Abstract
Male meiosis in higher organisms features synchronous cell divisions in a large number of cells. It is not clear how this synchrony is achieved, nor is it known whether the synchrony is linked to the regulation of cell cycle progression. Here, we describe an Arabidopsis mutant, named tardy asynchronous meiosis (tam), that exhibits a phenotype of delayed and asynchronous cell divisions during male meiosis. In Arabidopsis, two nuclear divisions occur before simultaneous cytokinesis yields a tetrad of haploid cells. In tam, cell divisions are delayed, resulting in the formation of abnormal intermediates, most frequently dyad meiotic products, or in rare cases, dyad pollen (two gametophytes within one exine wall). Temperature-shift experiments showed that the percentage of the abnormal intermediates increased at 27°C. Analysis of tam and the tam/quartet1 double mutant showed that most of these abnormal intermediates could continue through the normal rounds of cell divisions and form functional pollen, though at a slower than normal pace. The asynchrony of cell division started at the G2/M transition, with cells entering metaphase at different time points, during both meiosis I and II. In addition, chromosome condensation defects and mis-segregation were sometimes observed in tam. These observations suggest that the TAM protein positively regulates cell cycle progression, perhaps by promoting the G2/M transition. We speculate that there is a signal, perhaps TAM, that couples the normal pace of cell cycle progression with the synchrony of cell division during male meiosis.
Meiosis consists of two nuclear divisions (karyokinesis) and one simultaneous or two successive cytoplasmic divisions (cytokinesis). The final product of male meiosis in flowering plants is a tetrad of haploid microspores enclosed in a callose wall. Male meiosis usually occurs in a large enough population of synchronously dividing cells to ensure sufficient fertility of the organism. The synchrony in cell division is likely to be relevant to the effectiveness of sexual reproduction, but molecular mechanisms underlying this synchrony are poorly understood. However, molecular mechanisms controlling cell cycle progression have been extensively studied in eukaryotes (den Boer and Murray, 2000; Moser and Russell, 2000; Nebreda and Ferby, 2000; Walworth, 2000). Although it has not been shown, it is possible that the regulation of cell cycle progression is linked to the regulation of synchronous divisions in male meiosis of higher organisms.
The G2/M transition has been identified as one of the major checkpoints of cell cycle progression. The mitosis-promoting factor (MPF), which consists of B-type cyclins and cdc2-related cyclin-dependent kinases, is the key regulator of the G2/M transition in all eukaryotes (Ohi and Gould, 1999). The kinase component of the MPF phosphorylates numerous substrates; these phosphorylations are necessary for progression of multiple events during the onset of mitosis, including nuclear envelope breakdown, centrosome (spindle pole body) duplication and separation, spindle assembly, chromosome condensation, and Golgi fragmentation (Ohi and Gould, 1999; Nigg, 2001). The extensive literature on factors that activate and inactivate MPF indicates the importance and complexity of regulation of MPF activity. In yeast and animals, numerous kinases and phosphatases have been identified that regulate the function of MPF (Kishimoto, 1999; Ohi and Gould, 1999; Nigg, 2001). Regulation of MPF is also linked to MAP kinase-signaling pathways in yeast and mammalian cells (Wilkinson and Millar, 2000). Many other factors regulate the G2/M transition by directly or indirectly regulating the function of MPF. Examples of these factors include several cell cycle-regulating proteins in budding yeast (Saccharomyces cerevisiae; Goh and Surana, 1999; Loy et al., 1999; Richman et al., 1999), a cdc2-binding protein in Xenopus laevis oocytes (Ferby et al., 1999), cyclin A1 in male meiosis of mouse (Mus musculus; Liu et al., 2000), and cancer-related proteins in humans (Cajone and Sherbet, 1999; Rippmann et al., 2000). Developmental cues such as growth factors, hormones, cell adhesion status (Wilkinson and Millar, 2000), and other conditions such as stress (Wilkinson and Millar, 2000), DNA damage (Toyoshima et al., 1998), UV irradiation (Athar et al., 2000), and temperature (Kong et al., 2000), can all affect MPF function.
In higher plants, the G2/M transition is thought to operate via MPF (Joubès et al., 2000; Mészáros et al., 2000), and plant hormones such as auxin, cytokinin, and abscisic acid can apparently regulate the activity of plant MPFs (Stals et al., 2000). However, the underlying mechanisms of MPF regulation by plant hormones are unknown. Several recent studies have examined cell cycle progression and cell division patterns during specific stages of plant development. For example, in Arabidopsis, when pollen is released from the anther, the sperm cells are at G1, but their cell cycle continues to progress during pollen tube growth (Friedman, 1999). By monitoring the expression of the CYCLIN B1;1 gene, Boisnard-Lorig et al. (2001) demonstrated that cell divisions in developing endosperm are synchronous within discrete mitotic domains. However, to our knowledge, no groups have explored whether cell cycle progression is coupled with the synchronous cell divisions in male meiosis.
Together with other advantages as a model plant, Arabidopsis provides an excellent system for molecular genetic studies of meiosis. In each Arabidopsis flower, male meiosis occurs synchronously in about 500 microspore mother cells in the four medial anthers. Here, we describe a new mutation in Arabidopsis, tardy asynchronous meiosis (tam). The tam allele is temperature sensitive, but tam plants are fertile and even at the restrictive temperature can set seeds. We show that the tam mutant exhibits delayed and asynchronous cell divisions during male meiosis. The delay occurs in both meiosis I and II, at the G2/M transition. Our observations suggest a possible link between the control of G2/M transition and the synchrony of cell division in male meiosis.
