Abstract
Eight human isoforms of phosphoinositide 3-kinases (PI3Ks) exist, but their individual functions remain poorly understood. Here, we show that different human small cell lung carcinoma (SCLC) cell lines overexpress distinct subsets of class IA and II PI3Ks, which results in striking differences in the signalling cascades activated by stem cell factor (SCF). Over expression of class IA p85/p110α in SCLC cells increased SCF-stimulated protein kinase B (PKB) activation and cell growth, but did not affect extracellular signal-regulated kinase (Erk) or glycogen synthase kinase-3 (GSK-3). This effect was selective, since it was not observed in SCLC cell lines overexpressing p85/p110β or p85/p110δ. The SCF receptor associated with both class IA p85 and class II PI3KC2β, and both enzymes contributed to SCF-stimulated PKB activity. A dominant-negative PI3KC2β blocked both PKB activation and SCLC cell growth in response to SCF. Together our data provide novel insights into the specificity and functional significance of PI3K signalling in human cancer.
Keywords: cell growth/c-Kit/PI3K/PKB/SCLC
Introduction
The binding of a wide range of extracellular signals to their specific membrane receptors activates PI3K signalling in eukaryotic cells. These enzymes phosphorylate phosphatidylinositol (PI) on the D-3 position of the inositol ring, producing three distinct second messengers, PI(3)P, PI(3,4)P2 and PI(3,4,5)P3 (PIP3) (Vanhaesebroeck et al., 2001). The diverse cellular responses regulated by PI3Ks reflect the existence of different isoforms of these enzymes with specific regulatory mechanisms and functions. The eight PI3Ks identified in various species can be subdivided into three main classes, based on sequence homology and in vitro substrate specificity (Vanhaesebroeck et al., 2001).
Class IA includes three highly homologous isoforms: p110α, p110β and p110δ. These three p110s exist as a heterodimeric complex with a p85 regulatory subunit containing two src homology-2 (SH2) domains, which mediates their association with activated growth factor receptors. All the PI3Ks of class IA can phosphorylate PI, PI(4)P and PI(4,5)P2 in vitro. However, activation of the class IA PI3Ks by receptor tyrosine kinases (RTKs) has been demonstrated to lead mainly to the accumulation of PIP3 in vivo (Vanhaesebroeck et al., 2001). PIP3 is the key second messenger in the activation of phosphoinositide-dependent kinase-1 (PDK1) (Alessi et al., 1997), which controls various downstream cascades leading to cell growth and survival via protein kinase B (PKB)/Akt (Burgering and Coffer, 1995; Kauffmann-Zeh et al., 1997), ribosomal protein S6 kinase (S6K) (Alessi et al., 1998) and glycogen synthase kinase-3 (GSK-3) (Frame and Cohen, 2001). Accordingly, disruption of the p85α gene in mice impaired B-cell development and proliferation (Fruman et al., 1999).
Class II of PI3Ks comprises human PI3KC2α, PI3KC2β and PI3KC2γ (Vanhaesebroeck et al., 2001). The hallmarks of class II family members are a substrate specificity restricted to PI and PI(4)P in vitro, and a C-terminal C2 domain. Recent studies have begun to elucidate the regulation of class II PI3Ks in vivo. Human PI3KC2α is activated by insulin (Brown et al., 1999), and we have shown recently that both PI3KC2α and C2β associate with polypeptide growth factor receptors such as the epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR) (Arcaro et al., 2000).
The reason for the existence of eight different PI3Ks in higher eukaryotes is still unclear, and it remains to be clarified whether all isoforms have specific downstream effectors, or whether a certain degree of functional redundancy exists.
In addition to controlling growth and proliferation in normal eukaryotic cells (Kozma and Thomas, 2002), the PI3K/PKB pathway plays a crucial role in the tumorigenesis of various types of human cancer. Indeed, the tumour suppressor PTEN, which negatively controls PI3K signalling by dephosphorylating the D-3 position of polyphosphoinositides is defective in a large number of human cancers (Cantley and Need, 1999). Although PTEN mutations are rare in human small cell lung carcinoma (SCLC) (Forgacs et al., 1998), some SCLC cell lines were reported to express a constitutively active PI3K, indicating that these tumour cells may rely on an alternative mechanism for anchorage-independent growth (Moore et al., 1998).
Clearly, the identification of a cell system in which individual, rather than multiple, PI3K isoforms are expressed could provide useful insights into their specific functions. Indeed, in this report, we demonstrate that different human SCLC cell lines overexpress distinct subsets of class IA and class II PI3Ks. Our results in these and model cell systems demonstrate that distinct PI3Ks couple to different early signalling events, which affects the biological responses of these cells to polypeptide growth factors.
Results
Human SCLC cell lines overexpress different subsets of class I and II PI3Ks
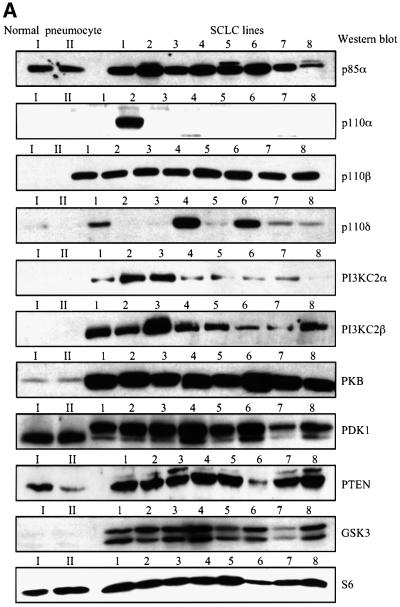
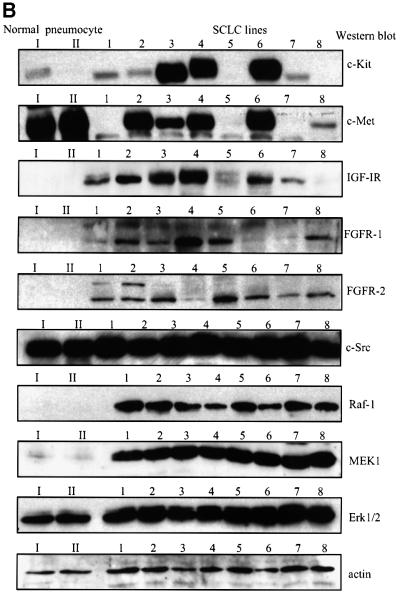
The protein expression of class IA and II PI3K isoforms in a panel of eight SCLC cell lines derived from primary human tumours was compared with type II pneumocytes purified from normal human lung tissue, which are believed to be precursors for SCLC (Pardo et al., 2001 and references therein). The p85α regulatory subunit was overexpressed in seven of eight SCLC cell lines, as compared with the type II pneumocytes (Figure 1A). In contrast, the expression of the class IA p110 catalytic subunits was more variable. Strikingly, p110α was strongly overexpressed only in the H-69 SCLC cell line (Figure 1A), which had previously been reported to express a constitutively activated PI3K (Moore et al., 1998). To further assess class IA PI3K isoform expression in type II pneumocytes, the p85 regulatory subunit was immunoprecipitated from cell lysates, and low amounts of p110α, but not p110β or p110δ (data not shown), could be detected complexed to p85 in these cells (Supplementary figure 1A available at The EMBO Journal Online). In contrast to the restricted p110α expression in SCLC cell lines, the p110β isoform was equally overexpressed in all the lines investigated (Figure 1A). The leucocyte-specific p110δ isoform was also overexpressed in some (HC-33, H-510 and H-1045), but not all SCLC cell lines (Figure 1A). The class II PI3KC2α and C2β were present in all the SCLC cell lines (Figure 1A). However, higher levels of II PI3KC2α were found in H-69 and H-209, while expression of PI3KC2β was strongest in H-209 cells. The tumour suppressor PTEN was highly expressed in seven of eight SCLC cell lines (Figure 1A). Compared with type II pneumocytes, SCLC cell lines also displayed overexpression of PKB, GSK-3α/β, Raf-1 and mitogen-activated Erk kinase-1 (MEK1), but not of PDK1, Erk1/2, c-Src, ribosomal S6 protein or actin (Figure 1A and B).
