Abstract
Bacterial chemotaxis receptors can detect a small concentration gradient of attractants and repellents in the environment over a wide range of background concentration. The clustering of these receptors to form patches observed in vivo and in vitro has been suspected as a reason for the high sensitivity, and such wide dynamic range is thought to be due to the resetting of the receptor sensitivity threshold by methylation/demethylation of the receptors. However, the mechanisms by which such high sensitivity is achieved and how the methylation/demethylation resets the sensitivity are not well understood. A molecular modeling of an intact bacterial chemotaxis receptor based on the crystal structures of a cytoplasmic domain and a periplasmic domain suggests an interesting clustering of three dimeric receptors and a two-dimensional, close-packed lattice formation of the clusters, where each receptor dimer contacts two other receptor dimers at the cytoplasmic domain and two yet different receptor dimers at the periplasmic domain. This interconnection of the receptors to form a patch of receptor clusters suggests a structural basis for the high sensitivity of the bacterial chemotaxis receptors. Furthermore, we present crystallographic data suggesting that, in contrast to most molecular signaling by conformational changes and/or oligomerization of the signaling molecules, the changes in dynamic property of the receptors on ligand binding or methylation may be the language of the signaling by the chemotaxis receptors. Taken together, the changes of the dynamic property of one receptor propagating mechanically to many others in the receptor patch provides a plausible, simple mechanism for the high sensitivity and the dynamic range of the receptors.
Keywords: transmembrane signaling|receptor signaling|receptor clustering| signal gain|signal amplification
All living organisms have sensory systems to detect the increase or decrease of attractants, repellents, and other changes in the environment. In these systems, the receptors are the first entry points of the environmental information, which is transduced into the cell interior to trigger chains of signaling pathways to induce cellular responses to the environment. For example, many bacteria move in response to their chemical environment by the mechanism of chemotaxis (for reviews, see refs. 1–3 and references therein), which involves a two-component signal transduction system [for reviews, see Bourret et al. (4), Falke et al. (5), and references therein]. Like other sensory receptors such as visual light receptors, bacterial chemotaxis receptors respond to the changes in the chemical environment with a high sensitivity and a broad dynamic range (1, 2, 6–8). The high sensitivity is manifested by, for example, an observation that the binding of attractants to less than 1% of the receptors can induce increased swimming motion of Escherichia coli (6, 8), and this high sensitivity is achieved at the beginning of the signal transduction pathway, not fully accountable by signal amplification downstream of the receptors (7, 8).
The clustering of the receptors has been implicated as related to the high sensitivity. Many evidences indicated that the chemotaxis receptors of E. coli form patches of clusters in vivo (9–12). The clusters comprise signaling teams that can contain difference receptor types acting collaboratively, and the signaling teams are based on a trimer of dimer format (13) observed in the crystal structures (14). Several mathematical models have been proposed for the high sensitivity (15–17). However, in these studies the structural basis for the receptor clustering is not described.
As for the dynamic range, the bacteria can detect the gradient of attractants such as aspartate under background concentration ranging from nanomolar to millimolar spanning five to six orders of magnitude (3, 6, 7). The wide dynamic range of bacterial chemotaxis receptors has been attributed to the adaptation process, by which the receptor sensitivity is reset on prolonged exposure to a new chemical environment. This adaptation process is associated with the methylation of the cytoplasmic domain of the receptor (18–23), although the mechanism by which the methylation affects the sensitivity is not understood.
As to the mechanism of transduction of signal from the ligand-binding domain to the cytoplasmic domain, several models have been advanced on the basis of biophysical and biochemical studies (24–27), none of which provides convincing insights to the mechanisms for the high sensitivity and the dynamic range described above.
Methods
Cloning, Mutagenesis, Protein Purification, and Crystallization.
