Scholar]