Fig. 1. Human SCLC cell lines overexpress different isoforms of class IA and II PI3Ks. (A) and (B) Purified human type II pneumocytes from normal lung tissue (I, II) or human SCLC cell lines (1–8) were lysed and detergent-soluble fractions analysed by western blotting with antibodies specific for various PI3K isoforms (A), polypeptide growth factor receptors (B) or the proteins indicated (A and B). The SCLC cell lines analysed were: 1, HC-33; 2, H-69; 3, H-209; 4, H-510; 5, H-524; 6, H-1045; 7, H-1622; 8, H-2171.
The apparent molecular weight of PDK1 on SDS– PAGE gels was higher in SCLC cells, as compared with type II pneumocytes (Figure 1A). In order to investigate whether this could be caused by differential phosphorylation of the enzyme, the impact of growth factor stimulation on the electrophoretic mobility of PDK1 was assessed. Epidermal growth factor (EGF), phorbol 12,13-dibutyrate (PDB), and to a lesser extent hepatocyte growth factor (HGF) and fibroblast growth factor-2 (FGF-2), induced PDK1 mobility shift in type II pneumocytes (Supplementary figure 1B). The extent of the shift correlated well with the ability of the growth factors to induce extracellular signal-regulated kinase (Erk) activation (Supplementary figure 1D). In contrast, stem cell factor (SCF) failed to induce a similar response in H-69 SCLC cells (Supplementary figure 1C), although it potently stimulated Erk (Figure 5B). The PI3K inhibitors wortmannin and LY294002 did not alter the electrophoretic mobility of PDK1 in H-69 cells (Supplementary figure 1C). However, in H-69 cells, okadaic acid (OA) induced PDK1 mobility shift, which was reversed by LY294002 (Supplementary figure 1C). Together these observations indicate that the difference in PDK1 electrophoretic mobility between type II pneumocytes and SCLC cells may be due to phosphorylation of the enzyme by the MEK/Erk pathway. Autocrine secretion of growth factors by SCLC cells may result in activation of the MEK/Erk pathway and constitutive PDK1 phosphorylation. How ever, activation of other signalling pathways may result in additional phosphorylations of PDK1 in SCLC cells, as shown by the responses observed with OA and LY294002. It is at present unclear whether the growth factor-stimulated shift in PDK1 electrophoretic mobility reflects activation, since its downstream target PKB was activated in response to EGF and FGF-2, but not PDB, in type II pneumocytes (Supplementary figure 1D). Growth factor-stimulated PKB S473 phosphorylation was much weaker in type II pneumocytes (Supplementary figure 1D), as compared with H-69 SCLC cells (Figure 5A), which may be the result of both PKB and PI3K overexpression in the tumour cells.
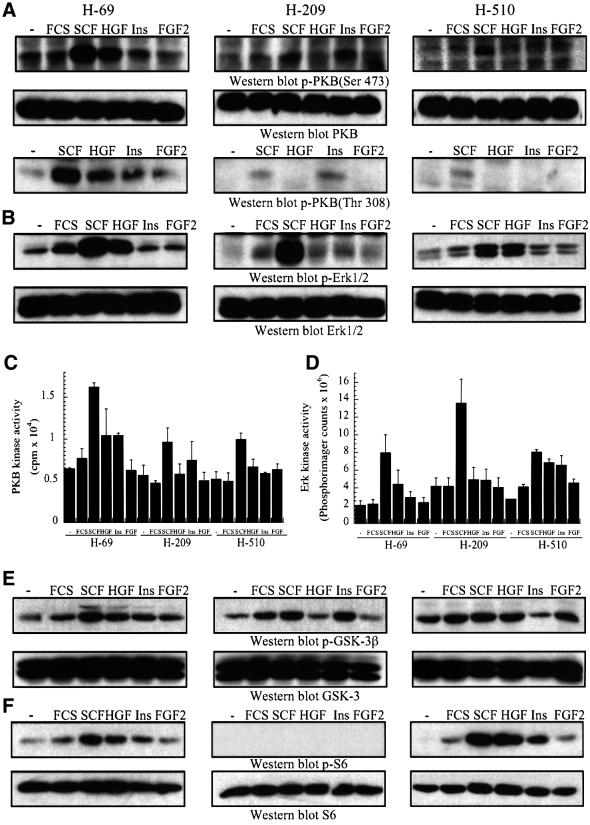
Fig. 5. Differential overexpression of PI3K isoforms affects downstream signalling cascades in human SCLC cells. (A–F) SCLC cells were treated with SCF (10 ng/ml), HGF (10 ng/ml), insulin (3 µg/ml), or FGF-2 (1 ng/ml) for 5 min or (F) 10 min, at 37°C. (A and B) and (E and F) The samples analysed by western blotting with antibodies specific for (A) activated PKB (p-PKB, Ser473 or Thr308) or total PKB, (B) activated Erk1/2 (p-Erk1/2) or total Erk, (E) Ser9-phosphorylated GSK-3β (p-GSK-3β) or total GSK-3, (F) Ser240/244-phosphorylated S6 protein (p-S6) or total S6 protein. Alternatively, anti-PKB (C) or anti-Erk (D) immunoprecipitates were assayed for in vitro protein kinase activity, using crosstide (C) or myelin basic protein (D) as substrates.
The expression pattern of polypeptide growth factor receptors in the SCLC cell lines was then investigated. The SCF receptor, c-Kit, was expressed in six SCLC cell lines, while c-Met, the receptor for HGF, was found in five of eight SCLC cell lines and in type II pneumocytes (Figure 1B). All the SCLC cell lines expressed the insulin-like growth factor-I receptor (IGF-IR), the fibroblast growth factor receptor-1 (FGFR-1) and FGFR-2, although there were some variations in protein levels between the lines. To investigate the impact of PI3K isoform overexpression on SCLC cell responses to polypeptide growth factors, we focused our study on the H-69, H-209 and H-510 cell lines, which selectively overexpress p110α, PI3KC2β and p110δ, respectively. These three cell lines displayed comparable levels of p85α and p110β, and all express the receptors for SCF, HGF, insulin/IGF and FGF-2. Since the expression levels of c-Kit appeared to be significantly lower in H-69 cells, as compared with H-209 and H-510 cells, we assayed c-Kit surface expression by FACS analysis. The normalized geometric mean intensities for c-Kit expression (staining: anti-c-Kit/R-phycoerythrin) were 56.61 (H-69), 51.06 (H-209) and 15.17 (H-510). This indicates that the levels of c-Kit expressed at the cell surface are less variable between the SCLC cell lines than total cellular c-Kit levels (Figure 1B).