The methods used for cloning, mutagenesis, protein purification, and crystallization are the same as those described (14). In brief, a DNA insert coding for the E. coli Tsr cytoplasmic domain spanning amino acids 286–526 was amplified by PCR from a plasmid containing the E. coli Tsr gene. For activated mutant, the plasmid was mutated to change the wild-type QEQE (amino acids at the four methylation sites) genotype to QQQQ (Q mutant). The mutation was verified by DNA sequencing. Both cytoplasmic domains of the serine receptor were purified on a Pharmacia HiTrap Q column and a phenyl-Sepharose hydrophobic interaction column (Bio-Rad). The crystals of both the wild-type and Q mutant domains were obtained at 4°C from a solution containing 0.1 M N-[2-acetamido]-2-iminodiacetic acid, pH 6.5, 15–20% 2-methyl-2,4-pentanediol, and 0.2 M sodium citrate. The crystals were transferred to the same mother liquor but with a higher concentration of 2-methyl-2,4-pentanediol by as much as 25% for more than 24 h before flash-freezing and data collection.
Model Building of the Intact Receptor and Receptor Cluster.
The intact Tsr model (Fig. 1) was built as described (14). The model for the “trussed slab” of the receptor cluster was built by repeated superpositioning of the cytoplasmic domain portion of the intact receptor model onto the cytoplasmic domains arranged in a single layer of the domain in the crystal structure of the Q mutant domain (14).
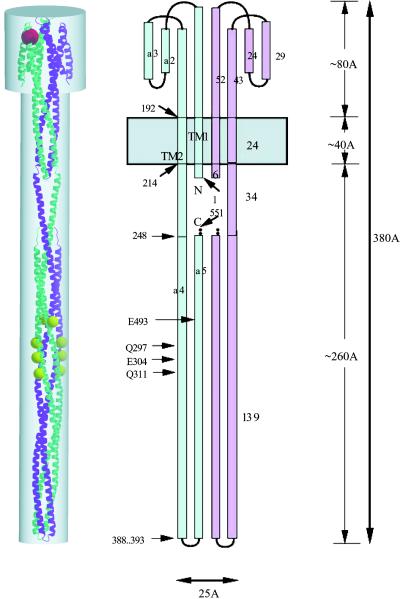
Figure 1.
A model of intact E. coli Tsr chemotaxis receptor dimer(14). (Center) Schematic drawing of the receptor, one monomer in blue and the other in pink. The presumed membrane bilayer is represented by a gray horizontal band. Landmark residues are labeled. (Right) The model is about 380 Å long and consists of an ≈80 Å long ligand-binding domain, about 40 Å of transmembrane domain, and about 260 Å of cytoplasmic domain. (Left) A ribbon diagram of the intact Tsr dimer model viewed perpendicularly to the noncrystallographic twofold symmetry axis. The dimensions are scaled to match those of the figure at the right. One monomer is in purple, and the other in cyan. Methylation sites are marked by yellow balls in one monomer and orange balls in the other, and the ligand serine is drawn as a red ball (partially hidden at the upper left corner).
X-Ray Diffraction Data Collection.
The x-ray diffraction data were collected at Advanced Light Source beam line 5.0.2. by using an ADSC Quantum 4 CCD-based x-ray detector. The x-ray wavelength was set to 0.977 Å, and the detector was placed 220 mm from the center of the goniometer head. A wild-type cTsr crystal was mounted to a Hampton cryo-loop directly from the crystallization drop. It was then flash-frozen in liquid nitrogen. An Oxford Cryosystem was used for temperature control. The frozen crystal was transferred to the gaseous nitrogen stream that was preset to 100 K. A complete data set, labeled as W100, was collected with 1° oscillation and 30-s exposure time. The temperature of the cold stream was then ramped up to 160 K at a speed of 120 K/h with the crystal staying in the stream. A second data set, labeled as W160, was collected at 160 K in the same manner as of W100. Similarly, two data sets were collected at 100 K and 160 K by using a Q mutant cTsr crystal. They are labeled as Q100 and Q160, respectively.