RESULTS
Isolation and Genetic Analysis of tam
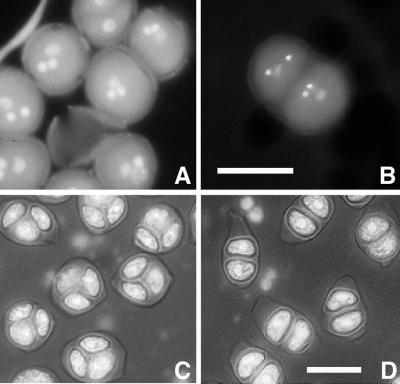
In a screen for mutations affecting pollen development, M2 plants from an ethyl methanesulfonate (EMS)-mutagenized population were screened for mutant phenotypes at the mature pollen stage. In one M2 plant, although the majority of the pollen grains appeared normal (Fig. 1A), we saw a low percentage (approximately 1%) of abnormal pollen grains that were composed of two gametophytes within one exine wall (Fig. 1B). This abnormal phenotype was heritable, indicating that it was caused by a mutation. To determine how these abnormal pollen grains arose in this mutant, we analyzed earlier stages of pollen development, starting at the stage when a callose wall surrounds the microspore mother cells. An apparent abnormality was first observed at the tetrad stage. The WT tetrads had four equal-sized spores enclosed in a callose wall (Fig. 1C). In the mutant, instead of tetrads, we saw many dyads (Fig. 1D). Other cell clusters in the mutant anthers contained different numbers of spores, ranging from three to seven, and some of them had more than one nucleus (Table I; see later description of the mutant phenotype). The aberrant clusters seen at this developmental stage indicate that the mutant has a meiotic defect. Because it is easier to score for aberrant clusters than to search for rare twin gametophytes in mature pollen, in most experiments we used tetrad stage anthers to identify the mutant plants. We named the mutant tam, for reasons discussed below.
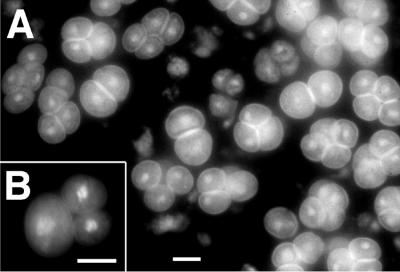
Figure 1.
Formation of double gametophytes and dyad meiotic products in tam. A, Normal-looking pollen grains in tam. B, Two pollen grains in one exine wall in tam. The two brightly fluorescent nuclei visible in the tricellular pollen grain correspond to the two sperm cells; the vegetative nucleus is out of the plane of focus. C, Wild-type (WT) tetrads, showing the characteristic tetrahedral arrangement of the four microspores enclosed in a callose wall. Usually one or two spores are out of focus. D, Dyads in tam. Note the callose wall. 4′,6-Diamidino-2-phenylindole (DAPI) staining was used. Scale bar, 10 μm.
Table I.
Dynamics of microspore mother cells and meiotic products in tam anthersa
| Developmental Stage | MMC | 2N 2C | 3N 2C | 3N 3C | 4N 2C | 4N 3C | 4N 4C | (4–8)N (3–7)C |
|---|---|---|---|---|---|---|---|---|
| % | ||||||||
| Class 1 (4)b | 15–46 | 54–64 | 0–9 | 0–4 | 0–5 | 0–5 | 0–3 | 0–4 |
| Class 2 (6) | 2–3 | 85–100 | 0–3 | 0–4 | 2–14 | 0–1 | 0–1 | 0–3 |
| Class 3 (3) | – | 48–62 | 2–9 | 0–8 | 2–42 | 3–17 | 2–13 | 3–4 |
| Class 4 (5) | – | 8–36 | 3–10 | 5–12 | 0–6 | 2–32 | 24–70 | 3–6 |
MMC, Microspore mother cells, which had one to two nuclei; N, nuclear no. in meiotic products; C, cell no. in meiotic products.
Anthers were assigned to four classes based on their developmental stages, as judged by the distribution of microspore mother cells and meiotic products. Class 1 was the least advanced in development because it had the highest percentage of microspore mother cells (one cell, one–two nuclei). Class 2 was more advanced than class 1 because it had a higher percentage of dyads (two cells) and a lower percentage of microspore mother cells. Class 3 was more advanced than class 2 because it no longer had microspore mother cells and had more tetrads than class 2. Class 4 was the most advanced because it had the highest percentage of tetrads.
No. of anthers examined.
Because tam was identified in a single M2 plant, it was unknown whether it was a dominant or recessive mutation. A plant with the tam phenotype was outcrossed to WT Columbia. All F1 plants had a WT phenotype, and selfing of each of these F1 plants gave roughly a 3:1 ratio of normal:tam progeny (data not shown), indicating that tam is a recessive mutation.
A mapping population of 100 plants and available simple sequence-length polymorphism (SSLP), cleaved-amplified polymorphic sequence (CAPS), and RFLP markers were used to map tam to the long arm of chromosome 1, between SSLP nF22K20 and CAPS agp64. The distance between the two markers is 550 kb (Arabidopsis Genome Initiative, 2000). Another meiotic mutant, mei1 (He and Mascarenhas, 1998), maps in this interval. We tested whether tam was allelic to mei1 by crossing tam/tam and mei1/mei1. All the F1 plants showed WT male meiosis; thus, tam is not allelic to mei1.
Mutant Phenotype Is Temperature Sensitive
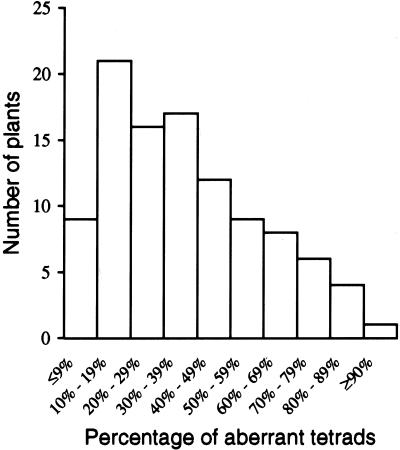
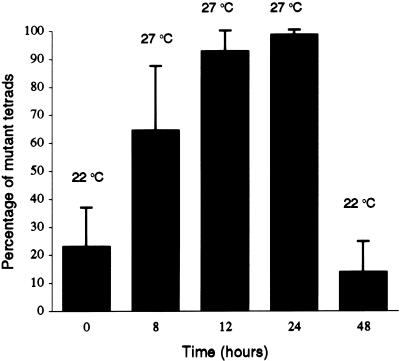
In addition to dyads (Fig. 1D), tam plants have, in less abundance, other types of aberrant tetrads, including polyads and nontetrahedral tetrads. For simplicity, we group all the types together and use the term aberrant tetrads to describe the phenotype. We scored the percentage of aberrant tetrads in tam plants. We noticed considerable variation in the percentage of aberrant tetrads between plants when plants were grown at 22°C. As shown in Figure 2, tam plants always had some aberrant tetrads, but the percentage varied from 5% to more than 90%. We also noticed that there were always close to 100% aberrant tetrads in greenhouse-grown tam plants during the warmer summer months, when the temperature could reach 27°C. Thus, the mutant phenotype appeared to be sensitive to higher temperature. To determine more precisely how temperature influences the percentage of aberrant tetrads, a temperature-shift experiment was conducted. As shown in Figure 3, 13 plants with less than 30% aberrant tetrads were first grown at 22°C and then at 27°C for 24 h. We point out that different buds from the same plant were dissected and scored before and after the temperature shift. If a bud is dissected and scored before the temperature shift, the microspore mother cells or tetrads within that bud cannot be followed through the temperature shift. However, because buds at the same stage on a plant have a similar percentage of aberrant tetrads on a particular day, we assumed that all the buds on the plant would respond equivalently to the temperature shift. The temperature shift dramatically increased the percentage of aberrant tetrads in each plant. A 12- to 24-h exposure to 27°C induced nearly 100% aberrant tetrads. After the plants were returned to 22°C for 24 h, the percentage of aberrant tetrads decreased to a value that was even lower than the starting value in each plant. In contrast, WT plants never showed aberrant tetrads in all tested growing conditions (data not shown). These results indicate that tam is temperature sensitive and suggest that the TAM protein in the mutant is partially active at the permissive temperature. In later descriptions of the mutant and WT phenotypes, unless noted, we used plants grown in a growth chamber at 27°C.
