Abstract
Most patients with the syndrome resistance to thyroid hormone (RTH) express a mutant thyroid hormone receptor β (TRβ) with transdominant negative transcriptional effects. Since no patient with a mutant TRα has been identified, we introduced a point mutation into the mouse thyroid hormone receptor (TRα1) locus originally found in the TRβ gene, that reduces ligand binding 10-fold. Heterozygous 2- to 3-week- old mice exhibit a severe retardation of post-natal development and growth, but only a minor reduction in serum thyroxine levels. Homozygous mice died before 3 weeks of age. Adult heterozygotes overcome most of these defects except for cardiac function abnormalities, suggesting that other factors compensate for the receptor defect. However, the additional deletion of the TRβ gene in this mouse strain caused a 10-fold increase in serum thyroxine, restored hormonal regulation of target genes for TRs, and rescued the growth retardation. The data demonstrate a novel array of effects mediated by a dominant negative TRα1, and may provide important clues for identification of a potentially unrecognized human disorder and its treatment.
Keywords: thyroid hormone receptor/transgenic mice
Introduction
The syndrome resistance to thyroid hormone (RTH) was first described during the 1960s (Refetoff et al., 1967), and has since been well characterized both in terms of disease and underlying molecular mechanisms. The majority of patients harbour a mutation in the thyroid hormone receptor β (TRβ) gene (Weiss and Refetoff, 1996, 2000). The TRα and TRβ genes, located on human chromosomes 17 and 3, respectively, belong to the family of nuclear hormone receptors (NRs) that function as ligand-modulated transcription factors (Mangelsdorf et al., 1995). The TRs, like many other NRs, regulate target gene expression in four different ways, depending on the availability to ligand (Cheng, 2000). One class of targets is repressed by the unliganded receptor and is usually activated in the presence of ligand (Nagy et al., 1999; Hermansson et al., 2002). A second class of target genes is activated by the aporeceptor, and is repressed by the thyroid hormone (TH)–TR complex (Love et al., 2000). About 50% of the target genes appear to be downregulated by the ligand-bound TR (Feng et al., 2000; Flores-Morales et al., 2002), indicating that transcriptional repression is a major feature of thyroid hormone action.
More than 250 RTH patient families have been analysed by DNA sequencing, and the mutations found have clustered in three main regions of TRβ (Chatterjee and Beck-Peccoz, 2001). One class of mutations predominantly affects binding of co-repressors, resulting in a relatively mild resistance to the action of thyroid hormone (TH). The two other classes of mutations reduce or abolish binding of either the co-activator or the ligand T3, which in turn also diminishes co-activator binding. These mutant receptors exhibit transdominant negative properties and fail to properly downregulate thyroid-stimulating hormone (TSH) production as a response to excess TH. The elevated TH level renders a tissue expressing the transdominant negative receptor along with products of normal TRβ or TRα1 alleles to become phenotypically hypothyroid, whereas tissues that express only TRα1 retain sensitivity to TH and appear hyperthyroid. Therefore, the patients can have goitre, learning disabilities and growth retardation in childhood, which are common hallmarks of hypothyroidism, and which can be accompanied by elevated heart rate (tachycardia) and increased metabolic rate as signs of hyperthyroidism (Yen, 2001).
Despite the large number of RTH patients analysed, none has been shown to have a mutant TRα1. There are several possible explanations for this: a mutant TRα1 could be innocuous or give mild effects that would not be regarded as abnormal in the general population; the mutant receptor may cause spontaneous abortions at embryonic or fetal stages of development, which are rarely investigated from a genetic endocrine point of view; or the symptoms of such a patient would not easily be thought to be associated with a defect in a thyroid hormone receptor, in contrast to RTH where abnormal circulating thyroid hormones and other clinical features readily signify a disorder of hormone action.
To understand the transdominant properties of a mutant TRα1, we have introduced a point mutation originally described in TRβ (R438C) of a RTH patient family (Adams et al., 1994) into the C-terminal region of mouse TRα1. The mutation was chosen because it still allowed T3 binding, albeit at a lower affinity, thus potentially reducing the risks for serious pre- or post-natal effects, as well as allowing hormone treatments to overcome any negative effects of the mutant receptor. Mice harbouring this mutation in the TRα1 locus had TH levels in the ‘low normal’ range, but exhibited serious retardation in post-natal development as judged by a number of different parameters. These deficiencies were largely absent in adult mice, suggesting that other mechanisms acted to overcome the impediment caused by the mutant receptor. However, an increase in circulating TH levels, accomplished by ablation of the TRβ gene, alleviated several of the deficiencies observed. Our data suggest that the mutant receptor acted in a dominant negative fashion, and thus emphasize the importance in normal physiology of gene repression by TRs. The results also indicate that an impairment in development, if seen in patients harbouring an equivalent mutation in the human TRα gene, could be overcome by treatment with TH during the critical periods of juvenile development.
Results
Introduction of a dominant negative TRα1 allele into the mouse genome
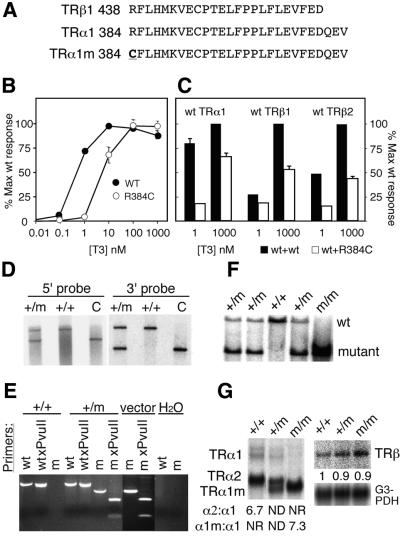
Since a strongly dominant negative TRα1 was expected to cause a severe phenotype if introduced into the mouse genome, we chose a weaker allele that retained residual hormone-binding capacity. The arginine to cysteine change (R438C) described in a TRβ RTH patient family (Adams et al., 1994) was therefore introduced into helix 12 of the C-terminal region of TRα1, yielding the equivalent TRα1R384C mutant receptor (Figure 1A). Transfection experiments (Figure 1B) showed that TRα1R384C failed to activate a reporter gene at 1 nM T3, whereas a 100-fold increase in concentration of ligand caused activation similar to that seen with a wild-type (wt) receptor. Furthermore, TRα1R384C cotransfected with equimolar amounts of wt receptors strongly suppressed transactivation by TRα1 and TRβ2 at 1 nM T3, whereas TRβ1 was less suppressed (Figure 1C). The inhibition was overcome in part at the higher ligand concentration tested, 1 µM, reducing transactivation by 25–50% (Figure 1C). We conclude that TRα1R384C has dominant negative properties in trans, similar to those described for its mutant TRβ counterpart.