The data sets were processed by using programs denzo and scalepack (28). Table 1 shows the statistics of data processing. The crystal diffraction limit was 2.8 Å, and data were processed to 3.0 Å with more than 99% completeness. To preserve temperature effects associated with the data sets, Wilson plot scaling was avoided during the process.
Table 1.
Statistics of x-ray diffraction data collection and structure refinement
| Data set | W100 | Q100 | W160 | Q160 |
|---|---|---|---|---|
| Redundancy | 4.4 | 5.2 | 4.4 | 3.7 |
| No. unique reflections | 27870 | 27233 | 28255 | 27513 |
| Completeness, % | 99.9 | 99.9 | 99.9 | 99.9 |
| I/σ | 10.4 | 10.8 | 10.1 | 8.4 |
| Unit cell, Å: a = b | 132.812 | 131.682 | 133.397 | 132.286 |
| C | 134.972 | 134.108 | 135.610 | 134.741 |
| B factor, Å2 | 77.9 | 60.45 | 81.0 | 62.50 |
| Refinement resolution, Å | 20–3.0 | 20–3.0 | 20–3.0 | 20–3.0 |
| R factor, % | 25.2 | 24.3 | 25.6 | 24.1 |
| Free R factors, % | 29.6 | 28.4 | 30.6 | 30.1 |
Space group of both crystals is P321. Each crystal has 83% solvent content. The crystal structures contain a dimer of 224 and 220 residues for the Q mutant and wild-type domain, respectively. Data were cut off at 3σ for refinement.
Structure Refinement and Temperature Factor Analysis.
The wild-type and Q mutant structures of cTsr (1QU7; ref. 14) were used as starting model for the refinement of the W and Q data sets, respectively. The refinement was performed by using program CNS (29). An identical test set of reflections (10% of data) for crossvalidation were used in refinement of all four structures. The x-ray diffraction pattern was highly anisotropic, expected for a crystal lattice containing long helical structures. To avoid the much higher noise level associated with the low-intensity diffractions, we applied an I/σ cut-off (3σ) to the data for refinement. Structure refinement process started with rigid-body refinement protocol where each monomer of the dimeric molecule was treated as separate rigid body, followed by a torsion angle dynamics at a constant temperature of 3,000 K. Thereafter, rounds of manual fitting (with program O; ref. 30) in combination with positional and individual B factor refinement resulted in progressive improvement of the electron density and R factors. An anisotropic model was used for bulk solvent correction. The refinement statistics are shown in Table 1.
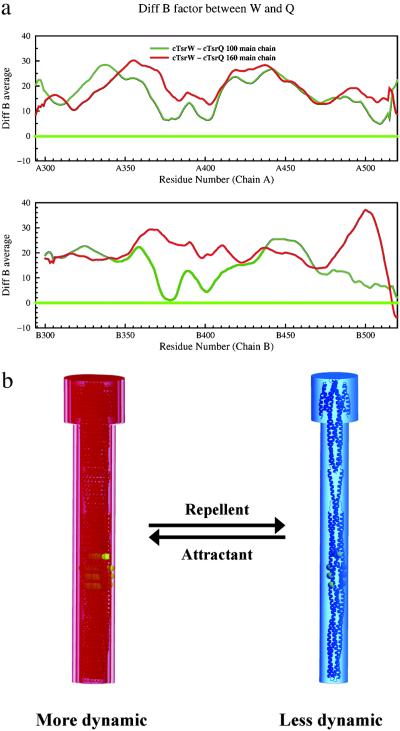
The average temperature factor of main-chain atoms (BMCavg) was calculated for each residue. The distribution of BMCavg across the polypeptide chain displays noisy variation, a common feature observed in many other protein structures. To represent the general trend of B factor distribution, we applied a smoothing algorithm that averages the BMcavg values of each residue and six neighboring residues on each side (a 13-residue sliding-window average). The window-averaged main-chain B factors were then subject to the systematic comparisons (Fig. 2a).
Figure 2.