Polypeptide growth factors induce the recruitment of class IA and II PI3Ks to phosphotyrosine-containing signalling complexes in SCLC cell lines
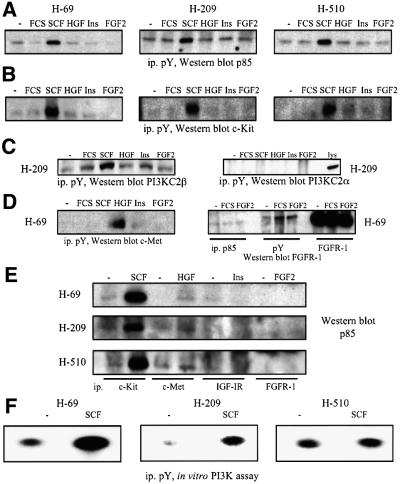
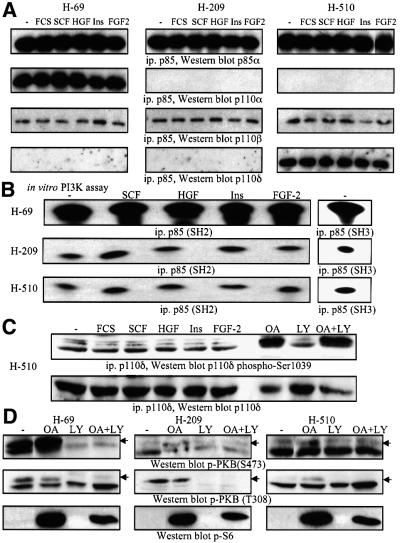
The classical activation mechanism of class IA PI3K involves binding to activated polypeptide growth factor receptors via the SH2 domains of the p85 regulatory subunit (Domin et al., 1996; Vanhaesebroeck et al., 2001). Consequently, we evaluated the ability of growth factors to induce the appearance of p85 or class II PI3Ks in anti-phosphotyrosine immunoprecipitates of H-69, H-209 and H-510 cells. SCF induced the association of p85 with phosphotyrosine-containing signalling complexes containing activated c-Kit (Figure 2A and B). This response was rapid (5 min) and selective for SCF, since similar results were not seen with HGF, insulin or FGF-2, although their respective RTKs could be activated, as evidenced by their recruitment to anti-phosphotyrosine immunoprecipitates (Figure 2A, B and D; data not shown). PI3KC2β also associated with phosphotyrosine-containing signalling complexes in the three SCLC cell lines (Figure 2C; data not shown), and the response was increased by SCF (Figure 2C). In contrast, PI3KC2α could not be found in anti-phosphotyrosine immunoprecipitates, even after prolonged (20 min) stimulation times in all three cell lines (Figure 2C; data not shown). SCF treatment of H-69, H-209 or H-510 cells also induced the association of p85 with c-Kit (Figure 2E). Strikingly, neither HGF, insulin nor FGF-2 was able to induce a comparable response in the three SCLC cell lines (Figure 2E). We then assayed anti-phosphotyrosine immunoprecipitates of resting and SCF-stimulated cells for in vitro PI3K activity, and found a substantially greater increase in enzyme activity in H-69 cells (Figure 2F), as compared with H-209 or H-510 cells. When Ca2+ was used instead of Mg2+ as a cofactor in the in vitro lipid kinase assay, significantly higher PI3K activity was detected in anti-phosphotyrosine immunoprecipitates from SCF-treated H-209 cells, as compared with H-69 and H-510 cells (data not shown). This can be explained by PI3KC2β association with phosphotyrosine-containing signalling complexes (Figure 2C), since this enzyme is expressed at higher levels in H-209 cells, and can use Ca2+ as a cofactor, in contrast to class IA PI3Ks (Arcaro et al., 1998). The SCF-stimulated increase in PI3K activity in samples from H-209 cells (Figure 2F) may thus reflect a contribution from PI3KC2β.
Fig. 2. Polypeptide growth factor stimulation of SCLC cell lines induces the recruitment of p85 to phosphotyrosine-containing signalling complexes. (A–D) SCLC cells were treated with SCF (10 ng/ml), HGF (10 ng/ml), insulin (3 µg/ml) or FGF-2 (1 ng/ml) for 5 min at 37°C. Anti-phosphotyrosine immunoprecipitates were analysed by western blotting with antibodies specific for the proteins indicated. In some cases lysates of the respective SCLC cell lines were analysed in parallel as positive controls (lys). (E) Lysates from SCLC cells prepared as in (A) were immunoprecipitated with antibodies against different receptors, and samples analysed by western blotting with anti-p85 antibodies. (F) SCLC cells (as indicated) were treated with SCF (10 ng/ml) for 5 min at 37°C. Anti-phosphotyrosine immunoprecipitates were assayed for in vitro PI3K activity. Radioactive phospholipids were separated by thin layer chromatography, and spots visualized by autoradiography.
Together these results demonstrate that PI3K is not constitutively active in SCLC cells, and can be regulated by growth factors. Moreover, the differences in lipid kinase activities present in phosphotyrosine-containing signalling complexes between the three tumour lines may reflect the expression of distinct PI3K isoforms.
To substantiate this hypothesis, we immunoprecipitated total p85 protein from H-69, H-209 or H-510 cells, and analysed both the identity of the associated p110 isoforms and their in vitro PI3K activity. While anti-p85 immunoprecipitates from H-69 cells contained both p110α and p110β, p85 was found associated with only p110β in H-209 cells, and with both p110β and p110δ in H-510 cells (Figure 3A). These results confirm the expression data presented in Figure 1A. Moreover, the levels of p110β associated with p85 were comparable between the three SCLC cell lines (Figure 3A). When anti-p85 immunoprecipitates were assayed for associated PI3K activity, significantly higher (19-fold, when normalized to protein content) lipid kinase activity was found in samples prepared from H-69 cells (Figure 3B), while there was no difference in p85-associated PI3K activity between H-209 and H-510 cells, although the latter overexpressed p110δ. These differences in p85-associated PI3K activity were observed in immunoprecipitates prepared with monoclonal antibodies directed against either the SH2 or the SH3 domain of p85 (Figure 3B). Similar results were also obtained when PI(4,5)P2 was used as a substrate instead of PI (data not shown). SCF treatment stimulated p85-associated PI3K activity (by 4-fold) in H-209 and H-510 cells (Figure 3B), in agreement with previous reports (Domin et al., 1996).
Fig. 3. SCLC cells overexpressing p110α display higher p85-associated PI3K activity than cells overexpressing p110β or p110δ. (A) and (B) SCLC cells (as indicated) were treated with SCF (10 ng/ml), HGF (10 ng/ml), insulin (3 µg/ml) or FGF-2 (1 ng/ml) for 5 min at 37°C. Anti-p85 immunoprecipitates (A) were analysed by western blotting with antibodies specific for class IA PI3Ks. (B) Anti-p85 immunoprecipitates prepared with antibodies to the SH2 or SH3 domain were assayed for in vitro PI3K activity as in Figure 2. (C) Lysates from H-510 cells stimulated as in (A) and (B) were analysed by western blotting with phospho-specific antibodies to Ser1039 of p110δ or anti-p110δ antiserum. Lysates from H-510 cells pre-treated for 2 h with vehicle (–), LY294002 (LY, 10 µM), okadaic acid (OA, 1 µM) or LY294002 and OA, were analysed in parallel. (D) Lysates from SCLC cells as in (C) were analysed by western blotting with antibodies specific for activated PKB (p-PKB, Ser473 or T308), or Ser240/244-phosphorylated S6 protein (p-S6).
The preceding results indicate that despite H-510 cells selectively overexpressing p110δ and its association with p85, PI3K activity is not enhanced compared with H-209 cells. To confirm this observation, we examined serine phosphorylation of p110δ at position 1039, which is known to negatively regulate this lipid kinase (Vanhaesebroeck et al., 1999). In H-510 cells, p110δ was constitutively phosphorylated on Ser1039 and this was not significantly affected by polypeptide growth factor stimulation (Figure 3C). This observation correlates well with the apparent lack of contribution of p110δ to p85-associated PI3K activity in H-510 cells (Figure 3B). OA treatment has been shown previously to enhance p110δ Ser1039 phosphorylation (Vanhaesebroeck et al., 1999), and we detected a significant increase in this phosphorylation in OA-treated H-510 cells (Figure 3C). Although Ser1039 phosphorylation was shown previously to be dependent on the protein kinase activity of p110δ (Vanhaesebroeck et al., 1999), the PI3K inhibitor LY294002 only marginally decreased basal and OA-stimulated levels of Ser1039 phosphorylation in H-510 cells (Figure 3C). This result indicates that a protein kinase activity distinct from p110δ may regulate Ser1039 phosphorylation in H-510 cells. OA strongly stimulated ribosomal S6 protein phosphorylation (Figure 3D) and Erk1/2 activation (data not shown) in all three SCLC cell lines, but had no significant effect on PKB activation (Figure 3D). In contrast, LY294002 decreased basal PKB activation in H-69 and H-209 cells (Figure 3D). Interestingly, although OA modulated the inhibitory Ser1039 phosphorylation of p110δ (Figure 3C) in H-510 cells, this did not correlate with any changes in PKB, S6K or Erk1/2 activity (Figure 3D). These results show that p110δ does not appear to regulate PKB, Erk or S6K signalling in H-510 cells.