Figure 2.
Variability in the percentage of aberrant tetrads among 103 tam plants grown at 22°C.
Figure 3.
Temperature sensitivity of the tam mutation. Thirteen plants were first grown at 22°C, then at 27°C for 24 h, and then returned to 22°C. The aberrant tetrads were scored before the temperature shift to 27°C, after the 8-, 12-, and 24-h time points at 27°C, and 24 h after return to 22°C. The data are expressed as the mean and se.
Male Meiotic Cell Cycle Progression Is Slower in tam Than in WT
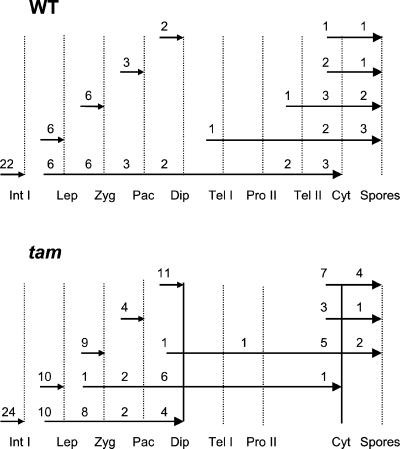
There are two types of cytokinesis in male meiosis in plants: successive cytokinesis and simultaneous cytokinesis. Successive cytokinesis, as seen in maize (Zea mays; Staiger and Cande, 1991), occurs after both the first and second nuclear divisions of meiosis, whereas simultaneous cytokinesis, as in Arabidopsis (Peirson et al., 1997), occurs only once, after meiosis II. The dyads observed in tam could result because an ectopic cytokinesis occurs after meiosis I, or because meiosis I is slower but cytokinesis occurs at the normal time. To determine how aberrant tetrads, mainly the dyads, are formed in tam plants, we examined the progression of the meiotic cell cycle in two consecutive buds in inflorescences from both WT and tam plants. During meiosis in a WT inflorescence, a smaller bud is always less advanced than the next larger bud in development. We reasoned that comparing the developmental stages in two consecutive buds might reveal different paces in stage progression between WT and tam buds. The younger and older buds are defined as bud 1 and 2, respectively. In WT, we found that when bud 1 was at interphase I, bud 2 could be at any stage from leptotene to tetrad (Fig. 4). When bud 1 in WT was at a later stage than interphase I, bud 2 stages ranged from telophase I to free spores (Fig. 4). In contrast, when bud 1 in tam is at interphase I, bud 2 was never found past the diplotene stage (Fig. 4, solid vertical bar on the left). When bud 1 in tam is at the leptotene stage, bud 2 could be at any stage from zygotene to cytokinesis. If cytokinesis had occurred in bud 2, it usually resulted in dyad formation (Fig. 4, solid vertical bar on the right). These results strongly suggest that in tam, cell cycle progression in male meiosis is significantly delayed but that the timing of cytokinesis is not dramatically altered. Figure 4 suggests that the first obvious delay occurs at the G2/M transition of meiosis I.
Figure 4.
Comparison of meiotic stages in two consecutive buds in both WT and tam plants. The lengths of the arrows represent the ranges of stages of the buds. The numbers above the arrows are the numbers of buds examined. Small arrowheads indicate bud 1, and large arrowheads indicate bud 2. Int I, Interphase I; Lep, leptotene; Zyg, zygotene; Pac, pachytene; Dip, diplotene; Tel I, telophase I; Pro II, prophase II; Tel II, telophase II; Cyt, cytokinesis; Spores, free spores.
Dyads Are Not Terminal in Development
Our initial analysis of tam was hampered by the fact that the percentage of aberrant tetrads fluctuated, even in the same plant grown under controlled temperature conditions. For example, one plant had 22% aberrant tetrads on the 1st d it was scored. On the 5th d, the percentage of aberrant tetrads had increased to 79%, but on the 7th d, the percentage of aberrant tetrads dropped to 22.5%. Later, we noticed that higher percentages of aberrant tetrads were usually found in tam anthers at early tetrad stages, as indicated by the presence of microspore mother cells without cytokinesis (Table I, class 2). Conversely, higher percentages of normal tetrads were found in older anthers, as indicated by the absence of microspore mother cells without cytokinesis (Table I, classes 3 and 4). We used Alexander's stain to assess pollen viability (Alexander, 1969). Dehiscent anthers of tam plants had approximately 74% viable pollen when grown at 22°C and still had approximately 59% viable pollen when grown at 27°C for 4 d. The tam plants have reasonable seed set at both the permissive and restrictive temperature.
