Abstract
In falciparum malaria, the malaria parasite induces changes at the infected red blood cell surface that lead to adherence to vascular endothelium and other red blood cells. As a result, the more mature stages of Plasmodium falciparum are sequestered in the microvasculature and cause vital organ dysfunction, whereas the ring stages circulate in the blood stream. Malaria is characterized by fever. We have studied the effect of febrile temperatures on the cytoadherence in vitro of P. falciparum-infected erythrocytes. Freshly obtained ring-stage-infected red blood cells from 10 patients with acute falciparum malaria did not adhere to the principle vascular adherence receptors CD36 or intercellular adhesion molecule-1 (ICAM-1). However, after a brief period of heating to 40°C, all ring-infected red blood cells adhered to CD36, and some isolates adhered to ICAM-1, whereas controls incubated at 37°C did not. Heating to 40°C accelerated cytoadherence and doubled the maximum cytoadherence observed (P < 0.01). Erythrocytes infected by ring-stages of the ICAM-1 binding clone A4var also did not cytoadhere at 37°C, but after heating to febrile temperatures bound to both CD36 and ICAM-1. Adherence of red blood cells infected with trophozoites was also increased considerably by brief heating. The factor responsible for heat induced adherence was shown to be the parasite derived variant surface protein PfEMP-1. RNA analysis showed that levels of var mRNA did not differ between heated and unheated ring-stage parasites. Thus fever-induced adherence appeared to involve increased trafficking of PfEMP-1 to the erythrocyte membrane. Fever induced cytoadherence is likely to have important pathological consequences and may explain both clinical deterioration with fever in severe malaria and the effects of antipyretics on parasite clearance.
The adherence of Plasmodium falciparum-infected erythrocytes to vascular endothelium and to other erythrocytes is considered central to the pathology of falciparum malaria. It is thought to involve a multistep interaction between parasite derived molecules expressed on the red blood cell surface (1–4) and receptor molecules present on the surface of endothelial cells (5, 6) or other red blood cells (7, 8). Although some sequestration of ring-stage-infected red blood cells has been observed in the brain of fatal cases of severe malaria (9), and ring-stage parasites, which bind to chondroitin sulfate A (CS-A), have been shown to cytoadhere in vitro (10), all red blood cells containing more mature parasites cytoadhere to vascular endothelium. Several ligands have been identified as potential receptors for parasitized red blood cell cytoadherence. The most important of these are CD36 (5), and intercellular adhesion molecule-1 (ICAM-1) (11, 12). CS-A has been identified recently as an important receptor in the brain (13) and placenta (14). Cytoadherence, and the resulting sequestration, are consistent features of falciparum malaria, although the vital organ distribution of sequestered parasitized erythrocytes varies between patients (15). Cerebral malaria, the major lethal manifestation of severe falciparum malaria, is associated particularly with cerebral sequestration. Expression of ICAM-1, the major parasite adherence receptor in the brain (13), is increased by proinflammatory cytokines including tumor necrosis factor (TNF). These proinflammatory cytokines are pyrogenic. TNF plays a central role in causing fever (16). Fluctuating fever is the essential clinical feature of symptomatic human malaria. In acute falciparum malaria, patients may have core temperatures which rise as high as 42°C, yet studies of parasitized red blood cell adherence are always conducted at the normal human body temperature of 37°C (2, 5–7, 17, 18). To reflect more closely the conditions in vivo, we have investigated the effect of febrile temperatures, in the range usually encountered during malaria, on the cytoadherence properties of P. falciparum in vitro.
Materials and Methods
Blood samples were obtained from 12 patients with acute falciparum malaria. These patients were admitted to hospital and were included in studies of antimalarial chemotherapy which will be reported in full elsewhere (unpublished observations). These studies were approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University. Fully informed consent was obtained for obtaining the 5-ml blood samples.
Effects of Febrile Temperatures on the Adherence of Ring-Stage P. falciparum-Infected Red Blood Cells.