Polypeptide growth factor regulation of PI3KC2β in human SCLC cells
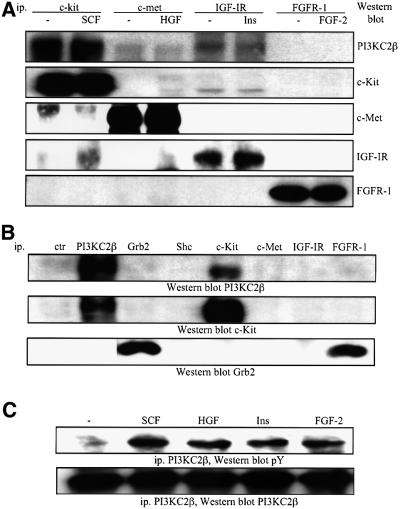
We next investigated the association of PI3KC2β with different polypeptide growth factor receptors. The enzyme selectively associated with c-Kit in the three SCLC cell lines and this interaction was not increased upon SCF stimulation (Figure 4A; data not shown). Association of PI3KC2β with c-Met and the IGF-IR was weaker, and an interaction could not be detected with the FGFR-1 (Figure 4A). PI3KC2α did not associate with any of the receptors under these conditions (data not shown).
Fig. 4. Polypeptide growth factor regulation of the class II PI3KC2β in human SCLC cells. (A) H-209 cells were treated with SCF (10 ng/ml), HGF (10 ng/ml), insulin (3 µg/ml) or FGF-2 (1 ng/ml) for 5 min at 37°C. The samples were immunoprecipitated with antibodies specific for various receptors and analysed by western blotting with antibodies specific for PI3KC2β or the receptors, as indicated. (B) Immuno precipitates from resting H-69 cells prepared with control antiserum (ctr), or antibodies specific for the proteins indicated were analysed by western blotting with antibodies specific for PI3KC2β or the receptors and adapters indicated. (C) H-209 cells were treated as in (A), and anti-PI3KC2β immunoprecipitates analysed by western blotting with antibodies specific for phosphotyrosine (pY) or PI3KC2β.
The adapter Grb2 has recently been implicated in the recruitment of PI3KC2β to the EGFR (Wheeler and Domin, 2001). We thus investigated whether Grb2 could be responsible for docking PI3KC2β to c-Kit, but failed to detect the adapter in anti-PI3KC2β or anti-c-Kit immunoprecipitates (Figure 4B), under conditions where the receptor–enzyme complex was formed in resting cells. Moreover, despite Grb2 associating with the FGFR-1, this receptor did not coprecipitate with PI3KC2β (Figure 4B).
Although cell stimulation with polypeptide growth factors did not modulate the interaction of PI3KC2β with the receptors, tyrosine phosphorylation of the enzyme could be observed upon SCF, HGF, insulin and FGF-2 treatment of H-209 cells (Figure 4C). In the case of SCF, HGF and insulin, growth factor-induced tyrosine phosphorylation of PI3KC2β correlated with receptor association. This is consistent with the response reported previously in EGF- or PDGF-stimulated A431 and NIH 3T3 cells (Arcaro et al., 2000).
Overexpression of class IA and II PI3Ks correlates with differential regulation of PKB in SCLC cells
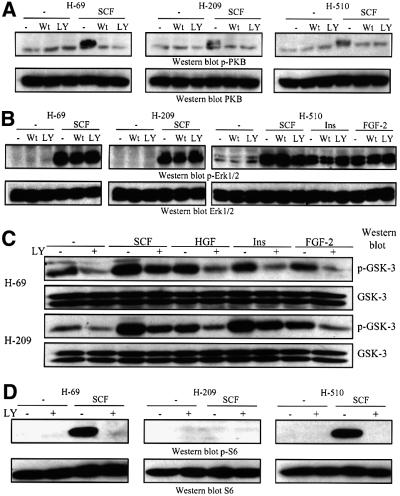
Having characterized the expression pattern of class IA and II PI3Ks, and their activation by polypeptide growth factors, we then investigated whether there were any obvious differences in the activation of downstream signalling cascades between H-69, H-209 and H-510 cells. PKB was activated by SCF, HGF and insulin in H-69 cells, and by SCF in H-209 and H-510 cells, as assessed by the induction of the phosphorylation of PKB T308 and S473 activation sites (Figure 5A). This response was rapid, since it was observed within 5 min of stimulation, which is in good agreement with other reports (Burgering and Coffer, 1995). The extent of PKB activation by SCF differed markedly between the SCLC cell lines, being most prominent in H-69 cells (Figure 5A), which overexpress p110α. However, the protein levels of p85, PDK1 and PKB were comparable between the three lines (Figure 1A). The increased PKB activation observed in H-69 is thus most likely the result of p110α overexpression. To confirm the results obtained by western blotting, PKB activation was also assessed by in vitro protein kinase assays on immunoprecipitates from resting or growth factor-stimulated SCLC cells. In H-69 cells, SCF stimulated a 3-fold increase in PKB protein kinase activity, while the responses to HGF and insulin were 2-fold (Figure 5C). The extent of SCF-stimulated PKB activation was lower in H-209 and H-510 cells (Figure 5C), and no detectable increase in PKB kinase activity was observed in response to other polypeptide growth factors in these cells.
In contrast to PKB, p110α overexpression did not impact on Erk activation, since all three SCLC lines displayed SCF-induced Erk activation, and the enzyme was activated by HGF to a similar extent in H-69 and H-510 cells, as assessed by western blotting with phospho-specific antibodies (Figure 5B). Essentially comparable results were obtained when Erk activation was assayed using an in vitro kinase assay on the enzyme immunoprecipitated from SCLC cells (Figure 5D).
We next investigated the activation status of two other downstream targets of PI3K signalling, S6K and GSK-3, in the three SCLC lines studied above. Surprisingly, and in contrast to PKB activity, there was no difference in the phosphorylation of GSK-3β on Ser9 between H-69, H-209 and H-510 cells (Figure 5E), but SCF was a slightly more potent stimulator of this response. Within 10 min of growth factor stimulation, phosphorylation of the ribosomal S6 protein on Ser240/244 could be observed in H-69 cells and H-510 cells, but not H-209 cells (Figure 5F). SCF and HGF were the strongest stimulators of S6 protein phosphorylation, as compared with serum, insulin and FGF-2.
In order to assess the dependence of various signalling pathways on PI3K activity, we used the two selective and structurally unrelated inhibitors wortmannin and LY294002. A short pre-incubation (15 min) with either inhibitor totally abolished SCF-stimulated PKB activation in H-69, H-209 and H-510 cells (Figure 6A). Similar results were obtained for HGF- and insulin-stimulated PKB activation (data not shown). In contrast, Erk1/2 activation induced by polypeptide growth factors was unaffected by either wortmannin or LY294002 in all three SCLC lines (Figure 6B).
Fig. 6. Pharmacological PI3K inhibitors abrogate polypeptide growth factor-stimulated PKB activation in human SCLC cells. (A–D) SCLC cells were pre-treated with vehicle (–), wortmannin (Wt, 50 nM) or LY294002 (LY, 10 µM) for 15 min at 37°C. (A–C) The cells were then stimulated with SCF (10 ng/ml), HGF (10 ng/ml), insulin (3 µg/ml) or FGF-2 (1 ng/ml) for 5 min or (D) 10 min, at 37°C. After stimulation the cells were lysed, and samples analysed by western blotting with antibodies specific for (A) activated PKB (p-PKB) or total PKB, (B) activated Erk1/2 (p-Erk1/2) or total Erk, (C) Ser9- phosphorylated GSK-3β (p-GSK-3β) or total GSK-3, (D) Ser240/244-phosphorylated S6 protein (p-S6) or total S6 protein.