Fig. 1. Characterization of the dominant negative TRα1 allele. (A) Comparison of the C-terminal sequences of mouse TRα1 and TRβ, and highlights the Arg to Cys change introduced into TRα1. (B) Dose response in transactivation resulting from cotransfecting human 293 cells with plasmids expressing either the wt or the mutant receptor, along with a reporter plasmid containing the TRE–PAL response elements in front of a TK–luciferase cassette. The data represent the average of five independent experiments. (C) Dominant-negative properties of TRα1R384C on wt TRs upon transfection of equimolar amounts of the respective receptor encoding plasmids. (D) Correct homologous recombination as determined by Southern blot analyses of BamHI cleaved genomic ES cell DNA hybridized with 5′ and 3′ probes that detect sequences adjacent to but outside the targeting vector. Lanes marked C are controls containing cleaved genomic DNA from homozygous TRα2–/– mice that yield fragments of the same size as the TRα1R384C targeted allele. (E) Verifies that the point mutation, which leads to the Arg to Cys change, is in the coding region of TRα1. Genomic DNA encoding exon 9 was PCR amplified and cleaved with PvuII, which cleaves only in the mutant allele and in the vector control. (F) The presence of all three possible genotypes after a heterozygous cross are shown. Tail DNA was digested with BamHI and hybridized with a 3′ probe after electrophoresis. (G) Examination of the relative levels of TRα1 and TRβ poly(A)+ RNA from brain in a northern blot analysis. The receptor bands were quantified by phosphoimager analysis and standardized to the G3PDH RNA levels. m indicates the primer for (E) or the allele encoding the mutant TRα1R384C. Error bars show SEM.
The R384C mutation was successfully introduced by homologous recombination (Saltó et al., 2001) into one of 1900 mouse embryonal stem cell clones, as determined by Southern blotting and PCR analyses (Figure 1D and E). Chimeric founder mice were bred for three generations against C57Bl, following which heterozygotes were intercrossed to yield mice hetero- and homozygous for the mutant allele (Figure 1F). Genotype analyses of the offspring showed that fewer mice hetero- (+/m) or homozygous (m/m) for the mutation were born or survived until genotyping was completed 1–2 weeks after birth: 80 were TRα1+/+, 120 TRα1+/m and only 19 TRα1m/m.
As expected (Wikström et al., 1998; Saltó et al., 2001; Gullberg et al., 2002), the introduction of the NeoR cassette into the TRα1 locus abrogated transcription into exon 10, which encodes the C-terminal domain of the non-hormone-binding variant receptor protein TRα2, as shown by northern analysis of brain RNA (Figure 1G). As an inevitable consequence, the expression level of TRα1R384C in homozygous mice was increased (6- to 7-fold) in comparison with TRα1 in wt animals, whereas no effect was seen on TRβ.
Function of the pituitary–thyroid axis
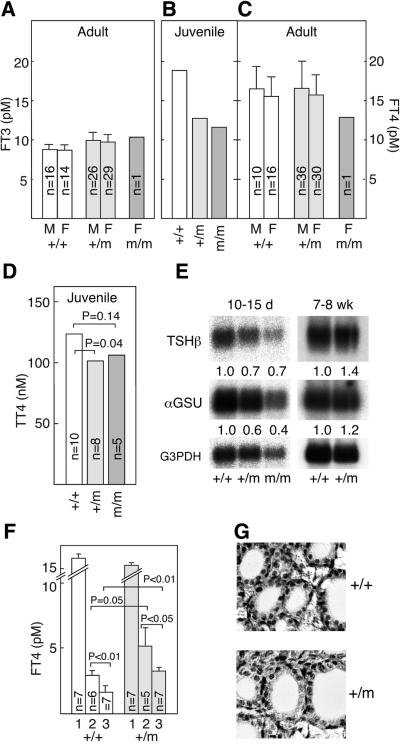
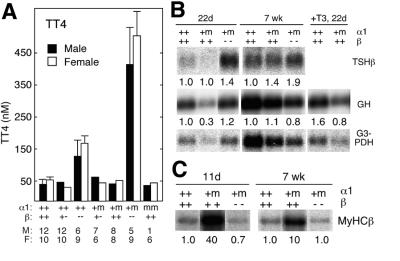
Adult (7- to 8-week-old) TRα1+/m mice of either sex and one TRα1m/m mouse (see below) exhibited normal serum levels of free T3 and T4 (Figure 2A and C). In contrast, juvenile (10- to 15-day-old) mutant mice showed reduced serum levels of both free T4 and total T4 (Figure 2B and D). However, the reduction was minor and falls within the low normal range in mice (data not shown). Surprisingly, the RNA expression levels of pituitary TSHβ and the common αGSU subunit were reduced in the juveniles, although they were normal in adults (Figure 2E). To test whether a residual impairment in the pituitary–thyroid axis persisted in the adults, TRα1+/m and wt mice were injected for three consecutive days with 0.2 or 5 µg of T3 and their free T4 levels determined. Figure 2F shows that the mutant mice had 30–40% higher T4 levels after the treatments, as compared with wt controls, suggesting that the mutant receptor interferes with the control of thyroid hormone production also in adult mice. Histological analyses of thyroid glands of adult (Figure 2G) or juvenile TRα1+/m mice (data not shown) revealed no obvious defects.
Fig. 2. Function of the pituitary–thyroid axis for control of TH production. (A–C) Levels of free T3 and T4 in serum from adult and juvenile mice. A statistically significant difference could be established between the samples in (A) and (C). Serum samples from five 10- to 15-day-old pups were pooled to generate enough material of the respective genotypes for the radioimmunoassay in (B). The numbers of animals tested are indicated in the figure. (D) Analysis of total T4 in individual 10- to 15-day-old pups. (E) Reduced expression in juvenile mice of RNAs for the TSHβ and αGSU subunits in TSH. Three (adults) to five (pups) pituitaries were pooled, and a northern blot with poly(A)+ was developed with the respective probes. Quantification was performed as described in Figure 1. (F) Demonstration of an impairment in downregulation seen in the TRα1+/m mice. Serum samples from the indicated numbers of mice were taken before (bars labelled 1) and after four daily injections of 0.2 (bars 2) or 5 µg (bars 3) of T3 per animal. The free T4 levels were determined at day 5. (G) Thyroid glands of heterozygotes have a normal appearance. Error bars show SEM.