Differences in dynamic properties as reflected by the temperature factors, B, of the cytoplasmic domains. The sliding window (of 13 residues)-averaged B factors are used for all calculations. (a) The difference between the window-averaged B factors of the wild type and the Q mutant at a given temperature. Red curves show the differences at 160 K, and green curves show the differences at 100 K. Residues 294–520 of A chain and 300–520 of B chain of the cTsr receptor dimer are shown in separate boxes. The wild-type cTsr displayed much higher temperature factors than the Q mutant (signal “on” state) throughout the entire dimeric structure at both temperatures, indicating that the overall structure of the Q mutant is in a much less dynamic state. (b) “Frozen dynamic” model for signaling. Phosphorylation signaling increases as the dynamic property of the receptor is reduced (blue) from more dynamic state (red).
Results and Discussion
Modulation of Dynamic Property as the Language of Signaling.
The most common mode of molecular signaling is attributed to either the changes in molecular conformation or in the oligomeric state of the molecules involved in the signaling. However, the crystal structures of the ligand domain of Salmonella typhimurium receptor in its ligand-bound and unbound states showed little conformational differences within monomeric domain, but small (about 4°) rotation of one domain with respect to the other in the dimeric structure of the domain, but no differences in oligomeric state (31, 32). A recent study with 13C19F REDOR NMR methods has indicated a small ligand-binding-induced change between helices α1 and α4 within the serine receptor (33, 34). Several models have been advanced to attribute the small conformational differences in the ligand domain dimer or monomer as the signal (24–27). None of these small conformational differences is without alternative explanations, or provides any convincing insights to the high sensitivity and the wide dynamic range discussed above. Similarly, comparison of the crystal structures of the cytoplasmic domain of E. coli serine chemotaxis receptor, cTsr, in signal “on” state (14) and wild-type states (see Table 1) shows very few differences (Table 2). [For cTsr in signal “on” state, we have used cTsr Q mutant with glutamine residue at all four methylation sites (QQQQ; ref. 35, and S. Parkinson, personal communication), and for wild type, the corresponding residues are QEQE.] However, the structure of the cTsr domain is much less dynamic in Q mutant than in wild type (Fig. 2a). The structural basis for the difference can be attributed to the fact that each glutamine residue on one helix of the coiled-coil structure of one Q mutant cTsr are at the positions that can make extensive hydrogen bonds to the residues or backbone of the helix in the other Q mutant cTsr in the same dimer, thus reducing dynamic mobility.
Table 2.
Root-mean-square distances (Å) between Cαs of the crystal structures of the cytoplasmic domain of Tsr
| W100 | W160 | Q100 | Q160 | |
|---|---|---|---|---|
| W100 | 0 | |||
| W160 | 0.967 | 0 | ||
| Q100 | 0.692 | 0.548 | 0 | |
| Q160 | 0.865 | 0.788 | 1.12 | 0 |
This observation suggests that the receptor in signal “off” state (attractant-bound state) is much more dynamic perhaps because of uncorrelated dynamic motion or correlated winding/unwinding motion of the coiled-coil structure; in signal “on” state (repellent-bound state), the dynamic property of the coiled-coil is modulated down (Fig. 2b) because of the favorable interactions (such as described above) between the monomers in a dimer receptor. We propose that this change or modulation of the dynamic property is the language of signaling, and CheA interact better and/or more productively with less dynamic state of the cytoplasmic domain to initiate the chain of phosphotransfer reaction downstream.
“Trussed Slab” Model for Receptor Cluster Patch.