The PI3K inhibitor only partially reduced GSK-3β Ser9 phosphorylation in response to polypeptide growth factors (Figure 6C). In contrast, pre-treatment with LY294002 totally abolished growth factor-stimulated S6 protein phosphorylation on Ser240/244 in H-69 and H-510 cell lines (Figure 6D). Together, these results indicate that although ribosomal S6 kinase activity is totally PI3K dependent, it is not specifically enhanced by p110α overexpression, in contrast to PKB. GSK-3β inhibition in response to growth factor stimulation was apparently not affected by the presence of p110α or p110δ, and only partially dependent on PI3K activity.
Transfection of class IA or II PI3Ks differentially affects downstream signalling cascades in mammalian cells
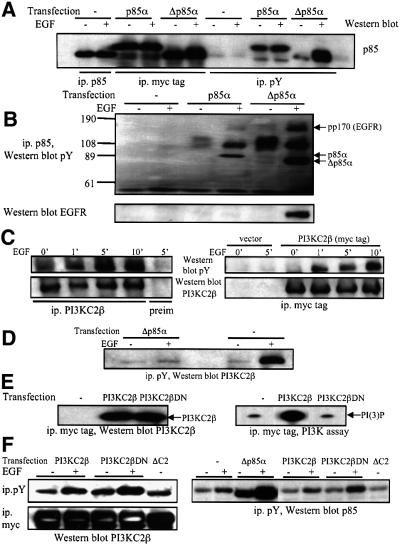
In order to confirm the data obtained from the comparative analysis of SCLC cell lines, we used transient transfections to study the impact of various PI3K cDNA constructs on downstream signalling cascades. The transfection efficiency of COS-7 and 293 cells was between 70 and 80%, as assessed by transfection of cells with an expression plasmid for green fluorescent protein and FACS analysis (data not shown). We first evaluated the dominant-negative Δp85α construct (Hara et al., 1994) for its ability to specifically block signalling through class IA PI3Ks. The protein expression levels of transfected myc-tagged p85α and Δp85α cDNA constructs in COS-7 cells were similar to the levels of endogenous p85 (Figure 7A). EGF stimulation of serum-starved cells induced the appearance of transfected p85α and Δp85α in anti-phosphotyrosine immunoprecipitates (Figure 7A), and the tyrosine phosphorylation of both proteins (Figure 7B). Intriguingly, the response to EGF observed with Δp85α was much stronger than that observed with wild-type p85α and with endogenous p85 (Figure 7A and B). A tyrosine- phosphorylated protein of 170 kDa corresponding to the EGF receptor selectively coprecipitated with Δp85α upon EGF stimulation (Figure 7B). The discrepancies in the responses observed with endogenous p85, recombinant p85α and Δp85α may reside in differences in intracellular localization and binding to p110, which could impact on their recruitment in response to EGF stimulation.
Fig. 7. Evaluation of the selectivity of dominant-negative PI3K constructs. (A) COS-7 cells transiently transfected with the constructs encoding myc-tagged p85α or Δp85α were stimulated with EGF, and anti-p85α or anti-phosphotyrosine immunoprecipitates analysed by western blotting with anti-p85 antibodies. (B) The blot was reprobed with anti-phosphotyrosine (top) or anti-EGFR (bottom) antibodies. The position of myc-tagged p85α and Δp85α, and the pp170 band corresponding to the EGFR are shown. (C) 293 cells (left) or 293 cells transfected with a construct encoding myc-tagged PI3KC2β (right) were stimulated with EGF for various lengths of time. Samples were immunoprecipitated with anti-PI3KC2β antiserum, pre-immune serum (left) or anti-myc tag antibodies (right), and analysed by western blotting with anti-phosphotyrosine (pY) or anti-PI3KC2β antibodies. (D) COS-7 cells were transiently transfected with the Δp85α construct and stimulated with EGF. Anti-phosphotyrosine immunoprecipitates were analysed by western blotting with anti-PI3KC2β antiserum. (E) 293 cells were transfected with a construct encoding myc-tagged PI3KC2β wild-type or DN. Anti-myc tag immunoprecipitates were analysed by western blotting with anti-PI3KC2β antiserum (left) or assayed for in vitro PI3K activity (right). (F) 293 cells transfected with the cDNA constructs indicated were stimulated with EGF. Anti- phosphotyrosine or anti-myc tag immunoprecipitates were analysed by western blotting with anti-PI3KC2β antiserum or with anti-p85 antibodies.
Mammalian cells were then transiently transfected with a cDNA construct encoding myc-tagged PI3KC2β (Arcaro et al., 1998) and its activation compared with the endogenous kinase. EGF stimulation of 293 cells induced rapid tyrosine phosphorylation of both myc-tagged and native PI3KC2β with similar time courses (Figure 7C). Strikingly, Δp85α-transfected cells showed a significant reduction in the amount of endogenous PI3KC2β associated with phosphotyrosine-containing signalling complexes (Figure 7D). Thus the Δp85α construct is not a selective inhibitor of class I PI3Ks, as it also interferes with class II PI3K activation.
To inhibit signalling via PI3KC2β, a kinase-inactive mutant construct was generated by site-directed mutagenesis. The PI3KC2β D1213A construct (PI3KC2βDN) was transiently transfected into HEK293 cells, purified and shown to have significantly (>99%) reduced lipid kinase activity towards PI and PI(4)P in vitro, when compared with the wild-type enzyme (Figure 7E; data not shown). EGF stimulation of transiently transfected 293 cells induced an increase in the amount of recombinant PI3KC2β wild-type and DN associated with phosphotyrosine-containing signalling complexes (Figure 7F), indicating that both constructs can couple to EGFR signalling with comparable efficiencies. Importantly, neither PI3KC2β wild-type, nor the DN mutant, impaired the EGF-stimulated recruitment of p85 to phosphotyrosine-containing signalling complexes in 293 cells (Figure 7F). This demonstrates that the PI3KC2βDN construct can be used to specifically investigate the involvement of the enzyme in the activation of downstream targets.
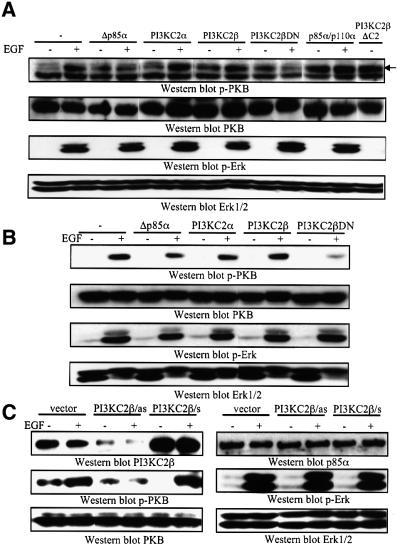
We then evaluated the impact of various transiently transfected class IA and II cDNA constructs on the activation of PKB and Erk. Human 293 cells displayed EGF-stimulated activation of PKB and Erk1/2 (Figure 8A), with time courses comparable to the responses observed in SCLC cells. The dominant-negative Δp85α construct impaired activation of PKB, but not Erk1/2, in response to EGF (Figure 8A). Moreover, transfection of p85α/p110α increased basal and EGF-stimulated PKB, without affecting Erk (Figure 8A). PKB and Erk activation were not affected by transfection of wild-type PI3KC2α or PI3KC2β, but surprisingly, the PI3KC2βDN construct blocked EGF-stimulated PKB activation as efficiently as Δp85α (Figure 8A). Growth factor-induced Erk activation, however, was not impaired by transfection of PI3KC2βDN (Figure 8A). We hypothesized that the inability of transfected PI3KC2β to activate PKB may be due to the fact that it is inactive as a lipid kinase, since its C-terminal C2 domain inhibits the catalytic activity of the enzyme (Arcaro et al., 1998). Indeed, in support of this notion, a transiently transfected C2 deletion mutant of the enzyme, expressed at levels equivalent to that of the wild-type (Figure 7F), stimulated PKB to an extent similar to the response observed with EGF (Figure 8A). Together, these data suggest that both p85/p110α and PI3KC2β can activate PKB in mammalian cells. To confirm these observations, we tested the effect of various PI3K constructs on EGF-stimulated PKB and Erk activation in COS cells. In these cells, both transiently transfected Δp85α and PI3KC2βDN blocked EGF-stimulated PKB activation, without affecting Erk (Figure 8B).