The blood specimens were washed in RPMI medium 1640 and resuspended at 1% red blood cell suspension in the medium containing 10% human serum and then kept for 6 h in 5% CO2 atmosphere at 37°C and 40°C. Adherence assays were performed hourly by using CD36 or ICAM-1- transfected mouse fibroblasts incubated with the infected red blood cell suspension for 1 h with gentle agitation. Adherence was assessed microscopically by staining these cells with Giemsa, and the number of infected red blood cells bound to 1,000 transfected cells were counted. To accommodate dependence on parasitaemia, the results were normalized to 1% infected red blood cells of each isolate. To compare the effects of different febrile temperatures, five different parasite isolates with parasites of similar maturation, estimated morphologically to be between 5 and 10 h after red blood cell invasion (9), were incubated at 37°, 38°, 39°, and 40°C for 6 h and tested in the same way.
Adherence of the P. falciparum Clone.
Purified CD36 isolated from platelets and purified ICAM-1 (19) were immobilized on plastic Petri dishes by incubating the protein solutions for 2 h at 37°C and blocking the uncoated plastic area overnight with 1% BSA. The A4 P. falciparum clone is well characterized, and binds to both CD36 and ICAM-1 (11, 17). Sorbitol lysis was used to synchronize A4 infected red blood cells. Ring stages of A4 at a mean age of 6 h old (9) were heated to 40°C for 2 h and then transferred to 37°C. Adherence assays were then carried out at 37°C every 2 h after the heating. Briefly, an aliquot of red blood cells was taken from the cultures and washed twice in 10 ml of RPMI medium 1640. The red blood cells were diluted to a final 1% cell suspension, and 3 ml was added into the protein coated Petri dish for 1 h with gentle agitation at every 15 min. Results were expressed as number of bound infected red blood cells per mm2.
Effects of Heat on Maturation of Parasites.
Ring stages of the A4-parasite from in vitro culture were maintained at 37°C or heated to 40°C for 2 h and then transferred to 37°C. Thin blood films were made immediately after the 2-h heating and at 6, 8, and 10 h after transfer to 37°C. A blood film from the 37°C parasite culture was made also at each time point. All thin films were stained with Giemsa. The stage of development of 100 parasites in the thin blood films was assessed and the size and development of the parasites compared by using computerized image processing (9). The differences in maturation of the parasites from both cultures were compared by using ANOVA.
P. falciparum Erythrocyte Membrane Protein 1 (PfEMP-1) Expression.
PfEMP-1 is considered the principle parasite cytoadherence ligand. PfEMP-1 expression on the ring-stage-infected red blood cells of A4 clone of P. falciparum was assessed by flow cytometry. The red blood cells were washed and stained sequentially with the mouse BC6 monoclonal antibody (mAb), which recognizes A4 PfEMP-1, and rabbit antibody to mouse Ig then swine antibody to rabbit Ig conjugated with fluorescein dye. An isotype-matched mouse antibody IgG1 was used as negative control. Mature stages of the A4 clone were used as positive controls for PfEMP-1 expression. The intracellular malaria parasites were stained with ethidium bromide.
mRNA Quantitation.
The total RNA of A4 parasites both cultured at 37°C, and after heating to 40°C for 2 h were prepared simultaneously as described (21). RNA content was assessed by Northern blot analysis. ≈5 μg RNA per lane was analyzed. The conserved probe for exon 2 of var (varC) was used to detect A4 var message (≈13 kb).
Results
Effects of Febrile Temperatures on Cytoadherence.