Post-natal development and growth
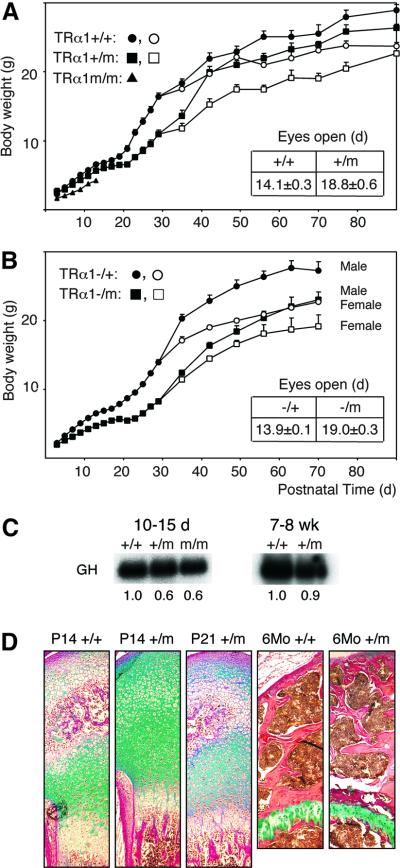
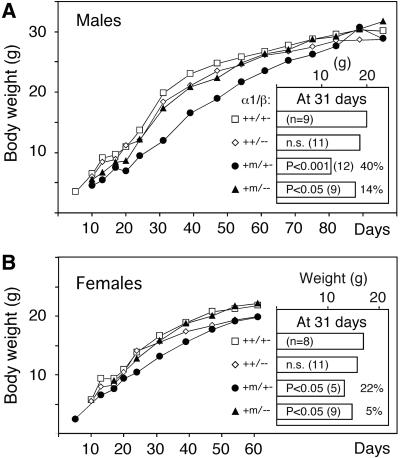
Figure 3A shows that most mice homozygous (TRα1m/m) for TRα1R384C died within 2 weeks after birth, although a few spontaneously survived to adulthood. Furthermore, 10-day-old TRα1+/m and m/m mice exhibited lower body weight, as compared with wt controls, a difference that was increased to 33% in +/m mice at 30 days of age. However, 90-day-old +/m mice weighed only 7% less than wt controls. The TRα1+/m mice also had delayed eye opening (by 4–5 days; Figure 3A, inset), indicating that the mutant allele affected juvenile development.
Fig. 3. Retarded growth and development. (A) Increase in body weight of wt, heterozygous and homozygous mice. The data for male and female mice were pooled until day 30 (closed symbols). The total number of mice until day 30 was 30 wt, 33–43 +/m and 8 m/m mice; after this time 15–22 wt and 16–23 +/m mice were used for each point. Closed symbols, males; open symbols, females. The inset shows the time when the mice had opened both eyes. (B and the inset) Similar retardation in growth and delay in eye opening in mice carrying both a null and an R384C TRα1 allele. Each point after day 30 represents 8–10 mice. (C) Reduced expression of pituitary RNA for GH in young but not adult mice. The filter described in Figure 2E was rehybridized with a GH probe and quantified. (D) Delayed maturation of the distal femur in juvenile mice. Histological sections of P14, P21 and 6-month-old mice were stained with Alcian Blue/Van Gieson dye. Note the similarity in maturation between P14 wt and P21+/m mice and the absence of differences between the adult mice. Cartilage area, green-blue; bone area, red. The growth plate is indicated by gp. Magnification, 20×; error bars show SEM.
To determine whether the difference in survival between the +/m and m/m mice was due to the presence of a counteracting wt allele in the former strain or two copies of the mutant in the latter, TRα1+/m mice were bred against TRα1–/– animals (which still express TRα2 at normal levels; Wikström et al., 1998). Figure 3B shows that TRα1–/m mice exhibited a similar juvenile growth retardation (35% at 35 days) and delayed eye opening to the TRα1+/m mice, and that the weight difference between mutant and control mice had decreased in the young adult mice (16% at 70 days). Next, we tested whether elevation of wt TRα1 expression would rescue the growth retardation. TRα1+/m mice were therefore crossed with TRα2–/– knockout mice, which overexpress the TRα1 receptor protein 3- to 6-fold, depending on the tissue (Saltó et al., 2001; Gullberg et al., 2002). The results show that at 30 days of age, the TRα2–/TRα1R384C mice weighed 27% less than TRα2+/– mice (n = 13), and that 70-day-old mice weighed only 5% less than the controls. Taken together, our data indicate that two copies of the TRα1R348C allele are incompatible with life, and that variations in the levels of wt TRα1 expression do not affect the physiological effects of a single mutant allele.
As TH directly regulates production of growth hormone (GH), we determined the levels of pituitary GH RNA by northern analysis. Figure 3C shows that 10- to 15-day-old TRα1+/m and m/m mice expressed significantly reduced GH RNA levels, whereas no reduction was seen in adults.
To verify that the delay in body weight increase represented a retardation in development and growth (as opposed to a persistent growth defect), the bone phenotype was analysed. Figure 3D shows that the femoral joints of P14 TRα1+/m mice showed little ossification as compared with wt mice, whereas the mutants at P21 approached the ossification seen in P14 controls (n = 4). No differences in ossification were seen between wt and mutant femurs from adult mice. Dual X-ray absorptiometry (DXA) scans of adult femora revealed a small but significant decrease in femur length in mutant mice, although no differences were seen in femoral bone mineral density or content (Table I). DXA analysis also showed a normal content of body fat in the TRα1+/m mice, and the serum levels of leptin and IGF-1 were within the normal range (data not shown). Peripheral quantitative computerized tomography (pQCT) measurements revealed minor but significant changes: a cortical bone decrease in volumetric density; an increased endosteal circumference; and an increase in trabecular volumetric density (Table II). The data indicate that the severe delay in bone maturation in juvenile mice is largely overcome in adult mice, although some changes persisted into adulthood.