Fig. 8. Transfection of class IA or II PI3Ks differentially affects downstream signalling cascades in mammalian cells. (A) HEK293 or (B) COS cells were transiently transfected with various PI3K constructs (as indicated). Serum-starved cells were stimulated with EGF (50 nM) for 5 min at 37°C, and samples analysed by western blotting for activated PKB (p-PKB), activated Erk1/2 (p-Erk1/2) or total respective proteins. (C) 293 cells were transfected with antisense PI3KC2β construct (as), wild-type PI3KC2β (s) or empty vector. Serum-starved cells were stimulated with EGF (50 nM) for 5 min at 37°C, and samples analysed by western blotting for PI3KC2β, p85α, activated PKB (p-PKB), activated Erk1/2 (p-Erk1/2) or total respective proteins.
To further substantiate the results obtained with the PI3KC2βDN mutant, an antisense approach was used to decrease the levels of endogenous PI3KC2β in 293 cells. The cDNA construct encoding wild-type PI3KC2β was subcloned in the antisense orientation into a mammalian expression vector and transfected into 293 cells. After 48 h of transfection, a significant decrease in the endogenous levels of PI3KC2β was observed (Figure 8C), while the protein levels of p85α, PKB and Erk were not significantly altered. EGF-stimulated PKB activation was impaired in the cells transfected with the antisense PI3KC2β construct, while no effect was observed on Erk activation (Figure 8C). As a control, 293 cells were transfected with a cDNA construct encoding PI3KC2β in the sense orientation. Increased levels of the enzyme were observed in lysates from transfected 293 cells, but this did not result in any effect on PKB or Erk activation in response to EGF (Figure 8C). Together, these results demonstrate that PI3KC2β contributes to PKB activation by EGF in 293 and COS cells.
Inhibition of PI3KC2β in SCLC cells selectively affects PKB activation by polypeptide growth factors
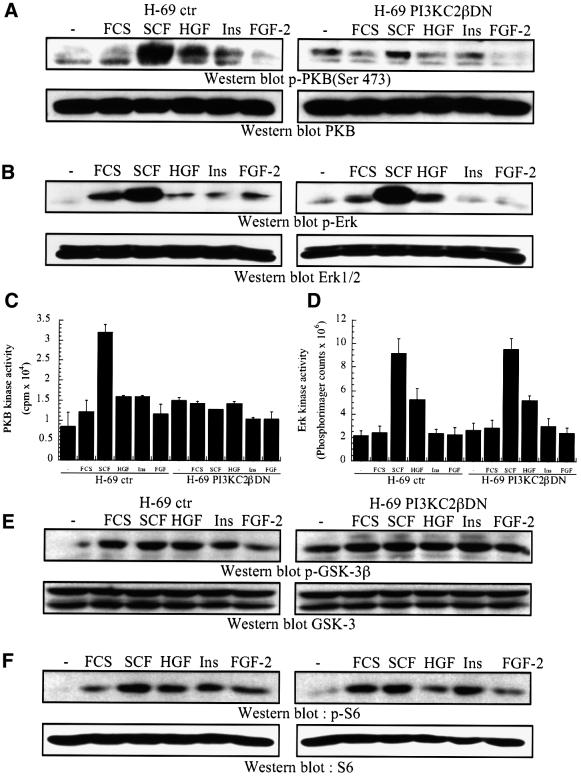
The cDNA construct encoding PI3KC2βDN was stably transfected into H-69 SCLC cells, and its impact on growth factor-stimulated responses evaluated. SCF- stimulated PKB S473 phosphorylation was impaired, as compared with control H-69 cells transfected with an empty vector (Figure 9A). This correlated with a dramatic inhibition of SCF-stimulated PKB activation, as assessed by in vitro immune complex kinase assays (Figure 9C). PKB activation in response to HGF and insulin were also impaired (Figure 9A and C).
Fig. 9. Transfection of kinase-inactive PI3KC2β selectively inhibits PKB activity. (A–F) H-69 cells transfected with an empty vector or PI3KC2βDN were treated with SCF (10 ng/ml), HGF (10 ng/ml), insulin (3 µg/ml) or FGF-2 (1 ng/ml) for 5 min, or (F) 10 min, at 37°C. (A and B) and (E and F) Samples were analysed by western blotting with antibodies specific for (A) activated PKB (p-PKB Thr308) or total PKB, (B) activated Erk1/2 (p-Erk1/2) or total Erk, (E) Ser9-phosphorylated GSK-3β (p-GSK-3β) or total GSK-3, (F) Ser240/244-phosphorylated S6 protein (p-S6) or total S6 protein. Alternatively, anti-PKB (C) or anti-Erk (D) immunoprecipitates were assayed for in vitro protein kinase activity, using crosstide (C) or myelin basic protein (D) as substrates.
In contrast, Erk activation by SCF was not significantly reduced in transfected compared with control H-69 cells, as assessed by western blotting with phospho-specific antibodies (Figure 9B) or in vitro immune complex kinase assays (Figure 9D).
The phosphorylation of either GSK-3β (Ser9) or ribosomal S6 protein (Ser240/244) in response to polypeptide growth factors was not affected by the transfection of PI3KC2βDN (Figure 9E and F). These results show that PI3KC2β mediates polypeptide-stimulated PKB activation in SCLC cells, confirming the results observed in model mammalian cells (Figure 8).
SCLC cell growth in response to polypeptide growth factors is dependent on PI3Ks
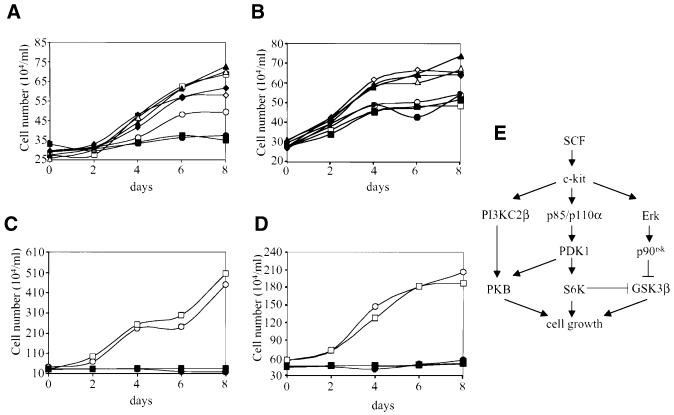
In order to assess the importance of PI3Ks in SCLC cell growth, we first studied the impact of pharmacological inhibitors on cell growth in liquid culture. The growth rate of H-69 cells was increased 2-fold upon addition of SCF or insulin, while the responses to FGF-2 and HGF were weaker (Figure 10A; data not shown). Strikingly, the inhibitor LY294002 completely blocked SCF-stimulated cell growth, but had no effect on the response to insulin or FGF-2 (Figure 10A). Basal H-69 cell growth was also inhibited by LY294002 (Figure 10A).
Fig. 10. SCF-stimulated SCLC cell growth is dependent on PI3K signalling. (A) H-69 cells or (B) H-69 transfected with PI3KC2βDN were treated with SCF (10 ng/ml, squares), insulin (3 µg/ml, triangles), FGF-2 (1 ng/ml, diamonds) or no growth factor (circles) in the presence (closed symbols) or absence (open symbols) of LY294002 (10 µM) and cell number determined by counting every 2 days. (C) H-209 or (D) H-510 cells were treated with SCF (squares) or no growth factor (circles) in the presence (closed symbols) or absence (open symbols) of LY294002 and cell number determined by counting every 2 days. The growth factors and inhibitor were added at 0 and 4 days. (E) Schematic representation of the PI3K-dependent signalling pathways involved in SCLC cell growth in response to SCF.