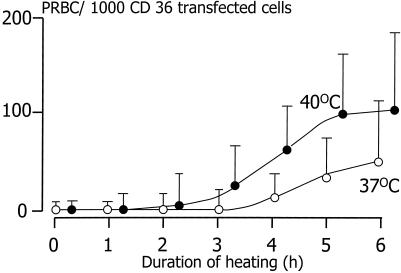
The effect of heating ring-stage-infected red blood cells on adherence was evaluated first with unselected P. falciparum parasite isolates obtained freshly from Thai patients with acute falciparum malaria. The range of parasitaemias was 2–26%. Ring-stage-infected red blood cells (average age estimated as 10 h after invasion; ref. 9) cultured at 40°C for as little as 1 h adhered to CD36 transfected cells (Fig. 1), whereas those grown at 37°C did not adhere at all. At both temperatures on some occasions, a few uninfected red blood cells also adhered. Brief heating to 40°C for 2 h also increased the adherence of red blood cells infected with more mature stages (trophozoites/schizonts) of P. falciparum (data not shown). Thus, heating to febrile temperatures enabled ring-stage-infected parasites to cytoadhere, and it augmented the binding of red blood cells infected with more mature parasite stages. Parasitized red blood cells from these clinical isolates rarely adhered to ICAM-1.
Figure 1.
The adherence of ring-stage P. falciparum-infected red blood cells from 12 clinical parasite isolates to CD36-transfected mouse fibroblasts during 6 h of heating at 40°C. Each circle represents the mean (95% confidence interval) adherence of 12 clinical parasite isolates incubated at 37°C (○) and 40°C (●).
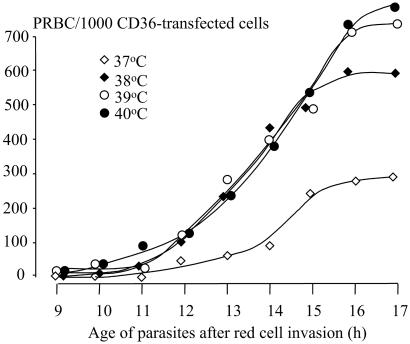
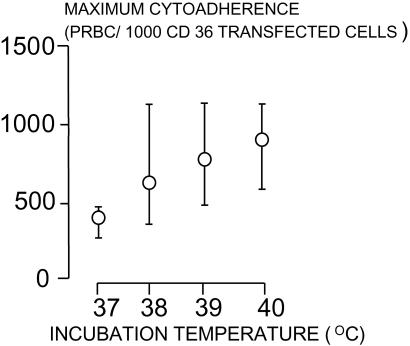
The effects of different febrile temperatures below 40°C on the adherence of ring-stage-infected red blood cells was then investigated in more detail in vitro. Five clinical parasite isolates of ring-stage parasites assessed microscopically (9) as being between 5 and 10 h after invasion were heated similarly to 38°C, 39°C, and 40°C, and adherence assays were performed before and hourly after heating and compared with the results at 37°C (Fig. 2). The increase in cytoadherence with these younger parasites was greater than with the unselected parasites in the first study. Heating, even to 38°C, increased cytoadherence. By the time the parasites were 11 h old (after invasion), there was a significant difference in the cytoadherence of parasites heated to 40°C (P = 0.014). Significant differences in adherence were observed by 12 h after heating to 38°C and 39°C (P ≤ 0.008). The median (range) maximum cytoadherence, measured as the number of parasitized erythrocytes (PRBC) bound per 1,000 CD36 transfected fibroblasts, was 262 (252–488) at 37°C, 597 (290–1,225) after heating to 38°C, 770 (350–1,232) after 39°C, and 827 (493–1,198) after 40°C (P ≤ 0.01) (Fig. 3). Median (range) times to reach cytoadherence levels of 10 and 100 PRBC per 1,000 cells were 12.2 (12–12.5) and 15.1 (13.5–16.2) h, respectively, at a temperature of 37°C, 11 (10.3–12.5) and 12.7 (12.5–13.1) h after heating to 38°, 10.5 (10.3–11.5) and 12.5 (12.0–13.3) h after 39°C, and 10.3 (9.5–11.5) and 12.1 (10.9–13) h after 40°C (P < 0.01).
Figure 2.