Table I. DXA measurements.
| wt | TRα1+/m | |
|---|---|---|
| Femur | ||
| Length (mm) | 16.8 ± 0.2 | 15.6 ± 0.2a |
| Areal BMDb (mg/cm2) | 63.7 ± 1.8 | 62.7 ± 3.3 |
| Area (cm2) | 0.51 ± 0.017 | 0.46 ± 0.018 |
| BMCc (mg) | 32.4 ± 1.9 | 29.4 ± 2.6 |
aP ≤ 0.01, eight animals per group were used.
bBMD, bone mineral density.
cBMC, bone mineral content.
Table II. pQCT measurements of the femur.
| wt | TRα1+/m | |
|---|---|---|
| Cortical volumetric density (mg/mm3) | 1.19 ± 0.01 | 1.10 ± 0.02a |
| Cortical area (mm2) | 1.09 ± 0.050 | 0.98 ± 0.056 |
| Cortical BMC (mg/mm) | 1.30 ± 0.072 | 1.08 ± 0.076 |
| Cortical thickness (mm) | 0.22 ± 0.007 | 0.19 ± 0.01b |
| Cortical periosteal circumference (mm) | 5.62 ± 0.12 | 5.82 ± 0.08 |
| Cortical endosteal circumference (mm) | 4.23 ± 0.11 | 4.65 ± 0.08a |
| Trabecular volumetric density (mg/mm3) | 0.37 ± 0.02 | 0.44 ± 0.04b |
aP ≤ 0.01, eight animals per group were used.
bP ≤ 0.05, eight animals per group were used.
Cardiac function
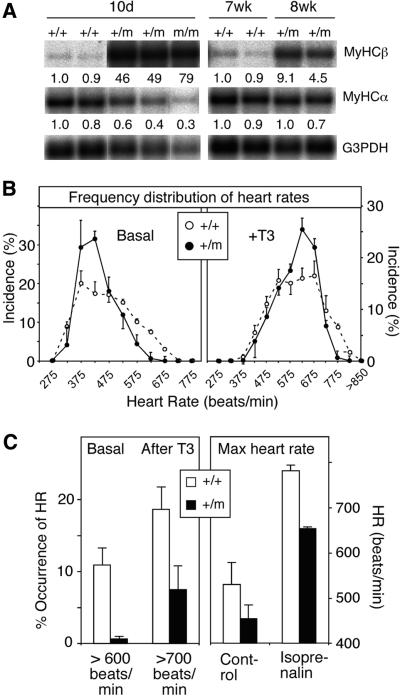
TH regulates the cardiac myosin heavy chain genes (MyHC) α and β positively and negatively, respectively. A comparison of their expression in mutant and wt mice shows that the MyHCβ gene was expressed at 40- to 80-fold elevated levels in 10-day-old mice, with only a 5- to 10-fold elevation in the adults (Figure 4A). MyHCα levels were decreased by 40–70% in the young mice, whereas no significant difference was seen in older mice.
Fig. 4. Cardiac function abnormalities in juvenile and adult mice. (A) Lack of repression of cardiac MyCHβ in 10-day-old and 7- to 8-week-old TRα1+/m mice, whereas expression of the positive regulated MyHCα gene was reduced only in young mice. Each lane represents poly(A)+ RNA from one animal. All the lanes shown are a montage of relevant lanes originating from the same filter that was rehybridized with the indicated probes. The strong signal in the background surrounding some of the RNA bands is due to a strong hybridization signal that yields stray radioactivity. Quantification was performed with a phosphorimager. (B) Distribution of heart rate frequencies recorded by telemetry during a 4 day period before and after injection of T3 (5 µg/mouse/day). Note the lower incidence of high heart rates in the TRα1+/m mice. Four male mice, 8 weeks old and weighing more than 20 g, were used in each group. (C) Left, a quantification of the high frequency heart rates before (>600 beats/min) and after (>700 beats/min) injection of T3. Right, heart rates after injection of isoprenalin. Error bars show SEM.
TRα1 has an important role in regulating heart rate (Wikström et al., 1998). However, the average heart rate in awake adult TRα1+/m mice was not significantly altered, as determined by telemetry (n = 4). Instead, a change in the incidence of low and high heart rates was noted (Figure 4B, left panel). The maximum heart rate during spontaneous activity rarely exceeded 600 beats/min in mutants, contrasting the 12% incidence frequency seen in wt mice (Figure 4C, left). The lower occurrence of high heart rate was not due to a reduced locomotor activity (data not shown). Injections of T3 (5 µg/animal for 4 days) increased the heart rate in both groups of animals (Figure 4B, right), although the mutant mice still exhibited a significantly lower incidence of high heart rate. To determine the maximal heart rate, the catecholamine isoprenalin was injected into the mice. Figure 4C shows that TRα1+/m mice achieved a heart rate of 660 beats/min, whereas the wt controls reached 800 beats/min. The data strongly suggest that adult mutant mice have an impaired cardiac function.
IgM-presenting B cells
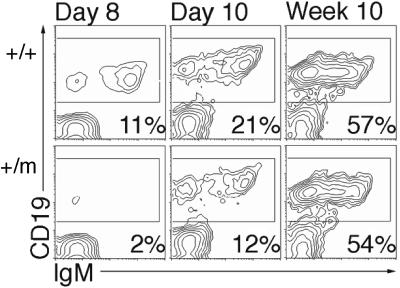
Histological analyses of spleens revealed decreased development of lymphoid cell nodules in young TRα1+/m mice (data not shown). Therefore a FACS analysis of spleen cells from juvenile and adult TRα1+/m mice was performed after labelling with B and T cell-specific markers. A 50–75% decrease in CD19/IgM-positive cells was seen in the 8- to 10-day-old mutant mice, whereas the adults were normal (Figure 5). No perturbances were seen with IgG markers or in T cell distributions (data not shown).
Fig. 5. Delayed appearance of IgM-positive B cells. Spleen cells from four mice of the indicated age and genotype groups were incubated with anti-CD19 antibodies, and subjected to FACS analysis.