We then investigated the effect of the PI3KC2βDN construct on H-69 cell growth. Stably transfected H-69 cells grew in response to insulin or FGF-2, but the response to SCF was completely abolished (Figure 10B). Intriguingly, LY294002 had no effect on basal growth in these transfected cells (Figure 10B). The SCF-stimulated growth of H-69 cells transfected with an empty expression vector was comparable to the response of untransfected cells (data not shown). In contrast to H-69 cells, SCF did not stimulate growth of H-209 or H-510 cells in liquid culture, but LY294002 inhibited basal growth (Figure 10C and D). Together these results show that p110α-overexpressing SCLC cells display enhanced growth in response to SCF, and that PI3KC2β activity is essential for SCF-stimulated cell growth.
Discussion
The overexpression of PI3K isoforms in SCLC cells may be relevant for the biology of the disease, since PTEN mutations have not commonly been found in this human malignancy (Forgacs et al., 1998). PTEN did not appear to be downregulated in the SCLC cells studied here, as compared with normal lung epithelial cells. Increased levels of PI3Ks may thus provide an alternative mechanism by which tumour cells gain the ability to grow in an anchorage-independent manner. Although a previous report has described constitutive activation of PI3K in human SCLC cell lines (Moore et al., 1998), we show here that polypeptide growth factors such as SCF regulate class IA PI3K activation by recruitment of the p85 regulatory subunit to phosphotyrosine-containing signalling complexes. The superior ability of SCF to activate PKB, as compared with other polypeptide growth factors, correlates well with its ability to induce the association of p85 with complexes of tyrosine-phosphorylated proteins.
The study of the impact of PI3K isoform overexpression on downstream effectors in SCLC cells revealed novel signalling specificities between the class IA catalytic subunits p110α, p110β and p110δ. Overexpression of p110α clearly enhanced SCF-stimulated PKB activation, but did not affect ribosomal S6 protein and GSK-3β phosphorylation, or Erk activation, which have all been shown in previous reports to be regulated by PI3K signalling (Duckworth and Cantley, 1997; Alessi et al., 1998; Wennstrom and Downward, 1999; Frame and Cohen, 2001). The phosphorylation of GSK-3β in response to growth factor stimulation was only partially inhibited by LY294002, which is consistent with the model that alternative signalling pathways such as MAPK/p90rsk can control GSK-3β inhibition (Frame and Cohen, 2001).
The observation that p110α-overexpressing SCLC cells have significantly higher p85-associated PI3K activity than cells expressing p110β alone is in agreement with a previous report which showed that recombinant p110α was found to have a significantly higher Vmax for PI than p110β (Beeton et al., 2000). Thus it is plausible that the selective activation of PKB by overexpressed p110α occurs because this isoform generates higher levels of PIP3 upon polypeptide growth factor stimulation.
Overexpression of p110δ did not impact on activation of PKB or inhibition of GSK-3β, which is surprising, given that this isoform displays identical p85 association and in vitro substrate specificity, as compared with p110α (Vanhaesebroeck et al., 2001). In SCLC cells, p110δ appeared to be phosphorylated on Ser1039, and polypeptide growth factors did not regulate this phosphorylation, which is consistent with the lack of increase in p85-associated lipid kinase activity upon p110δ overexpression. It is conceivable that p110α and p110δ are not activated under the same conditions in these cells, and that different growth factors or stimuli from those investigated here feed into p110δ.
We show here for the first time that class II PI3Ks participate in polypeptide growth factor signalling. The association of PI3KC2β with c-Kit in SCLC cells is clearly constitutive, as we have observed previously for the interaction between PI3KC2β and erbB1 or erbB2 (Arcaro et al., 2000). However, formation of the c-Kit–PI3KC2β complex does not appear to require Grb2, as has been reported for the EGFR (Wheeler and Domin, 2001).
Although transfection of the wild-type enzyme into mammalian cells did not impact on PKB activation, unlike p85/p110α, the PI3KC2βDN mutant inhibited both SCF- and EGF-stimulated PKB in three different cell systems. Moreover, an activated PI3KC2β variant lacking the inhibitory C2 domain induced PKB activation in the absence of growth factor stimulation. Decreased cellular levels of PI3KC2β resulting from transfection of an antisense construct also impaired PKB activation in response to growth factors. Together, these data demonstrate that both p85/p110α and PI3KC2β can transmit polypeptide growth factor signals leading to PKB activation. However, kinase-inactive PI3KC2β did not affect other downstream targets of class IA enzymes such as GSK-3β or S6K. It is striking to note that c-Kit (SCLC cells), the EGFR (293 cells) and the PDGFR (NIH 3T3 cells; Arcaro et al., 2000) recruit both p85/p110α and PI3KC2β. Thus, there appears to be little specificity in receptor coupling between class IA and II PI3Ks. A possible explanation for these findings is that both enzymes are required for optimal PKB activation in response to growth factor stimulation. Given the differences in substrate specificity between the two classes of enzyme, it can be speculated that the production both of PIP3 by p85/p110α and of PI(3,4)P2 by PI3KC2β participates in PKB activation by different mechanisms, which is consistent with recent reports (Scheid et al., 2002).
Numerous reports have implicated class IA PI3Ks in PKB activation by polypeptide growth factors (Vanhaesebroeck et al., 2001). However, commonly used reagents such as pharmacological inhibitors, or the dominant-negative Δp85α construct, are not selective for class IA PI3Ks, since both affect PI3KC2β signalling (Arcaro et al., 1998; this study). This suggests that the effects of wortmannin/LY294002 or Δp85α on PKB activation by growth factors may reflect the inhibition of two different PI3K isoforms.
In support of this notion, PKB activation in response to IGF-I was only partially impaired in cells derived from mice bearing a homozygous deletion of the Pik3r1 gene, which encodes the regulatory subunits of class IA PI3Ks (Ueki et al., 2002). These observations are consistent with the results presented here that show that polypeptide growth factors signal via two distinct PI3K isoforms to activate PKB.
PI3K activity is essential for SCLC cell growth in response to SCF, but other polypeptide growth factors such as insulin or FGF-2 stimulate cell growth in a PI3K-independent manner, as reported previously (Pardo et al., 2001). SCF-stimulated cell growth may involve distinct signalling pathways downstream of PI3K, involving S6K, GSK-3β and PKB (Figure 10E). Overexpression of p110α in SCLC cells increased cell growth in response to SCF, which correlated with selectively enhanced PKB activation. Moreover, in H-69 cells, PI3KC2βDN blocked both PKB activation and cell growth in response to SCF. Together these results imply that: (i) ribosomal S6 protein and GSK-3β phosphorylation are not sufficient for the induction of SCLC cell growth in response to SCF; and (ii) PKB activation is essential for SCF-stimulated cell growth, which is in good agreement with previous reports in other cell systems (Tuttle et al., 2001). On the other hand, the negative impact of LY294002 on basal SCLC growth may result from its ability to block basal PKB activity.
Thus, specific PI3K isoforms may represent novel targets for the development of drugs aimed at inhibiting SCLC cell growth.
Materials and methods
Cell culture
Human SCLC cell lines (NCI-HC-33, NCI-H-69, NCI-H-209, NCI-H-510, NCI-H-524, NCI-H-1045, NCI-H-1622 and NCI-H-2171) were cultured as described by Pardo et al. (2001). For experimental purposes the SCLC cells were diluted into serum-free medium (Pardo et al., 2001) and grown for 3–5 days. For liquid culture assays, SCLC cells were cultured in serum-free medium without insulin. HEK293 cells and COS-7 cells were cultured as described previously (Arcaro et al., 2000). For serum starvation, the cells were incubated for 16 h in medium containing 0.5% (v/v) heat-inactivated fetal calf serum.
Transient expression in mammalian cells
HEK293 or COS-7 cells were grown to 50–60% confluence on 100 mm dishes and transfected with cDNA constructs in pcDNA3 (Invitrogen) or pMT2 using calcium phosphate, exactly as described previously (Arcaro et al., 1998). The cells were harvested 48 h post-transfection and analysed for gene expression.
