Abstract
Splice variants (SVs) of receptors for growth hormone-releasing hormone (GHRH) have been found in primary human prostate cancers and diverse human cancer cell lines. GHRH antagonists inhibit growth of various experimental human cancers, including pancreatic and colorectal, xenografted into nude mice or cultured in vitro, and their antiproliferative action could be mediated in part through SVs of GHRH receptors. In this study we examined the expression of mRNA for GHRH and for SVs of its receptors in tumors of human pancreatic, colorectal, and gastric cancer cell lines grown in nude mice. mRNA for both GHRH and SV1 isoform of GHRH receptors was expressed in tumors of pancreatic (SW1990, PANC-1, MIA PaCa-2, Capan-1, Capan-2, and CFPAC1), colonic (COLO 320DM and HT-29), and gastric (NCI-N87, HS746T, and AGS) cancer cell lines; mRNA for SV2 was also present in Capan-1, Capan-2, CFPAC1, HT-29, and NCI-N87 tumors. In proliferation studies in vitro, the growth of pancreatic, colonic, and gastric cancer cells was stimulated by GHRH(1–29)NH2 and inhibited by GHRH antagonist JV-1–38. The stimulation of some gastroenteropancreatic cancer cells by GHRH was followed by an increase in cAMP production, and GHRH antagonist JV-1–38 competitively inhibited this effect. Our study indicates the presence of an autocrine/paracrine stimulatory loop based on GHRH and SV1 of GHRH receptors in human pancreatic, colorectal, and gastric cancers. The finding of SV1 receptor in human cancers provides an approach to an antitumor therapy based on the blockade of this receptor by specific GHRH antagonists.
Diverse antagonistic analogs of growth hormone (GH)-releasing hormone (GHRH) were synthesized in our laboratory in an endeavor to develop a new class of antineoplastic agents (1–3). GHRH antagonists strongly suppress the in vivo growth of various experimental human cancers including bone sarcomas; prostatic, mammary, and ovarian cancers; renal cell carcinomas; small-cell lung cancers (SCLC) and non-SCLC, pancreatic, and colorectal carcinomas; and malignant glioblastomas (reviewed in ref. 3). The antitumor effects of GHRH antagonists were initially thought to be exerted only indirectly through the inhibition of the pituitary GH/hepatic insulin-like growth factor-I (IGF-I) axis and reduction in serum IGF-I levels (4–7). However, it was later shown in cell cultures in vitro, where the contribution of the endocrine GH/IGF-I axis is clearly excluded, that GHRH antagonists directly inhibit the proliferation of various human cancer lines including MIA PaCa-2 and SW1990 human pancreatic cancers as well as HT-29 human colon cancers (8–12). Such an action would have to be mediated through specific GHRH receptors on the tumor cells (3, 13). GHRH antagonists also inhibit the autocrine production of IGF-I and/or IGF-II in many human cancer lines in vitro and in vivo, apparently through a direct action on the malignant cells (8, 10, 11, 14–20). IGF-II, like IGF-I, is a potent mitogen for many cancers and the suppression of its production would inhibit tumor growth, but unlike IGF-I, its synthesis does not depend on serum GH levels (1). The growth of human pancreatic cancers SW-1990 and human colorectal carcinomas HT-29 xenografted into nude mice and the autocrine synthesis of IGF-II were effectively inhibited by low doses of GHRH antagonists that did not significantly suppress the serum levels of IGF-I (10, 11). In other tumors such as SCLC and breast carcinomas, GHRH antagonists can inhibit the proliferation by blocking the action of autocrine/paracrine GHRH by a direct effect that may not involve IGFs (21–23).
The pituitary form of GHRH receptors could not be detected in tumors, but our investigations demonstrated that various human experimental cancers expressed the mRNAs corresponding to four truncated splice variants (SVs) of GHRH receptors denoted SV1 to SV4 (18, 23–29). Thus, the presence of SV1 has been shown in MIA PaCa-2 human pancreatic cancer, in some human prostatic, renal, ovarian, breast, lung, and bone cancer lines (3, 18, 23–29), as well as in primary specimens of human prostate cancer (30). Furthermore, the endogenous production of GHRH was detected in diverse human cancers such as endometrial, ovarian, breast, prostatic, pancreatic, gastric, colon cancers, SCLC, and sarcomas (21, 26–36). The proliferation of SCLC, sarcomas, and breast cancer cells can be stimulated by administration of GHRH or its agonistic analogs and conversely inhibited by GHRH antagonists. Together, these observations suggest that GHRH may serve as a growth factor for these cancers and that the direct antiproliferative action of GHRH antagonists could be exerted through the disruption of this autocrine/paracrine stimulatory loop (13, 21, 27, 29).