Rescue of the growth phenotype by an independent mutation in TRβ
Although injections of TH into suckling TRα1m/m pups inefficiently promoted their survival (data not shown), the results indicated that elevated hormone levels might restore growth by activating the mutant receptor. We therefore tested the hypothesis that an increase of serum T3 and T4 and a subsequent rescue of development and growth could be achieved by deleting the TRβ gene, thus allowing derepression of TSH. The TRα1+/m mice were crossed with the TRβ–/– mice, which have 3-fold elevated TH levels (Forrest et al., 1996), and double heterozygote progeny intercrossed to produce animals with the desired combinations of TR genes. The crosses yielded animals with all the possible genotypes except for TRα1m/mTRβ–/–, suggesting that this combination of TR genes is lethal. However, an increased survival of TRα1m/mTRβ+/+ mice was seen (Figure 7A), although this can be due to a hybrid vigour effect caused by the distinct genetic backgrounds of the TRα1+/m and TRβ–/– mice (Göthe et al., 1999). Growth curves showed that 31-day-old male TRα1+/mTRβ–/– mice weighed 40% less than TRα1+/+TRβ+/– controls, whereas TRα1+/–TRβ–/– males only showed a 14% per cent reduction (Figure 6A). As in the previous experiments, only small differences were seen between the adult mice of different genotypes. Similar results were obtained with female mice, although the growth retardation of the TRα1+/mTRβ+/– genotype was less, at 22% (Figure 6B). The delay in eye opening was partially rescued, occurring at day 15.3 (n = 8) in the TRα1+/mTRβ–/– mice compared with day 18.6 (n = 12) in the TRα1+/mTRβ+/– mice, and day 14 in the wt mice as well as in animals homozygous for wt TRα1 and one or two TRβ null alleles (n = 14).
Fig. 7. Normalization of gene expression in mutant mice with elevated TH levels. (A) The 10-fold increased serum levels of total T4 in the TRα1+/mTRβ–/– mice. The numbers below the diagram indicate the number of male (M) and female (F) mice analysed. (B) Northern blots illustrating the relief of TSH suppression by TRβ deficiency (upper) and an increase of pituitary GH RNA expression in young mice (lower). Pituitary RNA was prepared from pools of glands (three per genotype). The levels of expression were normalized against G3PDH. (C) Normalization of cardiac MyHCβ regulation in the TRα1+/mTRβ–/– mice. The right side of (B) shows the effect of T3 injected into young TRα1+/mTRβ+/+ mice on GH RNA expression. MyHCβ RNA levels were determined in three individual hearts; one representative lane is shown per genotype. Quantification was performed as described in the previous legends. The experiments in (B) and (C) were performed twice, obtaining similar results.
Fig. 6. Rescue of growth and development by an independent mutation in the TRβ gene. TRα1+/m males were bred with TRβ–/– females, and the resulting double heterozygotes intercrossed. Growth curves for male (A) and female (B) progeny were established as described in Figure 3. The differences in body weights at day 31 and the relevant statistical analyses are shown in the insets. The numbers in parentheses in the insets indicate the number of mice used.
To verify that the TH levels indeed were elevated in the adult TRα1+/mTRβ–/– mice, measurements of T4 levels were made. Figure 7A shows that the mutant mice had an ∼10-fold increase in serum total T4 as compared with wt mice, and an ∼3-fold higher level than the TRβ–/– animals. The other genotypes, including TRα1m/mTRβ+/+ mice, had similar or slightly reduced T4 levels as compared with wt mice.
Since the 10-fold elevation in serum T4 levels could potentially allow TRα1R384C regulation of target genes in a hormone-dependent manner, the expression of several genes relevant for the phenotype of TRα1+/mTRβ–/– mice was determined. Figure 7B shows that TSHβ gene expression was markedly elevated in adult TRα1+/mTRβ–/– mice, thus providing an explanation for the increased T4 levels. Mice of other genotypes expressed the TSHβ gene at levels compatible with their serum T4 levels (data not shown). GH mRNA expression (Figure 7B, middle panel) was normalized in the juvenile TRα1+/mTRβ–/– mice, indicating that the growth retardation seen in these animals is caused by reduced GH production. Figure 7C shows that the misexpression of the MyHCβ genes seen in animals carrying at least one wt TRβ allele was restored to normal in young and adult TRα1+/mTRβ–/– mice.
To substantiate that elevated TH levels can normalize target gene expression in TRα1+/mTRβ+/+ mice, 5 µg of T3 per animal were injected into 22-day-old mice, and pituitaries were explanted 2 h later. Figure 7B, right panels, shows that the GH RNA level was normalized. Taken together, the data suggest that the elevated thyroid hormone levels, caused by deletion of the pituitary TRβ function, rescue the growth retardation seen in TRα1+/mTRβ+/– mice by allowing hormone-dependent regulation of target genes.
Discussion
The data presented here show that a dominant negative TRα1 causes a severe delay in post-natal development and growth of the juvenile animal. Interestingly, the phenotype is quite distinct from that caused by a homozygous deletion of TRα1 (Wikström et al., 1998). The results thus highlight the effects of an unliganded TR and underscore the importance of proper control of TH action during post-natal development. Furthermore, the observation that adult mice exhibit very mild effects of TRα1R384C indicates that other mechanisms gradually interfere to compensate for the defective receptor.
Molecular properties of the mutant receptor
In transfection experiments, TRα1R384C exhibited dominant negative activity towards all the known TH-binding TRs, but could still be activated at higher concentrations of ligand. The properties are similar to those conferred by the original mutation, showing that the ligand-binding domains of TRα1 and TRβ are similarly affected by the introduced amino acid change. Analyses of expression of target genes known to be inhibited by a TR aporeceptor were also suppressed in the TRα1+/m mice, and were activated by excess circulating ligand. Furthermore, genes normally suppressed by the wt receptor–ligand complex were dysregulated in the mutant mice, unless TH levels were raised substantially. Thus, both the in vitro and in vivo data suggest that the dysregulation of target gene expression caused by the mutant receptor can be alleviated by excess TH.
Transient retardation in post-natal development and growth
A striking feature of the TRα1+/m mice is their low body weight at juvenile stages of post-natal development. However, a careful examination of the characteristics of their body weight gain revealed that the maximal weight difference, as compared with controls, occurred at ∼30 days of age, after which time the mutant mice slowly approached the weight of wt animals. At adult age, only a small difference in body weight and length of long bones was seen, indicating that the TRα1R384C mutation did not cause a persistent dwarfism, but rather a delayed development. This notion was confirmed by the analysis of several parameters of development in the juvenile mice: eye opening, bone ossification and development of IgM-positive B cells were delayed by 5–10 days. Other signs of delay included a late tooth eruption, necessitating a later weaning time (data not shown). Thus, the delay in development concerns not only growth-related parameters, but involves other distinct organs or tissue systems as well.