Stable expression in mammalian cells
H-69 SCLC cells were transfected with the cDNAs encoding myc-tagged PI3KC2βDN in pcDNA3, or with empty vector, using the lipofectin reagent (Life Technologies). The cells were split 1:4 into growth medium containing 1.0 mg/ml G418 (Calbiochem) 48 h after transfection. A population of resistant H-69 cells appeared after 1–2 weeks and was expanded in selection medium. The cells were tested for expression of recombinant PI3KC2β by anti-myc tag immunoprecipitation and western blot analysis.
Immunoprecipitations, PI3K assays and western blot analysis
These assays were performed exactly as described previously in Arcaro et al. (1998).
Generation of PI3KC2βDN mutant and antisense construct
A point mutation in the active site of PI3KC2β was introduced by site-directed mutagenesis at residue 1213, leading to a DFG to AFG substitution, which has been shown previously to lead to the generation of kinase-inactive p110α (Dhand et al., 1994). To generate the antisense construct, the cDNA fragment encoding wild-type PI3KC2β (Arcaro et al., 1998) was subcloned in the antisense orientation into pcDNA3.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Drs D.Alessi, R.C.Coombes, J.Downward, A.Gazdar, C.H.Heldin, R.Marais and A.Virmani for providing reagents and cell lines and Miss B.L.Ng for performing FACS analysis. This work was supported by a Program Grant from Cancer Research UK (M.J.S.) and a Research Grant from the Royal Society (A.A.).
References
- Alessi D.R., James,S.R., Downes,C.P., Holmes,A.B., Gaffney,P., Reece,C. and Cohen,P. (1997) Purification and characterization of a phosphatidylinositol 3,4,5 trisphosphate-dependent protein kinase (PDK1) which phosphorylates and activates protein kinase Bα. Curr. Biol., 7, 261–269. [DOI] [PubMed] [Google Scholar]
- Alessi D.R., Kozlowski,M.T., Weng,Q.P., Morrice,N. and Avruch,J. (1998) 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol., 8, 69–81. [DOI] [PubMed] [Google Scholar]
- Arcaro A., Volinia,S., Zvelebil,M.J., Stein,R., Watton,S.J., Layton,M.J., Gout, I., Ahmadi, K., Downward,J. and Waterfield,M.D. (1998) Human phosphoinositide 3-kinase C2β, the role of calcium and the C2 domain in enzyme activity. J. Biol. Chem., 273, 33082–33090. [DOI] [PubMed] [Google Scholar]
- Arcaro A., Zvelebil,M.J., Wallasch,C., Ullrich,A., Waterfield,M.D. and Domin,J. (2000) Class II phosphoinositide 3-kinases are downstream targets of activated polypeptide growth factor receptors. Mol. Cell. Biol., 20, 3817–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton C.A., Chance,E.M., Foukas,L.C. and Shepherd,P.R. (2000) Comparison of the kinetic properties of the lipid- and protein-kinase activities of the p110α and p110β catalytic subunits of class-Ia phosphoinositide 3-kinases. Biochem. J., 350, 353–359. [PMC free article] [PubMed] [Google Scholar]
- Brown R.A., Domin,J., Arcaro,A., Waterfield,M.D. and Shepherd,P.R. (1999) Insulin activates the α isoform of class II phosphoinositide 3-kinase. J. Biol. Chem., 274, 14529–14532. [DOI] [PubMed] [Google Scholar]
- Burgering B.M.T. and Coffer,P.J. (1995) Protein kinase B (c-akt) in phosphatidylinositol 3-kinase signal transduction. Nature, 376, 599–602. [DOI] [PubMed] [Google Scholar]
- Cantley L.C. and Need,B.G. (1999) New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl Acad. Sci. USA, 96, 4240–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand R. et al. (1994) PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J., 13, 522–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domin J., Dhand,R. and Waterfield,M.D. (1996) Binding to the platelet-derived growth factor receptor transiently activates the p85α–p110α phosphoinositide 3-kinase complex in vivo. J. Biol. Chem., 271, 21614–21621. [DOI] [PubMed] [Google Scholar]
- Duckworth B.C. and Cantley,L.C. (1997) Conditional inhibition of the mitogen-activated protein kinase cascade by wortmannin. Dependence on signal strength. J. Biol. Chem., 272, 27665–27670. [DOI] [PubMed] [Google Scholar]
- Forgacs E. et al. (1998) Mutation analysis of the PTEN/MMAC1 gene in lung cancer. Oncogene, 17, 1557–1565. [DOI] [PubMed] [Google Scholar]
- Frame S. and Cohen,P. (2001) GSK3 takes centre stage more than 20 years after its discovery. Biochem. J., 359, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman D.A., Snapper,S.B., Yballe,C.M., Davidson,L., Yu,J.Y., Alt,F.W. and Cantley,L.C. (1999) Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science, 283, 393–397. [DOI] [PubMed] [Google Scholar]
- Hara K. et al. (1994) 1-Phosphatidylinositol 3-kinase activity is required for insulin-stimulated glucose transport but not for RAS activation in CHO cells. Proc. Natl Acad. Sci. USA, 91, 7415–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann-Zeh A., Rodriguez-Viciana,P., Ulrich,E., Gilbert,C., Coffer,P., Downward,J. and Evan,G. (1997) Suppression of c-myc-induced apoptosis by Ras signaling through PI(3)K and PKB. Nature, 385, 544–548. [DOI] [PubMed] [Google Scholar]
- Kozma S.C. and Thomas,G. (2002) regulation of cell size in growth, development and human disease: PI3K, PKB and S6K. BioEssays, 24, 65–71. [DOI] [PubMed] [Google Scholar]
- Moore S.M., Rintoul,R.C., Walker,T.R., Chilvers,E.R., Haslett,C. and Sethi,T. (1998) The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorage-independent proliferation via a protein kinase B and p70s6k-dependent pathway. Cancer Res., 58, 5239–5247. [PubMed] [Google Scholar]
- Pardo O.E., Arcaro,A., Salerno,G., Tetley,T.D., Valovka,T., Gout,I. and Seckl,M.J. (2001) Novel cross talk between MEK and S6K2 in FGF-2 induced proliferation of SCLC cells. Oncogene, 20, 7658–7667. [DOI] [PubMed] [Google Scholar]
- Scheid M.P., Huber,M., Damen,J.E., Hughes,M., Kang,V., Neilsen,P., Prestwich,G.D., Krystal,G. and Duronio,V. (2002) Phosphatidyl inositol(3,4,5)P3 is essential but not sufficient for PKB activation: Phosphatidylinositol(3,4)P2 is required for PKB phosphorylation at Ser473. J. Biol. Chem., 277, 9027–9035. [DOI] [PubMed] [Google Scholar]
- Tuttle R.L., Gill,N.S., Pugh,W., Lee,J.P., Koeberlein,B., Furth,E.E., Polonsky,K.S., Naji,A. and Birnbaum,M.J. (2001) Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med., 7, 1133–1137. [DOI] [PubMed] [Google Scholar]
- Ueki K., Fruman,D.A., Brachmann,S.M., Tseng,Y.-H., Cantley,L.C. and Kahn,C.R. (2002) Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3-kinase regulates cell signaling and survival. Mol. Cell. Biol., 22, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Higashi,K., Raven,C., Welham,M., Anderson,S., Brennan,P., Ward,S.G. and Waterfield,M.D. (1999) Autophos phorylation of p110δ phosphoinositide 3-kinase: a new paradigm for the regulation of lipid kinases in vitro and in vivo. EMBO J., 18, 1292–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Leevers,S.J., Ahmadi,K., Timms,J., Katso,R., Driscoll,P.C., Woscholski,R., Parker,P.J. and Waterfield,M.D. (2001) Synthesis and functions of 3-phosphorylated inositol lipids. Annu. Rev. Biochem., 70, 535–602. [DOI] [PubMed] [Google Scholar]
- Wennstrom S. and Downward,J. (1999) Role of phosphoinositide 3-kinase in activation of ras and mitogen-activated protein kinase by epidermal growth factor. Mol. Cell. Biol., 19, 4279–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M. and Domin,J. (2001) Recruitment of the class II phosphoinositide 3-kinase C2β to the epidermal growth factor receptor: role of Grb2. Mol. Cell. Biol., 21, 6660–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]