The aim of the present study was to investigate the expression of GHRH and SVs of its receptors in established human pancreatic, gastric, and colorectal cancer lines. In addition, we studied the mitogenic actions of GHRH(1–29)NH2 and the antiproliferative effects of GHRH antagonist JV-1–38 in these cancer cell lines.
Materials and Methods
Peptides.
hGHRH(1–29)NH2, antagonistic GHRH analog JV-1–38, and GHRH agonist JI-38 were synthesized in our laboratory by solid-phase methods and purified as described (2, 37).
Cell Culture and Proliferation Assay.
Tumor cell lines were obtained from the American Type Culture Collection. The media for routine culture varied as follows, depending on the requirements of the cell lines: RPMI medium 1640 (RPMI)/10% FBS was used for Capan-2 cells; RPMI/10% heat-inactivated FBS for Colo 320DM cells; McCoy 5A medium/10% FBS for HT-29 cells; DMEM/10% FBS for SW1990, PANC-1, and HS746T cells; DMEM/1 mM pyruvate/10% FBS/2.5% horse serum for MIA PaCa-2 cells; Iscove's modified Dulbecco's medium/10% FBS for NCI N87 and CFPAC-1 cells; Iscove's modified Dulbecco's medium/15% FBS for Capan-1 cells; nutrient mixture F12 Ham (F12)/20% FBS for LoVo cells; and F12 Kaighn's modification/10% FBS for AGS cells. All of the media were supplemented with 100 units/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, and 0.25 μg/ml amphotericin B. The cultures were maintained in a humidified atmosphere containing 5% CO2/95% air at 37°C. Cells were passaged weekly and monitored routinely for mycoplasma contamination by using a detection kit (Roche Molecular Biochemicals). All culture media components were purchased from GIBCO unless otherwise stated.
In Vitro Proliferation Assay.
The culture medium for in vitro studies with SW1990, Capan-2, CFPAC1, COLO 320DM, HT-29, NCI-N87, and AGS cells was DMEM/F12 supplemented with 0.5% FBS, 100 units/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, and 0.25 μg/ml amphotericin B. For in vitro proliferation assays, the cells were seeded into 96-well microplates and cultured for 24 h before adding various test substances to the medium. After another 72 h, in vitro cell growth was estimated by using Crystal violet as described (38) or by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay as described (39). The results were calculated as percent T/C, where T = OD of treated cultures and C = OD of control cultures × 100. The measured absorbance is proportionate to cell number.
Other experiments were intended to test whether a frequent change of the reduced serum culture medium, resulting in the removal of autocrine growth factors secreted by the cells, can affect the rate of proliferation. In these experiments, the cancer cells were cultured for 72 h removing the medium from some wells and replacing it with fresh medium every 24 h, whereas in the control wells it was left unchanged during the entire 72-h period.
Animals and Tumors.
Male athymic (NCrnu/nu) nude mice, ≈6 weeks old on arrival, were obtained from the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD) and housed in laminar airflow cabinets under pathogen-free conditions with 12-h light/12-h dark schedule and fed autoclaved standard chow and water ad libitum. Xenografts of each cell line were initiated by s.c. injections of 1 × 107 cells into three nude mice. After 4 weeks, the tumors from donor animals were dissected aseptically and 3-mm3 tumor pieces were transplanted s.c. into experimental nude mice under isoflurane anesthesia. After another 4 weeks, the mice were killed and the tumors, which had grown to approximately 1,500 mm3 in size, were dissected, snap-frozen, and stored at −70°C for further analysis. All experiments were performed in accordance with institutional guidelines for the care and use of experimental animals.