The fact that juvenile, but not adult, mutant mice expressed lower levels of pituitary GH provides a possible reason for the growth deficiency, since GH exerts its growth promoting activities both directly and indirectly on target tissues (Kindblom et al., 2001). That the mice overcome the developmental delay suggests that compensatory mechanisms alleviate the negative effects of the mutant receptor. The details of these are unclear, but may involve the action of other maturation promoting factors, possibly in combination with a later occurring surge in post-natal TH levels.
Regulation of TH production
The adult mutant mice had normal levels of TH, as measured by a variety of criteria. This was accompanied by moderately elevated levels of pituitary RNA for the two subunits for TSH, which, in normal mice, should have resulted in increased levels of TH. Our observation that administration of T3 was less efficient in mutant mice than in the controls in lowering T4 levels suggests that TRα1R384C impedes the normal downregulation of TSH RNA, a hypothesis corroborated by the pronounced dysregulation of TH and TSHβ RNA seen in the TRα1+/mTRβ–/– mice. However, this can only provide a partial explanation for the apparent discrepancy between the elevated TSH RNA expression and the normal circulating TH levels. Recently, Saltó et al. (2001) showed that overexpression of TRα1 perturbs thyroid gland function. The TRα1+/m mice overexpress the mutant receptor to the same degree as the mice of Saltó et al. (2001) overexpress TRα1, and it is possible that this contributes to the anomaly.
In contrast to the adult mice, juvenile mutant animals exhibited both a reduction in TH levels and substantially decreased levels of RNAs for the TSH subunits. The reason why the TSH RNA failed to be upregulated is unclear, but may be related to the relative immaturity of the mutant mice as compared with the control pups; TH levels are known to reach a peak at days 10–16 post-natal (Campos-Barros et al., 2000), and it is possible that the mutant pups had not reached a developmental age comparable with that of the wt controls at the time of blood sampling. Nevertheless, the reduction in TH levels was transient and mild, in a range that could be considered ‘low normal’.
Cardiac activity
The adult mutant mice presented a cardiac phenotype reminiscent of that seen in mice with the TRα1 null allele: an inability to reach a high heart rate despite stimulation with a strong β-adrenergic receptor agonist (Johansson et al., 1999). However, mice deficient for TRα1 also exhibited a markedly lower average heart rate under normal conditions, a phenotype that could not be verified in the TRα1R384C mice. Therefore, the results may suggest that a dominant negative TRα1 yields effects qualitatively or quantitatively different from those caused by a lack of TRα1. The HCN-2 and -4 ion channel proteins have been suggested to be responsible for the hyperpolarizing ion channel activity in the SA node and therefore also for controlling heart rate (Ludwig et al., 1998). Both channel genes are induced by TH (Gloss et al., 2001), and it is possible that TRα1R384C constitutively reduces their expression, whereas a lack of wt TRα1 would have a less serious effect. This notion is supported by recent analyses with cDNA arrays, showing that few target genes require a TR for their basal expression and that many of them are suppressed by an unliganded TR instead (Feng et al., 2000; Flores-Morales et al., 2002).
The MyHCα and β chain genes were both highly dysregulated in the juvenile mice, whereas the β gene was only moderately overexpressed in the adults. As a high expression of MyHCβ relative to α is seen in late fetal/early post-natal stages of mouse development, the results support the conclusion that, when analysed, the mutant pups were developmentally younger than their wt controls. Furthermore, the data emphasize that the delay in acquiring a near normal expression of TR target genes was compensated for by other late-acting mechanisms. The dysregulation of these two target genes is unlikely to be the cause of the aberrant maximal heart rate.
Effect of gene dosage
The demonstration that the level of wt TRα1 expression was inconsequential to the effect of TRα1R384C indicates that the wt protein does not counteract the activity of the mutant receptor. Also, the TRα1+/mTRβ+/– strain exhibited growth similar to that of the TRα1+/mTRβ+/+ mice, thus indicating that TRβ also lacked the capacity to counteract the mutant receptor during post-natal growth. In contrast, the presence of two alleles encoding TRα1R384C seriously impeded survival and caused eye opening to occur in the few surviving pups as late as at days 25–30 (data not shown). This strongly suggests that the dosage of the mutant allele is important for elicitation of the phenotype.
Recently, Kaneshige et al. (2001) also described mice with a dominant negative TRα1, carrying the ‘PV’ mutation. This RTH mutation abolishes T3 binding completely, and is known to exert very strong dominant negative effects through TRβ in both mice and patients. However, the phenotype caused by the PV mutation differs substantially in many respects from that induced by TRα1R384C. The persistent dwarfism, infertility and elevated TH levels reported were not observed in the mice generated by us. Also, TRα1+/PV mice had a 50% mortality rate and no surviving homozygotes were seen, whereas the heterozygous TRα1R384C mice showed only a minor increase in mortality (data not shown), with homozygotes generally dying before 2–3 weeks of age. The targeting construct used in both instances was derived from the same basic vector (Wikström et al., 1998), and is known to cause a 3- to 6-fold overexpression of the targeted allele, as shown here and previously (Wikström et al., 1998; Saltó et al., 2001; Gullberg et al., 2002). Both mouse strains are therefore likely to express identical elevated levels of mutant receptors, suggesting that the discrepancies in phenotype are due to the differences in the dominant negative potencies of the respective mutant receptors. This supports the concept that gene dosage of a mutant TRα1 allele, as well as the potency of the mutation, determine the severity of the phenotype.
Recently, Flamant et al. (2002) generated mice that lack expression of all TRα products in combination with a thyroid gland ablation due to inactivation of the PAX8 gene (Mansouri et al., 1998). Lack of PAX8 normally leads to high mortality, but the results with the compound mice showed that deletion of the TRα gene specifically increased the survival rate, suggesting that the TRα1 aporeceptor can have deleterious effects on post-natal development. This is in accordance with the results obtained with mice carrying the TRα1R384C mutation. In contrast to these results, mice expressing dominant negative TRβ receptors have properties distinct from those of the TRα1R384C animals (Kaneshige et al., 2000; Hashimoto et al., 2001). Severe disturbances in the pituitary–thyroid axis were seen, accompanied by persistent bone and cerebellar maldevelopment, perturbed locomotor activity and deficiency in weight gain. Thus, the dominant negative TRα1 and TRβ receptors elicit distinct phenotypic characteristics.