The developmental fluctuation in the percentage of aberrant tetrads as well as the pollen viability suggested that aberrant tetrads in the mutant may not be terminal, i.e. that they may continue to divide to form normal spores even after release from the callose wall. To confirm this possibility, we constructed a double mutant with quartet1 (qrt1; Preuss et al., 1994), to keep the meiotic products together after the callose wall degradation. We examined male meiosis and pollen development in more than 20 tam/qrt1 double mutant inflorescences. The tam/qrt1 double mutant showed no morphological difference from tam before callose degradation. After callose degradation, clusters with different numbers of spores were observed (Fig. 5). The percentage of clusters with four cells increased along with the age of the buds, whereas the percentages of other clusters decreased. Figure 6 shows that in one of those inflorescences, the percentage of four-celled clusters in the youngest bud 1 was 8%, and it increased to 98% in the oldest bud 6. These results suggest that meiosis II in tam is often not finished before callose degradation but that it can, like meiosis I, be completed at a later time. The presence of twin gametophytes within one exine wall (Fig. 1B) implies that, in rare cases, meiosis II did not finish until after the exine was formed. It seems likely that the meiosis II delay also occurred at the G2/M transition because dyads with a nucleus in each cell could persist through the tetrad and later developmental stages (Table I, Figs. 5 and 6).
Figure 5.
Variation in number of meiotic products after callose degradation in the tam/qrt double mutant following a 1-d temperature shift to 27°C. A, Clusters with four, three, or two cells. B, Cluster with three cells. DAPI staining was used. Scale bars, 10 μm.
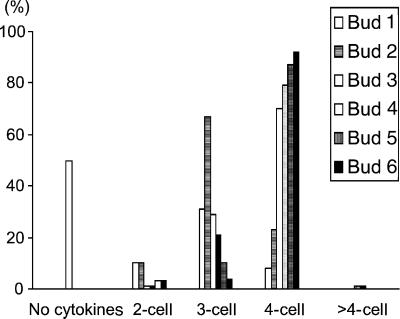
Figure 6.
Changes of abundance of clusters with different numbers of cells in tam/qrt1 double mutant during pollen development. Six consecutive buds were scored. The plants were grown at 22°C. A larger bud number corresponds to an older bud. Bud 1 was at the early cytokinesis stage, whereas bud 6 was at almost the mature pollen stage.
Male Meiotic Cell Division in tam Is Asynchronous during M-Phase
Cell divisions in all microspore mother cells in a WT Arabidopsis anther are highly synchronous at any given stage of the cell cycle (Fig. 1C; data not shown; Ross et al., 1996). In tam, however, we observed asynchronous cell divisions during both meiosis I and II. This asynchrony did not start until the onset of M-phase. Figure 7A shows a WT microspore mother cell with condensed chromosomes at the late diplotene stage. All tam microspore mother cells at the same stage were morphologically comparable and synchronous (examples shown in Fig. 7B). However, at the onset of M-phase, some cells in tam were still at diplotene stage, whereas others were at stages ranging from metaphase I to prophase II (Fig. 7, C–F), suggesting that microspore mother cells in tam entered M-phase at different time points. Chromosomal bridges were infrequently found in tam, indicating a defect in chromosome separation (Fig. 7D).
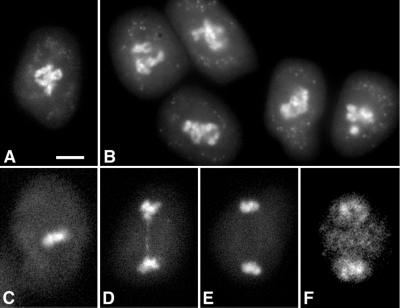
Figure 7.
Asynchronous M-phase during meiosis I in tam. A, WT microspore mother cell at the diplotene stage. The chromosomes are highly condensed. B, tam microspore mother cells at a stage similar to that in A. Microspore mother cells in C through F were found in the same anther but at different stages. C, At metaphase I. D, At early telophase I. A chromosome bridge was present. E, At late telophase I. F, At prophase II. DAPI staining was used. Scale bar, 5 μm.
In tam, dyads were usually formed before meiosis II, and thus tam anthers examined at this stage often appeared synchronous: Almost 100% of the cells were dyads for a period of time (Fig. 1D). This synchrony is consistent with a delay in cell cycle progression at prophase II. Figure 8A shows a dyad at this stage with both cells at prophase II. This dyad also contained a chromosome or chromosome fragment (Fig. 8A, arrow) that probably resulted from a defect in chromosome separation during meiosis I, as shown in Figure 7D. When meiosis II occurred, however, the asynchrony returned. In most of the dyads, cell division was more advanced in one of the cells, as shown in Figure 8, B through E, and only occasionally was cell division more or less synchronous for the two cells of the dyad, as shown in Figure 8, F and G. These results demonstrate that asynchrony in cell division also occurs at meiosis II, even for the two associated cells of the dyad. In addition, several abnormalities in mutant chromosome morphology and segregation were observed. As shown in Figure 8, A and C, chromosomes may be less condensed. Chromosome lagging (Fig. 8C, arrow) or scattering (Fig. 8E) was also apparent in some cells. The defects in chromosome morphology and segregation resulted in the occasional formation of aberrant tetrads with more than four spores and sometimes more than one nucleus in a cell (Table I; data not shown). Figure 8H shows an example of such an aberrant tetrad with five spores; one spore was much smaller than the others (Fig. 8H, arrow). We believe that in such cases, the final number of spores depends on whether there are additional clusters of chromosome fragments.
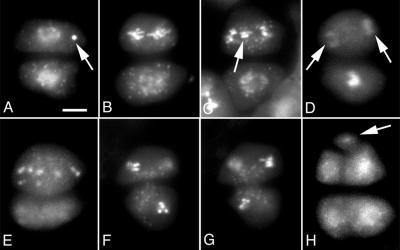
Figure 8.
Asynchronous M-phase during meiosis II in tam. A, Dyad contained a chromosome fragment (arrow). B, Dyad with one cell at anaphase (top) and the other cell at prophase. The chromosomes in the top cell were less condensed than is typical for anaphase. C, Dyad with a phenotype similar to that shown in B. The top cells contained lagging chromosomes. D, One cell has completed nuclear division (arrows) and the other cell was still at late prophase or early metaphase stage. E, An abnormal tetrad with three cells. The larger cell was at anaphase and contained scattered chromosomes. The two smaller cells at bottom are out of focus. F and G, Two focal planes of the same dyad, showing that both cells were at telophase. H, An abnormal tetrad with five cells. DAPI staining was used. Scale bar, 5 μm.