Reverse Transcription (RT)-PCR for GHRH and SVs of GHRH Receptors.
Total RNA was extracted from tumors belonging to each cell line by using Tri-Reagent (Sigma) according to the manufacturer's instructions. Poly(A)+ RNA was also purified by using oligo(dT)-cellulose MicroPolyAPure mRNA isolation kit (Ambion, Austin, TX). One microgram of poly(A)+ RNA from COLO 320DM, LoVo, Hs746T, PANC-1, and Capan-1 tumors and 5 μg of total RNA from HT-29, NCI-N87, AGS, SW1990, MIA PaCa-2, Capan-2, and CFPAC1 tumors (for GHRH receptors) or 1 μg of poly(A)+ RNA (for GHRH) were reverse-transcribed by using the reagents and protocol of the GeneAmp RNA PCR Core kit (Perkin–Elmer). The PCR amplifications of the cDNAs for SVs of GHRH receptors were performed by using the GeneAmp RNA PCR Core kit (Perkin–Elmer) and PCR for GHRH was performed by using the GeneAmp Gold RNA PCR Reagent kit (Perkin–Elmer). Five microliters of cDNA (for GHRH receptors) or 8 μl of cDNA (GHRH) was amplified by RT-PCR in 25 μl of mixture containing 1× PCR buffer, 2 mM MgCl2 (GHRH receptors), or 2.5 mM MgCl2 (GHRH), 200 μM each dNTP, 0.5 μM each primer (GHRH receptors), or 0.75 μM each primer (GHRH), and 2.5 units/100 μl AmpliTaq DNA polymerase (GHRH receptors) or 2.5 units/50 μl AmpliTaq Gold DNA polymerase (GHRH). For GHRH receptors, samples were denatured at 95°C for 3 min followed by 35 cycles of 95°C for 30 s, 58°C for 30 s, 72°C for 2 min, and a final extension at 72°C for 7 min; a secondary PCR of 20 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 45 s was subsequently performed on 1 μl of the primary PCR product. For GHRH, samples were denatured at 95°C for 10 min and subjected to 35 cycles of 94°C for 35 s, 57°C for 40 s, and 72°C for 40 s with a final extension at 72°C for 7 min. The primers used for SVs of GHRH receptor were 5′-CCT ACT GCC CTT AGG ATG CTG G-3′ (sense) and 5′-ATC TCA CGG AAG TGG CAT GGC C-3′ (antisense) for the first PCR, and 5′-GCA CCT TTG AAG CCA GAG AAG G-3′ (sense) and 5′-CAC GTG CCA GTG AAG AGC ACG G-3′ (antisense) for the second PCR (24, 25, 27–30). The primers for GHRH have been described (35). The PCR products were electrophoresed on 1.5% agarose gel and stained with ethidium bromide.
Measurement of Intracellular cAMP Levels.
Cell lines were seeded in 12-well plates (2 × 104 cells per well) with their routine medium and cultured until reaching 70–80% confluency. Subsequently, cells were incubated at 37°C for 30 min with DMEM containing 1 mg/ml bacitracin, 0.5% (wt/vol) BSA, 0.05 mM phenylmethylsulfonyl fluoride, 0.4 mM 3-isobutyl-1-methylxanthine, and GHRH(1–29)NH2 or GHRH agonist JI-38 (10 nM) alone or in combination with JV-1–38 (1 μM). The medium was then removed, and cold 0.1 M HCl was added to each well and harvested. Cell lysates were centrifuged for 10 min at 5,000 rpm at 4°C to remove protein; the supernatants were neutralized with an equal volume of 150 mM Tris⋅HCl (pH 8.6) containing 4 mM EDTA, and acetylated with triethylamine/acetic anhydride (2:1; 25 μl per 500 μl of sample). The levels of cAMP in acetylated supernatants were determined by double-antibody RIA (40). 2′-O-monosuccinyl-cAMP tyrosil methyl ester (Sigma) was used for the iodination. The antiserum for cAMP was obtained from the National Institute of Diabetes and Digestive and Kidney Diseases (CV-27). cAMP from Sigma was used as the standard.
Statistical Analysis.