Rescue of the developmental delay
A preliminary attempt to test whether the delay in development could be rescued by injections of T3 into pups was inconclusive, probably due to the difficulties in reproducibly administering the hormone to maintain sufficiently high T3 levels in a sustained fashion. Since it is known that lack of TRβ leads to dysregulated TSH production and hence elevated T3 levels, the introduction of a TRβ null allele into the TRα1R384C mice was considered an alternative approach to achieving sustained levels of increased T3. The fact that the 10-fold raised TH levels were accompanied by an almost complete restoration of body weight gain as well as a near normalization of the time for eye opening suggests that ligand activation of the mutant receptor was achieved. This was corroborated by the findings that these mice efficiently suppressed MyHCβ and normalized GH RNA expression.
Can this aid the identification of patients with a mutant TRα1?
The approach we have chosen for identifying a possible phenotype of patients with a mutant TRα1 gene involved generating a mouse model harbouring a mutation with properties commonly found in RTH, i.e. hormone binding to the mutant receptor was reduced but not abolished. The main features observed, a severe retardation in maturation that is largely overcome in the adult mouse, combined with a minor dysfunction of the pituitary–thyroid axis, would not easily be ascribed to a TR defect in humans. It should also be kept in mind that we report an overexpression of the mutant allele caused by the gene targeting, an event that may have exacerbated the phenotype described. Despite this, the data indicate that a potent dominant negative mutation could cause a delay in maturation, and that replacement treatment with TH may alleviate such a deficiency. Given the central role of TH in the brain, other parameters of TRα1 action need to be examined in the TRα1R384C mice. Important aspects of cerebellar development and hippocampal function were recently described to be under the influence of TRα1 (Itoh et al., 2001; Guadaño-Ferraz et al., 2002; Liu et al., 2002; Morte et al., 2002), indicating that a study of the TRα1R384C neurological phenotype is warranted.
Materials and methods
Introduction of the TRα1R384C mutation into the mouse genome
Codon 384 of a mouse TRα1 cDNA was changed by standard site-directed mutagenesis to encode a cysteine instead of an arginine, thereby also introducing a new and diagnostic PvuII restriction cleavage site. The change was verified by nucleotide sequencing. Transfection analyses with 293 cells were performed as described previously (Collingwood et al., 1997). A fragment of the coding region containing the point mutation was introduced into the targeting vector described by Wikström et al. (1998) and Saltó et al. (2001). The procedures used for generation of chimeric founder mice from embryonic stem (ES) cells were as described by Wikström et al. (1998). The founders were mated with C57BlCR mice, and the heterozygotes from an additional two consecutive crosses with C57BlCR mice were intercrossed to yield animals for experiments. Pups carrying the mutant TRα1 allele were weaned at 4–5 weeks of age while being fed semi-solid chow due to their delayed development and tooth rupture. The other TR-deficient mouse strains used for generation of double mutant mice, the genotyping procedures and animal handling have been described previously (Forrest et al., 1996; Wikström et al., 1998; Saltó et al., 2001). All animal experiments were performed with permission from the local animal ethics committee.
Flow cytometric analysis
Single-cell suspensions were prepared from spleens and treated with geys solution to remove red blood cells. Cells (1 × 106) were incubated on ice in 100 µl of staining solution (phosphate-buffered saline supplemented with 2% fetal calf serum) together with 0.25 µg/ml anti-CD16/CD32 (Fc block, PharMingen) to reduce background. These cells were stained with the following conjugated antibodies, purchased from PharMingen: phycoerythrin-conjugated anti-CD19 (1D3) and biotin-conjugated anti-IgM (AF6-78). Biotin staining was revealed by the addition of streptavidin–fluorescein isothiocyanate (FITC) second reagent (Sigma). After a 30 min incubation, cells were washed, resuspended in staining solution and applied to a FACScaliber flow cytometer (Becton Dickinson). Dead cells were excluded by propidium iodide staining and lymphocyte populations gated on forward and side scatter.
Analyses of physiological parameters
All the procedures used have been described in detail previously (Wikström et al., 1998; Göthe et al., 1999; Saltó et al., 2001).
Acknowledgments
Acknowledgements
We are grateful to Drs Douglas Forest and Ola Hermansson for constructive criticism on the manuscript. We thank Mrs Marie-Louise Spångberg for histological analyses, and the Karolinska Institute Transgene Facility for injections of ES cells. This project was supported by funds from the Swedish Cancer Society, the Swedish Medical Research Council, the Swedish Foundation for Strategic Research, the Lundberg Foundation, the Swedish Association for Rheumatic Diseases, the Wallenberg Consortium North, the Novonordisk Foundation, Astrazeneca R&D, Torsten and Ragnar Söderbergs Stiftelser, the Göteborg Medical Society, NIH grant (DC 03441) and the Wellcome Trust (M.A., O.R. and K.C.).
References
- Adams M., Matthews,C., Collingwood,T.N., Tone,Y., Beck-Peccoz,P. and Chatterjee,K.K. (1994) Genetic analysis of 29 kindreds with generalized and pituitary resistance to thyroid hormone. Identification of thirteen novel mutations in the thyroid hormone receptor β gene. J. Clin. Invest., 94, 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Barros A., Amma,L.L., Faris,J.S., Shailam,R., Kelley,M.W. and Forrest,D. (2000) Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc. Natl Acad. Sci. USA, 97, 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee V.K.K. and Beck-Peccoz,P. (2001) Resistance to thyroid hormone. In DeGroot,L.J. and Jameson,J.L. (eds), Endocrinology. 4th edn. pp. 1609–1615.