Effects of febrile temperatures on the adherence of ring-stage P. falciparum-infected red blood cells from five fresh clinical parasite isolates. The adherence assays were performed hourly at 37°C. Each circle represents the median value for the number of parasitized red blood cells (PRBC) bound to 1,000 CD36 transfected fibroblasts.
Figure 3.
Median (range) maximum values of cytoadherence after incubation at different temperatures.
The effects of febrile temperatures were consistent for CD36. ICAM-1 binding is intrinsically more variable. Only one of the clinical isolates studied showed increased adherence to ICAM-1; increasing from 72 to 261 at 40°C and increasing from 3 to 98 at 39°C infected erythrocytes per 1,000 ICAM-1-transfected cells after 8–10 h of heating. There was no increased adherence to ICAM-1 at 37°C and 38°C.
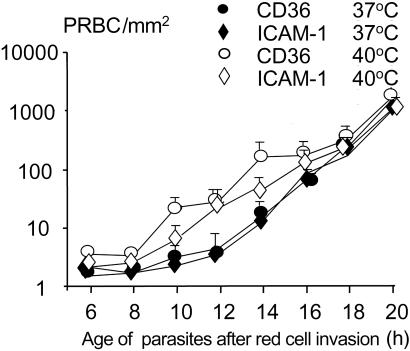
Heating could either have changed the membrane properties of the infected red blood cells, accelerated parasite development, or altered the surface expression of the main cytoadherence ligand PfEMP-1. If PfEMP-1 was involved primarily, then synthesis could have increased either as a result of augmented gene transcription or translation, or there could have been increased trafficking of the protein from its putative cytoplasmic pool to the red blood cell membrane. The mechanism by which heating induced cytoadherence was investigated by using the well-characterized A4 parasite clone (17, 19) expressing PfEMP-1, which adheres to both CD36 and ICAM-1. The effects of heating 6-h-old ring stages of A4 to 40°C for 2 h and then returning them to 37°C are shown in Fig. 4. Ring stages of the A4 parasite clone did not adhere to either CD36 or ICAM-1 when maintained at 37°C, but cytoadherence was increased from 2 h after heating. Binding to both CD36 and ICAM-1 increased synchronously, reaching a maximum 4 h after returning the parasites to 37°C.
Figure 4.
Parasitized erythrocyte (PRBC) adherence to immobilized CD36 and ICAM-1. Ring-stage P. falciparum-infected red blood cells of the A4var clone were heated to 40°C for 2 h and transferred to 37°C. The adherence assay was carried out at 37°C every 2 h after the heating. Results were expressed as number of infected red blood cells per mm2. Each circle represents the mean of 2 or 3 experiments and the bars are SD.
PfEMP-1 Expression.
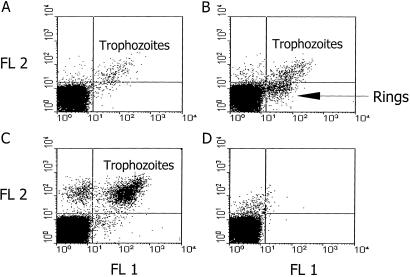
Flow cytometry was conducted to assess the role of PfEMP-1 involvement in adherence of the ring-stage parasites. A murine BC6 mAb to PfEMP-1 (20) was used to stain the heated and nonheated A4 ring-stage parasites in parallel semisynchronous cultures after the above heating protocol. Unheated ring-stage-infected red blood cells were not stained by BC6 mAb (Fig. 5A), whereas the heated ring-stage-infected red blood cells and the more mature trophozoites did stain (Fig. 5 B and C). The same results were observed by using the A4 parasite at the trophozoite stage. Control IgG subclass unrelated to malaria did not stain (Fig. 5D). BC6 mAb positivity of the heated ring-infected red blood cells correlated well with the adherence of the parasites to CD36 and ICAM-1. (Figs. 1 and 5). This finding suggests that PfEMP-1 is expressed on the membrane of these heated ring-infected red blood cells and mediates their cytoadherence, whereas it is not expressed on the surface of the unheated parasitized erythrocytes.