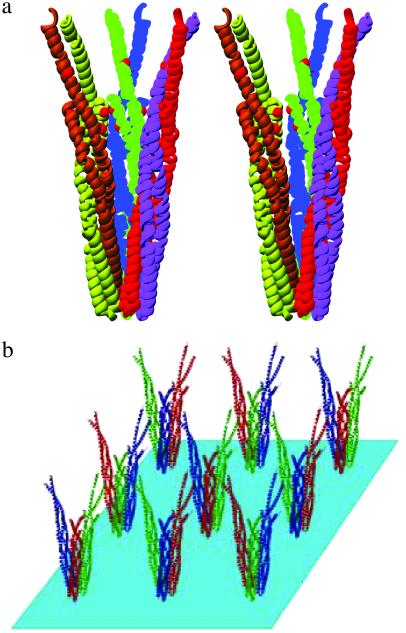
The crystal structures of cTsr (14) showed that each cytoplasmic domain is a coiled-coil of two antiparallel helices, and two of such coiled-coils form a supercoiled four-helix bundle to form a dimer. The core of the supercoil is stabilized by extensive interactions among hydrophobic residues and a few hydrogen-bonding interactions. The crystal structure also revealed that three dimers of the domain form a trimer of the dimers (Fig. 3a) by contacting one end of each domain (14), which contains a stretch of amino acid sequence strictly conserved among all bacterial chemotaxis receptors (36). In the crystal, these trimers of dimeric domains are separated by 132 Å in a close-packing arrangement on a two-dimensional plane, where each trimer is surrounded by six other trimers at equidistance (Fig. 3b).
Figure 3.
(a) Trimeric clustering of the cytoplasmic domain dimer of cTsr as determined by x-ray crystallography (14) and (b) two-dimensional lattice packing of the clusters in crystal of cTsr.
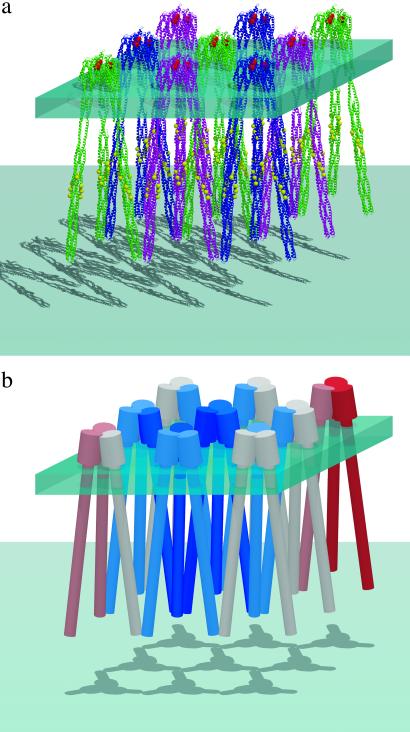
When the cytoplasmic domain of the intact receptor model (ref. 14; Fig. 1) is superpositioned to each cytoplasmic domain in one crystallographic unique layer in the crystal lattice (Fig. 3b) of cTsr, a surprising arrangement of the receptor-clustering model emerges (Fig. 4a). In this arrangement, not only the extreme ends of three cytoplasmic domain dimers are in contact by the sequence-specific interactions at the cytoplasmic side, as observed in the cTsr crystal structure, but each ligand-binding domain dimer is also in contact, by sequence nonspecific interactions, with two other ligand-binding domain dimers at the periplasmic side. Thus, each receptor dimer is in contact with four other receptor dimers forming a two-dimensional slab with the interconnected receptors acting as trusses (Fig. 4b). We further propose that the modulation of the dynamic property of one receptor dimer on ligand binding is “felt” by and transmitted to many others through the receptor network similar to this two-dimensional truss model, thus, modulating the dynamic properties of a large number of receptors resulting in a high sensitivity.
Figure 4.
(a) A model of clustering of a bacterial chemotaxis receptor. The model of intact E. coli Tsr receptor dimer (ref. 14; Fig. 3) was superimposed on each dimer of Tsr cytoplasmic domain as in the crystal of Tsr cytoplasmic domain (see Fig. 2a Lower). Six receptors in a hexameric cluster have the same color. This modeling suggests that the intact receptor models can form a two-dimensional “trussed slab,” where each receptor dimer makes contacts to two other receptor dimers at the tip of the cytoplasmic domain (by sequence-specific interactions) and another two at the top of the ligand-binding domain (by sequence nonspecific helix–helix contact); thus, each receptor dimer is in contact to every other receptor dimer in the sheet either directly or indirectly. (b) On repellent binding to or departure of bound attractant from one receptor, the dynamic property of the receptor is reduced (shown in blue) and the reduction is “felt” progressively by the first- and second-order neighbors (indicated by milder blue colors) propagating through the trussed slab of the receptors. The cluster cast by a vertical light source reveals the contact network in two dimensions graphically.