- Cheng S.Y. (2000) Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev. Endocr. Metab. Disord., 1, 9–18. [DOI] [PubMed] [Google Scholar]
- Collingwood T.N. et al. (1997) A natural transactivation mutation in the thyroid hormone β receptor: impaired interaction with putative transcriptional mediators Proc. Natl Acad. Sci. USA, 94, 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Jiang,Y., Meltzer,P. and Yen,P.M. (2000) Thyroid hormone regulation of hepatic genes in vivo detected by complementary DNA microarray. Mol. Endocrinol., 14, 947–955. [DOI] [PubMed] [Google Scholar]
- Flamant F., Poguet,A.L., Plateroti,M., Chassande,O., Gauthier,K., Streichenberger,N., Mansouri,A. and Samarut,J. (2002) Congenital hypothyroid Pax8(–/–) mutant mice can be rescued by inactivating the TRα gene. Mol. Endocrinol., 16, 24–32. [DOI] [PubMed] [Google Scholar]
- Flores-Morales A., Gullberg,H., Fernandez,L., Ståhlberg,N., Lee,N.H., Vennström,B. and Norstedt,G. (2002) Patterns of liver gene expression governed by TRβ. Mol. Endocrinol., 16, 1257–1268. [DOI] [PubMed] [Google Scholar]
- Forrest D., Hanebuth,E., Smeyne,R.J., Everds,N., Stewart,C.L., Wehner,J.M. and Curran,T. (1996) Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor β: evidence for tissue-specific modulation of receptor function. EMBO J., 15, 3006–3015. [PMC free article] [PubMed] [Google Scholar]
- Gloss B. et al. (2001) Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor α or β. Endocrinology, 142, 544–550. [DOI] [PubMed] [Google Scholar]
- Göthe S., Wang,Z., Ng,L., Kindblom,J.M., Barros,A.C., Ohlsson,C., Vennström,B. and Forrest,D. (1999) Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary–thyroid axis, growth, and bone maturation. Genes Dev., 13, 1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadaño-Ferraz A., Benavides-Piccione,R., De Felipe,J., Lancha,C., Venero,C., Sandi,C., Vennström,B. and Bernal,J. (2002) The lack of thyroid hormone receptor α1 isoform induces alterations in hippocampal circuits and behavior. Mol. Psych., in press. [DOI] [PubMed] [Google Scholar]
- Gullberg H., Rudling,M., Saltó,C., Forrest,D., Angelin,B. and Vennström,B. (2002) Requirement for thyroid hormone receptor β in T3 regulation of cholesterol metabolism in mice. Mol. Endocrinol., 16, 1767–1777. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Curty,F.H., Borges,P.P., Lee,C.E., Abel,E.D., Elmquist,J.K., Cohen,R.N. and Wondisford,F.E. (2001) An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc. Natl Acad. Sci. USA, 98, 3998–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansson O., Glass,C. and Rosenfeld,M. (2002) Nuclear receptor coregulators: multiple modes of modification. Trends Endocrinol. Metab., 13, 55–60. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Esaki,T., Kaneshige,M., Suzuki,H., Cook,M., Sokoloff,L., Cheng,S.Y. and Nunez,J. (2001) Brain glucose utilization in mice with a targeted mutation in the thyroid hormone α or β receptor gene. Proc. Natl Acad. Sci. USA, 98, 9913–9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C., Göthe,S., Forrest,D., Vennström,B. and Thoren,P. (1999) Cardiovascular phenotype and temperature control in mice lacking thyroid hormone receptor-β or both α1 and β. Am. J. Physiol., 276, H2006–H2012. [DOI] [PubMed] [Google Scholar]
- Kaneshige M. et al. (2000) Mice with a targeted mutation in the thyroid hormone β receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc. Natl Acad. Sci. USA, 97, 13209–13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshige M., Suzuki,H., Kaneshige,K., Cheng,J., Wimbrow,H., Barlow,C., Willingham,M.C. and Cheng,S. (2001) A targeted dominant negative mutation of the thyroid hormone α 1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc. Natl Acad. Sci. USA, 98, 15095–15100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindblom J.M., Göthe,S., Forrest,D., Törnell,J., Vennström,B. and Ohlsson,C. (2001) Growth hormone substitution reverses the growth phenotype but not the defective ossification in the thyroid hormone receptor α1–/–β–/– mice. J. Endocrinol., 171, 15–22. [DOI] [PubMed] [Google Scholar]
- Liu Y.Y., Tachiki,K.H. and Brent,G.A. (2002) A targeted thyroid hormone receptor α gene dominant-negative mutation (P398H) selectively impairs gene expression in differentiated embryonic stem cells. Endocrinology, 143, 2664–2672. [DOI] [PubMed] [Google Scholar]
- Love J.D., Gooch,J.T., Nagy,L., Chatterjee,V.K. and Schwabe,J.W. (2000) Transcriptional repression by nuclear receptors: mechanisms and role in disease. Biochem. Soc. Trans., 28, 390–396. [PubMed] [Google Scholar]
- Ludwig A., Zong,X., Jeglitsch,M., Hofmann,F. and Biel,M. (1998) A family of hyperpolarization-activated mammalian cation channels. Nature, 393, 587–591. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D.J. et al. (1995) The nuclear receptor superfamily: the second decade. Cell, 83, 835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A., Chowdhury,K. and Gruss,P. (1998) Follicular cells of the thyroid gland require Pax8 gene function. Nat. Genet., 19, 87–90. [DOI] [PubMed] [Google Scholar]
- Morte B., Manzano,J., Scanlan,T., Vennström,B. and Bernal,J. (2002) Deletion of the thyroid hormone receptor α1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc. Natl Acad. Sci. USA, 99, 3985–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L. et al. (1999) Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev., 13, 3209–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refetoff S., DeWind,L.T. and DeGroot,L.J. (1967) Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J. Clin. Endocrinol. Metab., 27, 279–294. [DOI] [PubMed] [Google Scholar]
- Saltó C. et al. (2001) Ablation of TRα2 and a concomitant overexpression of α1 yields a mixed hypo- and hyperthyroid phenotype in mice. Mol. Endocrinol., 15, 2115–2218. [DOI] [PubMed] [Google Scholar]
- Weiss R.E. and Refetoff,S. (1996) Effect of thyroid hormone on growth. Lessons from the syndrome of resistance to thyroid hormone. Endocrinol. Metab. Clin. North Am., 25, 719–730. [DOI] [PubMed] [Google Scholar]
- Weiss R.E. and Refetoff,S. (2000) Resistance to thyroid hormone. Rev. Endocr. Metab. Disord., 1, 97–108. [DOI] [PubMed] [Google Scholar]
- Wikström L., Johansson,C., Salto,C., Barlow,C., Campos Barros,A., Baas,F., Forrest,D., Thoren,P. and Vennström,B. (1998) Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α1. EMBO J., 17, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen P.M. (2001) Physiological and molecular basis of thyroid hormone action. Physiol. Rev., 81, 1097–1142. [DOI] [PubMed] [Google Scholar]