Figure 5.
Flow cytometric analysis of PfEMP-1 expression (FL1) on the ring-stage-infected red blood cells of A4var clone of P. falciparum (A) cultured at 37°C, and after heating to 40°C for 2 h and kept subsequently at 37°C for 4 h (B). (C) Mature stages of the A4var clone. (D) A mouse antibody IgG1 isotype stained A4var ring-stage-infected red blood cells was used as control. The intracellular parasites were stained with ethidium bromide (FL2).
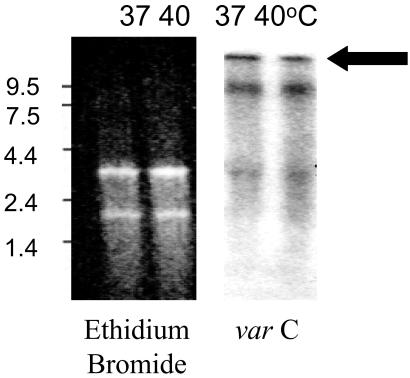
To investigate further whether heating affected the transcription of PfEMP-1, the expression of var mRNA in the ring-infected red blood cells from heated and nonheated cultures was compared. When the var exon2 probe in a simple RNA analysis method was used (21) (Fig. 6), there was no difference in the level of var mRNA in the ring stages from both cultures. The var mRNA is detectable shortly after merozoite invasion. At ≈3 h, var mRNA can be detected and it is expressed maximally by 12 h of ring-stage development (21). In this study, the var mRNA of the heated and nonheated ring-stage parasites appeared with similar time courses, suggesting that the effect of heating is not related to accelerated gene transcription.
Figure 6.
Northern blot analysis of A4 parasite total RNA at 37°C and 40°C. Ethidium bromide staining shows equivalent loading of RNA. varC, probe for exon2 of var detects A4var message (≈13 kb). See ref. 18 for details of the methods.
Effects of Heat on Parasite Growth.
To determine whether this phenomenon of fever-augmented cytoadherence could be explained simply by heat promotion of the growth rate of the ring-stage parasites to more mature stages, a stage and size comparison of the parasites was performed. As described (9), parasite cytoplasm and nuclear areas were determined from their film microscope slides using the NIH image program. Immediately after heating parasites from 40°C, blood parasites were smaller (mean size ratio 40°C/37°C = 0.89) than those from 37°C cultures (P = 0.02). Mean size ratios of the parasites at 6 and 8 h after heating were 0.75 and 0.83, respectively. The heated parasites showed significantly slower growth, defined by the fractional increases in cytoplasm volume (P = 0.0002 at 6 h and 0.0006 at 8 h) than unheated ones, though by 10 h after the heating, size ratios were not significantly different (0.94, P = 0.54). These results suggest that heat-accelerated cytoadherence is not related to an increase in parasite growth rate.
Discussion
Fever is the hallmark of malaria. This study shows that fever accelerates and increases the cytoadherence of parasitized erythrocytes to the two major vascular receptors CD36 and ICAM-1. The time to cytoadherence was accelerated by ≈2–3 h (20%), and the maximum value was doubled. These large effects, which occurred over a narrow temperature range, may explain some of the considerable variance in cytoadherence observed between different laboratories, and even between experiments. They could also have several pathological consequences. Relatively rapid synchronous sequestration in patients with high circulating parasitemias would be expected to result in clinical deterioration. In cerebral malaria, deterioration in the level of consciousness with high fever is well known, but had been attributed to exacerbated supply–demand inequalities related to increased metabolic demand and to the toxic effects of pyrogenic cytokines. However, the finding in this study that febrile temperatures increase cytoadherence to ICAM-1, the main adherence receptor in the brain (13), provides an alternative and plausible explanation. Recent pathology studies indicate a considerable increase (on average, 9-fold) in the numbers of ring-stage parasites in the capillaries and venules of the brain compared with those in the circulation in fatal cerebral malaria (9). This finding could result either from fever augmented cytoadherence by means of PfEMP1 or the recently described ring-stage adhesion (10), or a combination of the two. These data suggest that fever may have adverse consequences in patients with high parasitaemias
In highly synchronous infections, the fever spike at the time of synchronous schizogony would not be expected to affect sequestration, as there would be very few circulating parasitized cells capable of adhering. But acute falciparum malaria is usually not highly synchronous, and may even have a bimodal distribution of parasite ages. In this context, if a proportion of the infecting circulating parasites were ≥10 h old, a spike of fever would be expected to accelerate and increase sequestration. This would result in an increase in the sequestered biomass, with its associated pathology, and a fall in parasitemia.