On the basis of numerous crystal structures of the periplasmic domains (25, 31, 32, 37, 38) of bacterial chemotaxis receptors with and without ligands and of the cytoplasmic domain in its wild-type state and activated state (14) at two different temperatures (this work) we suggest:
(i) The chemotaxis receptor molecule in signal “active state” (Q mutant) is more “frozen” conformationally than the wild type. The observed differences in the dynamic properties of the wild-type and Q mutant of cTsr (Fig. 2a) cannot be caused by possible differences in the crystalline environment, because both domains crystallize with the same crystal lattice, the same space group, and the same cell parameters (Table 1). This modulation of the dynamic property of the receptor (“frozen dynamic model”; ref. 24) is proposed to be the mechanism of signaling in bacterial chemotaxis receptors rather than the change in conformation (“conformational change model”) or the change in oligomerization state (“oligomerization model”) of the receptors. It is further assumed that CheA/CheW binds to the “frozen” receptor better and/or in a more productive mode and activates phosphotransfer reaction, but it dissociates from the receptors with increased dynamic motion or remains bound but in nonproductive mode, and deactivates the reaction. Thermosensing properties of some chemoreceptor mutants (39) may be the manifestation of the changes in dynamic properties of the receptors on temperature changes.
(ii) In a two-dimensional “trussed slab” of chemoreceptors, where each receptor dimer, as a truss, makes contacts with two other receptor dimers at the tip of the cytoplasmic domain (by sequence-specific interactions as observed in cTsr; ref. 14) and another two at the side of the periplasmic domain [by sequence nonspecific helix–helix contact, where a side chain of an amino acid from one helix fits into a concave surface of the contacting helix-like “knobs into triangles” (40) or “knobs into holes” (41)], each receptor dimer is in contact with every other receptor dimer in the slab either directly or indirectly. This model suggests that a mixture of different receptors can coexist in a slab as observed by Ames et al. (13), because the trimer of dimers can be formed by the sequence-specific interactions by the conserved sequences at the cytoplasmic side and by sequence nonspecific interactions at the periplasmic side as described above. It further suggests that a weaker trimer of dimers may be formed by helix–helix contacts at the periplasmic side if such clustering at the cytoplasmic side is blocked or the periplasmic interactions are augmented (12). It also suggests that, in the presence of a high concentration of “receptors” with no or small ligand-binding domains, the “trussed slab” size may be smaller. The observation that clustered receptors are found mostly at two poles of the bacterial cell (9–11) may imply that the curvature, membrane fluidity, and/or pole-specific proteins at the cell poles are more compatible with the trussed patch of receptors.
The receptors in a slab or two-dimensional patch are more in a “frozen” state in the presence of repellents (or absence of attractants) compared with those in the presence of attractants. As one bound attractant leaves from a receptor on the slab, the receptor becomes less dynamic (represented in blue in Fig. 4), and the reduced or frozen dynamic property of this receptor is propagated to nearby receptors through the “trussing” contacts, thus, propagating the decrease in dynamic property of the receptors further away from the central receptor. This coupling of dynamic property of one receptor to many on the same receptor patch is proposed as the mechanism for the observed high sensitivity of the chemotaxis.