Cytochalasin D Partially Suppresses Meiotic Defect in tam
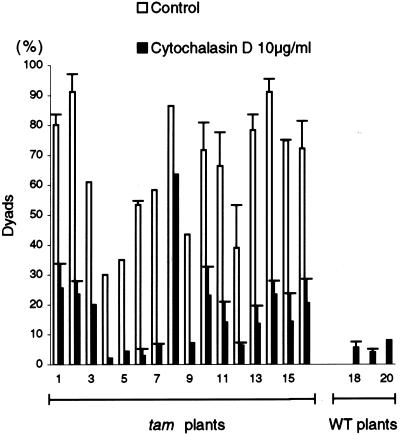
The slow G2/M transition, but subsequent normal cytokinesis, leads to the formation of dyads in tam. We postulated that devising some way to artificially delay cytokinesis in tam male meiosis might suppress the dyad phenotype. It is known that cytochalasin D treatment can arrest mitosis or cytokinesis of tobacco (Nicotiana tabacum) BY-2 cells (Nebenführ et al., 2000). We tested cytochalasin D on tam to see what effect it might have on the formation of dyads. We adapted an in vitro inflorescence culture system (Lardon et al., 1993) to deliver the cytochalasin D. We found that 10 μg mL−1 cytochalasin D treatment of the WT inflorescences (from four different plants) often resulted in tetrads with misoriented cell walls (Fig. 9A) that were not found in the WT control (not shown), indicating that this concentration of cytochalasin D was effective. The same concentration of cytochalasin D, compared with the tam control (Fig. 9B), resulted in a marked decrease in the number of dyads in the tam inflorescences and an increase in tetrahedral, i.e. WT-like, tetrads (Figs. 9C and 10). Cytochalasin D (2.5 or 5 μg mL−1) was still effective in causing misoriented cell walls in tetrads of WT inflorescences, and it was mildly effective in reducing dyad formation in tam inflorescences (not shown). However, 1 μg mL−1 cytochalasin D had no effect on the organization of tetrads in WT inflorescences and was not effective in reducing the percentage of dyads in tam plants (not shown). Together, these data suggest that cytochalasin D can significantly suppress dyad formation in tam.
Figure 9.
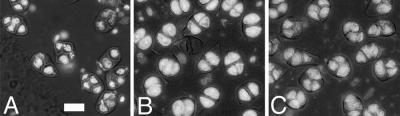
Effect of cytochalasin D during meiosis. A, Cells from a WT inflorescence that was maintained for 24 h in medium supplemented with 10 μg mL−1 cytochalasin D. Note irregularly shaped tetrads. B, Cells from a tam inflorescence that was maintained for 24 h in control medium. The percentage of dyads was 96% in this bud. C, Cells from a tam inflorescence from the same plant as in B, cultivated in medium supplemented with 10 μg mL−1 cytochalasin D. The percentage of dyads was 20% in this bud. Scale bar, 10 μm.
Figure 10.
Effect of cytochalasin D on the percentage of dyads in tam. Inflorescences from 16 tam plants and four WT plants were maintained for 24 h in control medium or in medium supplemented with 10 μg mL−1 cytochalasin D. One to two inflorescences were used for each treatment. The percentage of aberrant tetrads was scored for each inflorescence (≥1 bud) and is expressed as the mean and se. Where no se value is presented, only one bud was scored. The control treatment for the WT plants yielded no dyads.
DISCUSSION
The tam mutant is defective in cell cycle progression during male meiosis, and as a consequence dyads are formed when cytokinesis occurs. By comparing progression through the cell cycle in consecutive buds of WT and tam flowers, we were able to detect a delay at the G2/M transition in tam male meiosis. Although progression through meiosis can be followed in culture in maize (Yu et al., 1997), analysis of consecutive buds appears to be an attractive alternative for studying progression through male meiosis in Arabidopsis because it is difficult to track the development of the same bud through time. It is possible that tam also exhibits a cell cycle progression defect during female meiosis, but we did not test for this for technical reasons and because no obvious phenotype was seen in mature siliques.
The dyad phenotype in male meiosis has also been observed in the Arabidopsis ask1-1 mutant (Yang et al., 1999). The ask1-1 mutation is in an Arabidopsis homolog of the human and yeast SKP1 genes. Skp1p in yeast has been found to regulate both the G1/S and G2/M transitions (Bai et al., 1996; Connelly and Hieter, 1996), and thus it is plausible that the dyads found in ask1-1 are also formed due to a defect in cell cycle progression. In the Arabidopsis mutant dyad, dyads are the terminal phenotype seen during female meiosis. Siddiqi et al. (2000) therefore concluded that the dyad phenotype was due to a defect in cell cycle progression. In the Arabidopsis dmc1 mutant, dyads are formed in both male and female meioses (Couteau et al., 1999). Taken together, it seems that defects in cell cycle progression during meiosis I often lead to the formation of dyads. However, tam is clearly distinct from the other mutants that form dyads during meiosis because those mutants arrest at the dyad stage and are nearly always sterile. Despite the delay in meiosis and consequently the delayed formation of microspores, tam has reasonable fertility. The difference in severity of the other dyad-forming mutants and tam might be because tam, even at the restrictive temperature, does not behave like a null allele.
Cytokinesis is successive in most monocots (Skvarla and Larson, 1966; Hogan, 1987; Staiger and Cande, 1991) and simultaneous in most dicots, including Arabidopsis (Hogan, 1987; Brown and Lemmon, 1988; Traas et al., 1989; Peirson et al., 1997). Cytokinesis in tam meiosis is superficially similar to successive cytokinesis because dyads are first formed after meiosis I. Each dyad can divide again to form spores, and each spore, when released from the callose wall, can form functional pollen. The fact that a single mutation can convert the mode of cell division from simultaneous cytokinesis to almost successive cytokinesis raises the possibility that the different modes of cytokinesis during meiosis might be achieved by minimal genetic modifications. Furthermore, the temperature sensitivity of the cell cycle progression revealed by the tam mutant allele might suggest that such different modes of cytokinesis are influenced by environmental factors such as temperature. Some species of orchid can undergo either simultaneous or successive cytokinesis (Brown and Lemmon, 1991). It will be interesting to determine whether the variation in the modes of cytokinesis in such species depends on environmental factors and whether the choice is attributable to changes in the pace of cell cycle progression.