Fever-induced cytoadherence was associated with increased expression of PfEMP-1 on the infected red blood cell surface. This suggests that PfEMP-1 was mainly responsible for the phenomenon, but the possibility that there may be other proteins expressed on heating, which partly account for the adhesion of these ring-stage-infected red blood cells, cannot be ruled out. Febrile temperatures both accelerated and increased the expression of the main cytoadherence ligand, which could have resulted either from augmented gene transcription or translation, or there could have been increased trafficking of the protein from its cytoplasmic pool to the red blood cell membrane. As there were no effects of heating on PfEMP-1 mRNA transcripts, this suggests that febrile temperatures increased the trafficking of preformed PfEMP1.
Recent studies (22) indicate that antipyretic drugs slow parasite clearance, an effect attributed to impairment of host-defense mechanisms and considered harmful. This interpretation has created a therapeutic dilemma for the health worker, as reducing fever also has potentially beneficial effects in reducing seizure risk and metabolic supply–demand inequalities in compromised microcirculations (23). These data provide an alternative and more plausible explanation. By reducing fever, antipyretics will attenuate fever-induced cytoadherence. This results in an apparent prolongation of parasite clearance when compared with uncontrolled fever. Thus, antipyretic drugs are not harmful, but in contrast are likely to be beneficial, both by reducing maximum cytoadherence and also by providing a greater opportunity for the antimalarial drugs to prevent parasite development and thus sequestration (24). This distinction is of considerable therapeutic relevance, as antipyretics are the most widely used drugs in the treatment of malaria apart from antimalarial drugs themselves. The antimalarial drugs with the broadest stage specificity of action are the artemisinin derivatives. These result in the clearance of ring-stage parasites before they mature to sequester. The precise relationship between cytoadherence in vitro and sequestration in vivo is not known. Presumably as parasitized red blood cells become increasingly adhesive, the probability of their adhering to vascular endothelium increases in parallel. If, when artemisinin treatment was started in acute falciparum malaria, core temperature was prevented from rising above 37°C, then delaying cytoadherence by 3 h would allow up to 25% of the circulating ring-stage parasites to be removed before they sequestered. This finding would argue in favor of giving antipyretics with antimalarial treatment to hyperparasitemic patients, whether or not they were febrile.
Acknowledgments
We are grateful to the staff at the Bangkok Hospital for Tropical Diseases for patient care, to Dr. Britta Urban and Neline Kriek for the help in flow cytometry, and to Dr. John Elliott for his kind gift of the transfected cells. This work was supported by the World Bank/World Health Organization/TDR Special Program for Research and Training in Tropical Diseases, and was part of the Wellcome Trust–Mahidol University–Oxford Tropical Medicine Research Programme supported by the Wellcome Trust of Great Britain.