(iii) On persistent exposure to an attractant the receptors are methylated, and phosphotransfer activity increases. It is proposed that each methylated glutamate at the methylation sites of one helix of a receptor favorably interact with parts of the other receptor in the same receptor dimer and reduces the dynamic property of the receptor dimer, thus increasing the activity of the phosphotransfer reaction (increased tumbling). This damping effect is expected to propagate (Fig. 4b) through the “trussed slab” of receptor patch by transmitting the reduction in dynamic property along the interconnected trusses in the receptor patch as described above. Mutations at the methylation sites may influence the phosphotransfer reaction positively or negatively depending on the balance between favorable and unfavorable interactions the substituted side chains may have on stabilizing the receptor dimmer, respectively.
Acknowledgments
We thank Dr. Kenji Komata of Banyu Pharmaceuticals, Inc., Tsukuba, Japan, for his help with the structural studies of the cytoplasmic domain of the wild-type Tsr while he was a visiting scientist at the University of California, Berkeley. We also thank Hisao Yokota for cloning and Dr. Sandy Parkinson for in vitro activity assay of the cytoplasmic domains used in this work and his very constructive advice. We thank the members of Advanced Light Source of Lawrence Berkeley National Laboratory (funded by the U.S. Department of Energy) for assistance in data collection. This work was supported by grants from the National Institutes of Health (CA78406) and from the Office of Biological and Environmental Research, Office of Science, U.S. Department of Energy. The authors declare that they have no competing financial interests.
References
- 1.Adler J. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- 2.Koshland D E., Jr Annu Rev Biochem. 1981;50:765–782. doi: 10.1146/annurev.bi.50.070181.004001. [DOI] [PubMed] [Google Scholar]
- 3.Berg H C, Tedesco P M. Proc Natl Acad Sci USA. 1975;72:3235–3239. doi: 10.1073/pnas.72.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourret R B, Borkovich K A, Simon M I. Annu Rev Biochem. 1991;60:401–441. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- 5.Falke J J, Bass R B, Butler S L, Chervitz S A, Danielson M A. Annu Rev Cell Dev Biol. 1997;13:457–512. doi: 10.1146/annurev.cellbio.13.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segall J E, Block S M, Berg H C. Proc Natl Acad Sci USA. 1986;83:8987–8991. doi: 10.1073/pnas.83.23.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim C, Jackson M, Lux R, Khan S. J Mol Biol. 2001;307:119–135. doi: 10.1006/jmbi.2000.4389. [DOI] [PubMed] [Google Scholar]
- 8.Sourjik V, Berg H C. Proc Natl Acad Sci USA. 2002;99:123–127. doi: 10.1073/pnas.011589998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maddock J R, Shapiro L. Science. 1993;259:1717–1723. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- 10.Sourjik V, Berg H C. Mol Microbiol. 2000;37:740–751. doi: 10.1046/j.1365-2958.2000.02044.x. [DOI] [PubMed] [Google Scholar]
- 11.Lybarger S R, Maddock J R. J Bacteriol. 2001;183:3261–3267. doi: 10.1128/JB.183.11.3261-3267.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gestwicki J E, Kiessling L L. Nature (London) 2002;415:81–84. doi: 10.1038/415081a. [DOI] [PubMed] [Google Scholar]
- 13.Ames P, Studdert C A, Reiser R H, Parkinson J S. Proc Natl Acad Sci USA. 2002;99:7060–7065. doi: 10.1073/pnas.092071899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K K, Yokota H, Kim S H. Nature (London) 1999;400:787–792. doi: 10.1038/23512. [DOI] [PubMed] [Google Scholar]