The tam inflorescences cultivated in vitro at 22°C in the presence of cytochalasin D showed a marked reduction in the percentage of aberrant tetrads. In higher plants, it is known that cytochalasin D can interfere with cytokinesis (Traas et al., 1989; Dinis and Mesquita, 1993; Nebenführ et al., 2000), and assembly of short actin filaments seems to be a crucial process in normal plant cytokinesis (Endle et al., 1998; Smith, 1999). Cytochalasin D prevents extension or new polymerization of microfilaments by binding to the ends of existing microfilaments (Flanagan and Lin, 1980; Gibbon et al., 1999). Thus, cytochalasin D might interfere with cytokinesis by halting the assembly of short actin filaments. In our experiments, cytochalasin D clearly affected cytokinesis as shown in the treated WT inflorescences. It is possible that cytochalasin D delayed cytokinesis in the treated tam inflorescences, making cytokinesis better coupled with meiosis II and resulting in an increase of normal tetrad formation. Alternatively, cytochalasin D might somehow hasten nuclear division in tam and thereby make meiosis II better coupled with cytokinesis. However, the dyad phenotype was not corrected if the cultured inflorescences were maintained at 27°C during treatment with 10 μg mL−1 cytochalasin D (data not shown). This suggests that the effect of the cytochalasin D is limited and could not overcome the more severe delay of cell cycle progression seen in tam at 27°C.
Although meiosis in tam exhibits abnormal intermediates, most are able to continue through the normal rounds of cell divisions and form functional pollen. The occasional reduced chromosome condensation, chromosome nondisjunction, and mis-segregation defects are consistent with the notion that some mutant cells are not ready for the M-phase, even when the nuclear envelope has broken down. It is possible that the primary defect in tam occurs either before asynchrony is apparent, or at the G2/M transition. There are two possible explanations for the asynchronous M-phase, if we assume that the temperature sensitivity of the tam allele allows a partially active TAM protein at the permissive temperature. First, it is possible that the mutant allele is leaky: Even at 27°C, perhaps some cells have higher residual activity of the mutated TAM protein than others and are thus able to enter M-phase earlier than other cells. Alternatively, all cells could have lost TAM function at 27°C, and the timing of entry into M-phase is stochastic. We think that the second scenario is more likely. An asynchronous M-phase during meiosis II was predominant, even between the two adjoined cells of a dyad. It is unlikely that these two adjoined cells have different levels of the TAM protein. If the second scenario is true, it would suggest a pathway that links a signal for synchronous cell division with the activation of the G2/M transition. The MPF has been established as the universal regulator of G2/M transition in all eukaryotes (Ohi and Gould, 1999). In fission yeast (Schizosaccharomyces pombe), a cdc2 mutant (encoding the kinase component of the MPF) produces dyads during meiosis, providing a direct link between MPF and the dyad phenotype (Niwa and Yanagida, 1988). If the TAM protein is a positive regulator of the G2/M transition, it might directly or indirectly regulate the function of the MPF in Arabidopsis.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
M2 plants from an EMS-mutagenized population (Columbia ecotype, generated in Dr. Robert Fischer's laboratory, Berkeley, CA) were initially grown in the greenhouse under continuous fluorescent light. Once we discovered that the tam mutation was temperature sensitive, plants were grown in a growth chamber at 22°C or at 27°C, as noted, under a 16-h-light/8-h-dark regime.
To test for allelism with mei1, an mei1/mei1 plant was crossed as female by a tam/tam male. Meiotic stage buds of six F1 plants were scored. To construct the double mutant between tam and qrt1, tam/tam was crossed as a female by qrt1/qrt1 (Landsberg ecotype). The F1 plants were selfed to obtain the F2 progeny. The F2 was scored for the qrt phenotype. Homozygous qrt1/qrt1 F2 plants were then exposed to 27°C for 24 h and tetrad stage anthers were scored to identify tam/tam plants.
Light Microscopy
Both fresh and fixed anthers were used. For fresh anthers, their contents were released into 2 μL of DAPI (Molecular Probes, Eugene, OR; 1 μg mL−1 in water) staining solution, using a needle under a dissecting scope. An additional 5 μL of DAPI was then added to the sample. Fixed anthers were analyzed as previously described (Yang et al., 1999). An Axiophot compound microscope (Zeiss, Jena, Germany) was used for the light and fluorescent microscopy. Images were photographed with Kodak Ektachrome 160T color slide film (Eastman Kodak, Rochester, NY) or with a digital camera. Stages of meiosis were determined according to Ross et al. (1996). Pollen viability was assessed with Alexander's stain (Alexander, 1969).
Inflorescence Culture and Treatment with Cytochalasin D
Inflorescences were cultured using a culture system developed for Brassica napus flowers (Lardon et al., 1993). The culture medium was 1× Murashige and Skoog salt mixture (Invitrogen, Carlsbad, CA), 3% (w/v) Suc, and 1× vitamins (Feldmann, 1991). The pH was adjusted to 5.8 with NaOH before filter sterilization. Each well of a 24-well tissue culture plate (Corning, Palo Alto, CA) was filled with approximately 3 mL of culture medium. For each well, an opening was drilled into the lid of the culture plate with a hot needle. The inflorescences were cut from the plants and all open flowers were removed. The peduncles of the inflorescences were immediately placed in the culture medium through the hole in the lid of the plate. The plate was incubated in the 22°C growth chamber. New flowers opened 1 d after the cultures were started and were then continuously produced. These flowers were fertile, and up to 25 siliques could be produced per peduncle. The inflorescence culture system thus mimics development on the intact plant.
In some experiments, the peduncle of the inflorescence was surface-sterilized for 10 min in a sodium hypochlorite solution (1% [v/v] available chlorine) containing traces of Triton X-100 and was subsequently washed four times with sterile water. Surface sterilization was not necessary for cultures maintained 24 h or less.
For treatment with cytochalasin D, the tissue culture plate was covered with aluminum foil to prevent photodegradation of the chemical. The stock solution of 10 mg mL−1 cytochalasin D (Sigma, St. Louis) was prepared in dimethyl sulfoxide. When appropriate, dimethyl sulfoxide was added to the control.