Abbreviations
- ICAM-1
intercellular adhesion molecule-1
- PfEMP-1
P. falciparum erythrocyte membrane protein 1
References
- 1.Howard R J. Prog Allergy. 1988;41:98–147. doi: 10.1159/000415221. [DOI] [PubMed] [Google Scholar]
- 2.Baruch D I, Gormely J A, Ma C, Howard R J, Pasloske B L. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith J D, Kyes S, Craig A G, Fagan T, Hudson-Taylor D, Miller L H, Baruch D I, Newbold C I. Mol Biochem Parasitol. 1998;97:133–148. doi: 10.1016/s0166-6851(98)00145-5. [DOI] [PubMed] [Google Scholar]
- 4.Buffet P A, Gamain B, Scheidig C, Baruch D, Smith J D, Hernandez-Rivas R, Pouvelle B, Oishi S, Fujii N, Fusai T, et al. Proc Natl Acad Sci USA. 1999;96:12743–12748. doi: 10.1073/pnas.96.22.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnwell J W, Asch A S, Nachman R L, Yamaya M, Aikawa M, Ingravallo P. J Clin Invest. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treutiger C J, Heddini A, Fernandez V, Muller W A, Wahlgren M. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 7.Carlson J, Wahlgren M. J Exp Med. 1992;176:1311–1317. doi: 10.1084/jem.176.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowe J A, Moulds J M, Newbold C I, Miller L H. Nature (London) 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 9.Silamut K, Phu N H, Whitty C, Turner G D, Louwrier K, Mai N T, Simpson J A, Hien T T, White N J. Am J Pathol. 1999;155:395–410. doi: 10.1016/S0002-9440(10)65136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pouvelle B, Buffet P, A, Lepolard C, Scherf A, Gysin J. Nat Med. 2000;6:1264–1268. doi: 10.1038/81374. [DOI] [PubMed] [Google Scholar]
- 11.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Nature (London) 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 12.Robert C, Pouvelle B, Meyer P, Muanza K, Fujioka H, Aikawa M, Scherf A, Gysin J. Res Immunol. 1995;146:383–393. doi: 10.1016/0923-2494(96)81042-x. [DOI] [PubMed] [Google Scholar]
- 13.Turner G D, Morrison H, Jones M, Davis T M, Looareesuwan S, Buley I D, Gatter K C, Newbold C I, Pukritayakamee S, Nagachinta B, et al. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 14.Fried M, Duffy P E. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 15.MacPherson G G, Warrell M J, White N J, Looareesuwan S, Warrell D A. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 16.van Hensbroek M B, Palmer A, Onyiorah E, Schneider G, Jaffar S, Dolan G, Memming H, Frenkel J, Enwere G, Bennett S, et al. J Infect Dis. 1996;174:1091–1097. doi: 10.1093/infdis/174.5.1091. [DOI] [PubMed] [Google Scholar]
- 17.Roberts D J, Craig A G, Berendt A R, Pinches R, Nash G, Marsh K, Newbold C I. Nature (London) 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udomsangpetch R, Aikawa M, Berzins K, Wahlgren M, Perlmann P. Nature (London) 1989;338:763–765. doi: 10.1038/338763a0. [DOI] [PubMed] [Google Scholar]
- 19.Gardner J P, Pinches R A, Roberts D J, Newbold C I. Proc Natl Acad Sci USA. 1996;93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson-Taylor D E, Peterson D S, Pinches R, Newbold C I, Miller L H. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyes S, Pinches R, Newbold C. Mol Biochem Parasitol. 2000;105:311–315. doi: 10.1016/s0166-6851(99)00193-0. [DOI] [PubMed] [Google Scholar]
- 22.Brandts C H, Ndjave M, Graninger W, Kremsner P G. Lancet. 1997;350:704–709. doi: 10.1016/S0140-6736(97)02255-1. [DOI] [PubMed] [Google Scholar]
- 23.White N J. In: Malaria: Parasite Biology, Pathogenesis, Protection. Sherman I, editor. Washington, DC: Am. Soc. Microbiol.; 1998. pp. 371–385. [Google Scholar]
- 24.Udomsangpetch R, Pipitaporn B, Krishna S, Angus B, Pukrittayakamee S, Bates I, Suputtamongkol Y, Kyle D E, White N J. J Infect Dis. 1996;173:691–698. doi: 10.1093/infdis/173.3.691. [DOI] [PubMed] [Google Scholar]