- 15.Bray D, Levin M D, Morton-Firth C J. Nature (London) 1998;393:85–88. doi: 10.1038/30018. [DOI] [PubMed] [Google Scholar]
- 16.Duke T A, Novere N L, Bray D. J Mol Biol. 2001;308:541–553. doi: 10.1006/jmbi.2001.4610. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Kim S-H. Pacific Symp Biocomput. 2000;5:350–361. [Google Scholar]
- 18.Russo A F, Koshland D E., Jr Science. 1983;220:1016–1020. doi: 10.1126/science.6302843. [DOI] [PubMed] [Google Scholar]
- 19.Borkovich K A, Alex L A, Simon M I. Proc Natl Acad Sci USA. 1992;89:6756–6760. doi: 10.1073/pnas.89.15.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cochran A G, Kim P S. Science. 1996;271:1113–1116. doi: 10.1126/science.271.5252.1113. [DOI] [PubMed] [Google Scholar]
- 21.Surette M G, Stock J B. J Biol Chem. 1996;271:17966–17973. doi: 10.1074/jbc.271.30.17966. [DOI] [PubMed] [Google Scholar]
- 22.Djordjevic S, Stock A M. Nat Struct Biol. 1998;5:446–450. doi: 10.1038/nsb0698-446. [DOI] [PubMed] [Google Scholar]
- 23.Yi T M, Huang Y, Simon M I, Doyle J. Proc Natl Acad Sci USA. 2000;97:4649–4653. doi: 10.1073/pnas.97.9.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S H. Protein Sci. 1994;3:159–165. doi: 10.1002/pro.5560030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeh J I, Biemann H P, Prive G G, Pandit J, Koshland D E, Jr, Kim S H. J Mol Biol. 1996;262:186–201. doi: 10.1006/jmbi.1996.0507. [DOI] [PubMed] [Google Scholar]
- 26.Chervitz S A, Falke J J. Proc Natl Acad Sci USA. 1996;93:2545–2550. doi: 10.1073/pnas.93.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ottemann K M, Xiao W, Shin Y K, Koshland D E., Jr Science. 1999;285:1751–1754. doi: 10.1126/science.285.5434.1751. [DOI] [PubMed] [Google Scholar]
- 28.Otwinowski Z, Minor W. Methods Enzymol. 1996;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 29.Brünger A T, Adams P D, Clore G M, DeLano W L, Gros P, Grosse-Kunstleve R W, Jiang J S, Kuszewski J, Nilges M, Pannu N S, et al. Acta Crystallogrogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 30.Jones T A, Zou J-Y, Cowan S W, Kjeldgaard M. Acta Crystallogrogr A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 31.Milburn M V, Prive G G, Milligan D L, Scott W G, Yeh J, Jancarik J, Koshland D E, Jr, Kim S H. Science. 1991;254:1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- 32.Scott W G, Milligan D L, Milburn M V, Prive G G, Yeh J, Koshland D E, Jr, Kim S H. J Mol Biol. 1993;232:555–573. doi: 10.1006/jmbi.1993.1411. [DOI] [PubMed] [Google Scholar]
- 33.Murphy O J, III, Kovacs F A, Sicard E L, Thompson L K. Biochemistry. 2001;40:1358–1366. doi: 10.1021/bi0015109. [DOI] [PubMed] [Google Scholar]
- 34.Danielson M A, Falke J J. Annu Rev Biophys Biomol Struct. 1996;25:163–195. doi: 10.1146/annurev.bb.25.060196.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunten P, Koshland D E., Jr J Biol Chem. 1991;266:1491–1496. [PubMed] [Google Scholar]
- 36.Le Moual H, Koshland D E., Jr J Mol Biol. 1996;261:568–585. doi: 10.1006/jmbi.1996.0483. [DOI] [PubMed] [Google Scholar]
- 37.Yeh J I, Biemann H P, Pandit J, Koshland D E, Kim S H. J Biol Chem. 1993;268:9787–9792. [PubMed] [Google Scholar]
- 38.Chi Y I, Yokota H, Kim S H. FEBS Lett. 1997;414:327–332. doi: 10.1016/s0014-5793(97)01027-2. [DOI] [PubMed] [Google Scholar]
- 39.Nishiyama S, Nara T, Homma M, Imae Y, Kawagishi I. J Bacteriol. 1997;179:6573–6580. doi: 10.1128/jb.179.21.6573-6580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C, Hou J, Kim S H. Proc Natl Acad Sci USA. 2002;99:3581–3585. doi: 10.1073/pnas.052003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crick F H C. Acta Crystallogr. 1953;6:689–697. [Google Scholar]