Mapping
tam/tam plants were outcrossed as female by WT Landsberg erecta ecotype. The F1 plants were backcrossed either as females or males to tam/tam to generate the mapping population. The genotypes of the plants of the backcross progeny (tam/tam or +/tam) were determined by examination of the tetrad stage after a 24-h temperature shift to 27°C. The backcross progeny was tested for linkage of tam to CAPS (Konieczny and Ausubel, 1993), SSLP (Bell and Ecker, 1994), and RFLP markers available from The Arabidopsis Information Resource (http://www.Arabidopsis.org) following standard protocols. The RFLP marker agp64 was converted into a CAPS marker. The sequences of primers used for DNA amplification for CAPS agp64 were as follows: CGAGGTATGTTCGGCTTGAT and CAAGGTGAGATTTCCCATTGAG. The PCR conditions were: 95°C for 5 min (initial denaturation); 95°C for 40 s, 50°C for 40 s, and 72°C for 1 min 30 s (25 cycles); and 72°C for 10 min (final elongation). The polymorphism was detected after digestion of the PCR products with EcoRI: The Columbia ecotype gives bands of 175, 300, and 800 bp, and the Landsberg erecta ecotype gives bands of 175 and 1,100 bp.
ACKNOWLEDGMENTS
We thank Robert Fischer for the M2 seeds of the EMS-mutagenized population, Andrea Prescott and the John Innes Centre for agp64 and agp15e markers, Daphne Preuss for providing the qrt1 seeds, David Hantz and his staff for excellent care of the plants, and Abdul Jandali for assistance with mapping. We also thank Robyn Cotter, Ines Ezcurra, and Sheila Johnson for helpful discussions during the course of this work.
Footnotes
This work was supported by the U.S. Department of Agriculture Current Research Information System (grant no. 5335–21000–011–00D). M.L. was a participant in the Undergraduate Research Apprentice Program (URAP) at the University of California (Berkeley) and was supported by a URAP fellowship.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010473.
LITERATURE CITED
- Alexander MP. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969;44:117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Athar M, Kim AL, Ahmad N, Mukhtar H, Gautier J, Bickers DR. Mechanism of ultraviolet B-induced cell cycle arrest in G2/M phase in immortalized skin keratinocytes with defective p53. Biochem Biophys Res Commun. 2000;277:107–111. doi: 10.1006/bbrc.2000.3436. [DOI] [PubMed] [Google Scholar]
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Ecker JR. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Boisnard-Lorig C, Colon-Carmona A, Bauch M, Hodge S, Doerner P, Bancharel E, Dumas C, Haseloff J, Berger F. Dynamic analysis of the expression of the HISTONE::YFP fusion protein in Arabidopsis shows that syncytial endosperm is divided in mitotic domains. Plant Cell. 2001;13:495–509. doi: 10.1105/tpc.13.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE. Microtubules associated with simultaneous cytokinesis of coenocytic microsporocytes. Am J Bot. 1988;75:1848–1856. [Google Scholar]
- Brown RC, Lemmon BE. Pollen development in orchids: I. Cytoskeleton and the control of division plane in irregular patterns of cytokinesis. Protoplasma. 1991;163:9–18. [Google Scholar]
- Cajone F, Sherbet GV. Stathmin is involved in S100A4-mediated regulation of cell cycle progression. Clin Exp Metastasis. 1999;17:865–871. doi: 10.1023/a:1006778804532. [DOI] [PubMed] [Google Scholar]
- Connelly C, Hieter P. Budding yeast SKP1 encodes an evolutionarily conserved kinetochore protein required for cell cycle progression. Cell. 1996;86:275–285. doi: 10.1016/S0092-8674(00)80099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteau F, Belzile F, Horlow C, Grandjean O, Vezon D, Doutriaux M-P. Random chromosome segregation without meiotic arrest in both male and female meiocytes of a dmc1 mutant of Arabidopsis. Plant Cell. 1999;11:1623–1634. doi: 10.1105/tpc.11.9.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boer BGW, Murray JAH. Triggering the cell cycle in plants. Trends Cell Biol. 2000;10:245–250. doi: 10.1016/s0962-8924(00)01765-7. [DOI] [PubMed] [Google Scholar]
- Dinis AM, Mesquita JF. The F-actin distribution during microsporogenesis in Magnolia soulangeana Soul. (Magnoliaceae) Sex Plant Reprod. 1993;6:57–63. [Google Scholar]
- Endle M-C, Stoppin V, Lambert A-M, Schmit A-C. The growing cell plate of higher plants is a site of both actin assembly and vinculin-like antigen recruitment. Eur J Cell Biol. 1998;77:10–18. doi: 10.1016/S0171-9335(98)80097-6. [DOI] [PubMed] [Google Scholar]
- Feldmann KA. T-DNA insertion mutagenesis in Arabidopsis: mutational spectrum. Plant J. 1991;1:71–82. [Google Scholar]
- Ferby I, Blazquez M, Palmer A, Eritja R, Nebreda AR. A novel p34(cdc2)-binding and activating protein that is necessary and sufficient to trigger G(2)/M progression in Xenopus oocytes. Genes Dev. 1999;13:2177–2189. doi: 10.1101/gad.13.16.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan MD, Lin S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J Biol Chem. 1980;255:835–838. [PubMed] [Google Scholar]
- Friedman WE. Expression of the cell cycle in sperm of Arabidopsis: implications for understanding patterns of gametogenesis and fertilization in plants and other eukaryotes. Development. 1999;126:1065–1075. doi: 10.1242/dev.126.5.1065. [DOI] [PubMed] [Google Scholar]
- Gibbon BC, Kovar DR, Staiger CJ. Latrunculin B has different effects on pollen germination and tube growth. Plant Cell. 1999;11:2349–2363. doi: 10.1105/tpc.11.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh PY, Surana U. Cdc4, a protein required for the onset of S phase, serves an essential function during G(2)/M transition in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:5512–5522. doi: 10.1128/mcb.19.8.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Mascarenhas JP. MEI1, an Arabidopsis gene required for male meiosis: isolation and characterization. Sex Plant Reprod. 1998;11:199–207. [Google Scholar]
- Hogan CJ. Microtubule patterns during meiosis in two higher plant species. Protoplasma. 1987;138:126–136. [Google Scholar]
- Joubès J, Chevalier C, Dudits D, Heberle-Bors E, Inzé D, Umeda M, Renaudin J-P. CDK-related protein kinases in plants. Plant Mol Biol. 2000;43:607–620. doi: 10.1023/a:1006470301554. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Activation of MPF at meiosis re-initiation in starfish oocytes. Dev Biol. 1999;214:1–8. doi: 10.1006/dbio.1999.9393. [DOI] [PubMed] [Google Scholar]
- Kong WH, Zheng G, Lu JN, Tso JK. Temperature dependent expression of cdc2 and cyclin B1 in spermatogenic cells during spermatogenesis. Cell Res. 2000;10:289–302. doi: 10.1038/sj.cr.7290056. [DOI] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- Lardon A, Triboi-Blondel AM, Dumas C. A model for studying pollination and pod development in Brassica napus: the culture of isolated flowers. Sex Plant Reprod. 1993;6:52–56. [Google Scholar]
- Liu D, Liao C, Wolgemuth DJ. A role for cyclin A1 in the activation of MPF and G2-M transition during meiosis of male germ cells in mice. Dev Biol. 2000;224:388–400. doi: 10.1006/dbio.2000.9776. [DOI] [PubMed] [Google Scholar]
- Loy CJ, Lydall D, Surana U. NDD1, a high-dosage suppressor of cdc28–1N, is essential for expression of a subset of late-S-phase-specific genes in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:3312–3327. doi: 10.1128/mcb.19.5.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mészáros T, Miskolczi P, Ayaydin F, Pettkó-Szandtner A, Peres A, Magyar Z, Horváth GV, Bakó L, Fehér A, Dudits D. Multiple cyclin-dependent kinase complexes and phosphatases control G2/M progression in alfalfa cells. Plant Mol Biol. 2000;43:595–605. doi: 10.1023/a:1006412413671. [DOI] [PubMed] [Google Scholar]
- Moser BA, Russell P. Cell cycle regulation in Schizosaccharomyces pombe. Curr Opin Microbiol. 2000;3:631–636. doi: 10.1016/s1369-5274(00)00152-1. [DOI] [PubMed] [Google Scholar]
- Nebenführ A, Frohlick JA, Staehelin LA. Redistribution of Golgi stacks and other organelles during mitosis and cytokinesis in plant cells. Plant Physiol. 2000;124:135–151. doi: 10.1104/pp.124.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda AR, Ferby I. Regulation of the meiotic cell cycle in oocytes. Curr Opin Cell Biol. 2000;12:666–675. doi: 10.1016/s0955-0674(00)00150-2. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- Niwa O, Yanagida M. Universal and essential role of MPF/cdc2+ Nature. 1988;336:430. doi: 10.1038/336430a0. [DOI] [PubMed] [Google Scholar]
- Ohi R, Gould KL. Regulating the onset of mitosis. Curr Opin Cell Biol. 1999;11:267–273. doi: 10.1016/s0955-0674(99)80036-2. [DOI] [PubMed] [Google Scholar]
- Peirson BN, Bowling SE, Makaroff CA. A defect in synapsis causes male sterility in a T-DNA-tagged Arabidopsis thaliana mutant. Plant J. 1997;11:659–669. doi: 10.1046/j.1365-313x.1997.11040659.x. [DOI] [PubMed] [Google Scholar]
- Preuss D, Rhee SY, Davis RW. Tetrad analysis possible in Arabidopsis with mutation of the QUARTET (QRT) genes. Science. 1994;264:1458–1460. doi: 10.1126/science.8197459. [DOI] [PubMed] [Google Scholar]
- Richman TJ, Sawyer MM, Johnson DI. The Cdc42p GTPase is involved in a G2/M morphogenetic checkpoint regulating the apical-isotropic switch and nuclear division in yeast. J Biol Chem. 1999;274:16861–16870. doi: 10.1074/jbc.274.24.16861. [DOI] [PubMed] [Google Scholar]
- Rippmann JF, Hobbie S, Daiber C, Cuilliard B, Bauer M, Birk J, Nar H, Garin-Chesa P, Rettig WJ, Schnapp A. Phosphorylation-dependent proline isomerization catalyzed by Pin1 is essential for tumor cell survival and entry into mitosis. Cell Growth Differ. 2000;11:409–416. [PubMed] [Google Scholar]
- Ross KJ, Fransz P, Jones GH. A light microscopic atlas of meiosis in Arabidopsis thaliana. Chromosome Res. 1996;4:507–516. doi: 10.1007/BF02261778. [DOI] [PubMed] [Google Scholar]
- Siddiqi I, Ganesh G, Grossniklaus U, Subbiah V. The dyad gene is required for progression through female meiosis in Arabidopsis. Development. 2000;127:197–207. doi: 10.1242/dev.127.1.197. [DOI] [PubMed] [Google Scholar]
- Skvarla JJ, Larson DA. Fine structural studies of Zea mays pollen I: cell membranes and exine ontogeny. Am J Bot. 1966;53:1112–1125. [Google Scholar]
- Smith LG. Divide and conquer: cytokinesis in plant cells. Curr Opin Plant Biol. 1999;2:447–453. doi: 10.1016/s1369-5266(99)00022-9. [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Cande WZ. Microfilament distribution in maize meiotic mutants correlates with microtubule organization. Plant Cell. 1991;3:637–644. doi: 10.1105/tpc.3.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stals H, Casteels P, Van Montagu M, Inzé D. Regulation of cyclin-dependent kinases in Arabidopsis thaliana. Plant Mol Biol. 2000;43:583–593. doi: 10.1023/a:1006409907831. [DOI] [PubMed] [Google Scholar]
- Toyoshima F, Moriguchi T, Wada A, Fukuda M, Nishida E. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traas JA, Burgain S, Dumas De Vaulx R. The organization of the cytoskeleton during meiosis in eggplant (Solanum melongena (L)): microtubules and F-actin are both necessary for coordinated meiotic division. J Cell Sci. 1989;92:541–550. [Google Scholar]
- Walworth NC. Cell-cycle checkpoint kinases: checking in on the cell cycle. Curr Opin Cell Biol. 2000;12:697–704. doi: 10.1016/s0955-0674(00)00154-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson MG, Millar JBA. Control of the eukaryotic cell cycle by MAP kinase signaling pathways. FASEB J. 2000;14:2147–2157. doi: 10.1096/fj.00-0102rev. [DOI] [PubMed] [Google Scholar]
- Yang M, Hu Y, Lodhi M, McCombie WR, Ma H. The Arabidopsis SKP1-LIKE1 gene is essential for male meiosis and may control homologue separation. Proc Natl Acad Sci USA. 1999;96:11416–11421. doi: 10.1073/pnas.96.20.11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HG, Hiatt EN, Chan A, Sweeney M, Dawe RK. Neocentromere-mediated chromosome movement in maize. J Cell Biol. 1997;139:831–840. doi: 10.1083/jcb.139.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]