The data are expressed as the mean ± SEM. The differences between the groups in the cAMP production study were evaluated by two-tailed Student's t test; P < 0.05 was considered statistically significant. The results of in vitro proliferation experiments were compared by one-way ANOVA followed by Dunnett's test. P values shown are against the control group unless otherwise stated.
Results
Expression of mRNA for SVs of GHRH Receptor in Human Pancreatic, Colon, and Gastric Cancers.
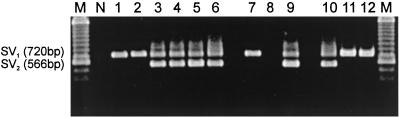
The expression of mRNAs for SVs of GHRH receptor in tumors grown in nude mice was assessed by RT-PCR. By using mRNA isolated from tumors and gene-specific primers for amplifying cDNAs of SVs of GHRH receptors, we were able to detect a 720-bp PCR product in SW1990, PANC-1, MIA PaCa-2, Capan-1, Capan-2, and CFPAC1 pancreatic cancers; COLO 320DM and HT-29 colonic tumors; as well as in NCI-N87, HS746T, and AGS gastric cancers (Fig. 1). This 720-bp product corresponds to SV1 of GHRH receptors described (25). A second 566-bp PCR product was also detected in MIA PaCa-2, Capan-1, Capan-2, and CFPAC1 pancreatic tumors, HT-29 colorectal tumors, and NCI-N87 gastric cancers (Fig. 1). This 566-bp product corresponds to SV2. No expression of mRNA for GHRH receptor splice variants SV1 or SV2 was detected in LoVo colon cancer (Fig. 1). In addition, no bands representing SV3 and SV4 (25) were obtained in any of the human pancreatic, colon, and gastric tumor models.
Figure 1.
RT-PCR analysis of the expression of mRNA for SVs of GHRH receptors in human pancreatic, colonic, and gastric cancer cell lines grown in nude mice. Products were of the expected size of 720 bp corresponding to SV1 and 566 bp corresponding to SV2. Lane 1, SW1990; lane 2, PANC-1; lane 3, MIA PaCa-2; lane 4, Capan-1; lane 5, Capan-2; and lane 6, CFPAC-1 pancreatic cancer cell lines. Lane 7, Colo 320DM; lane 8, LoVo; and lane 9, HT-29 colon cancers. Lane 10, NCI-N87; lane 11, HS 746T; and lane 12, AGS gastric cancer cell lines. Lane N, negative control; lane M, 100-bp DNA molecular weight marker. A representative PCR from three separate experiments is shown.
Expression of mRNA for GHRH in Human Pancreatic, Colonic, and Gastric Cancers.
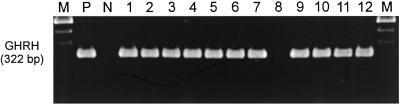
RT-PCR with primers specific for GHRH yielded a product of the expected size of 322 bp in tumors of SW1990, PANC-1, MIA PaCa-2, Capan-1, Capan-2, and CFPAC1 pancreatic cancer lines; COLO 320DM and HT-29 colonic; and NCI-N87, HS746T, and AGS gastric cancer lines grown in nude mice (Fig. 2). This band corresponds to mRNA for GHRH, identified in the positive control (H69 SCLC) (21) (Fig. 2). However, no expression of mRNA for GHRH was detected in LoVo colon tumors.
Figure 2.
The expression of mRNA for GHRH in human pancreatic, colonic, and gastric tumors grown in nude mice. Products were of the expected size of 322 bp. Lane 1, SW1990; lane 2, PANC-1; lane 3, MIA PaCa-2; lane 4, Capan-1; lane 5, Capan-2; and lane 6, CFPAC-1 pancreatic cancer lines. Lane 7, Colo 320DM; lane 8, LoVo; and lane 9, HT-29 colonic cancers. Lane 10, NCI-N87; lane 11, HS 746T; and lane 12, AGS gastric cancer lines. Lane P, H69 human SCLC (positive control); lane N, negative control; lane M, 100-bp DNA molecular weight marker. A representative PCR from three separate experiments is shown.
Effect of GHRH and GHRH Antagonist JV-1–38 on the Proliferation of Pancreatic, Colonic, and Gastric Cancer Cell Lines.
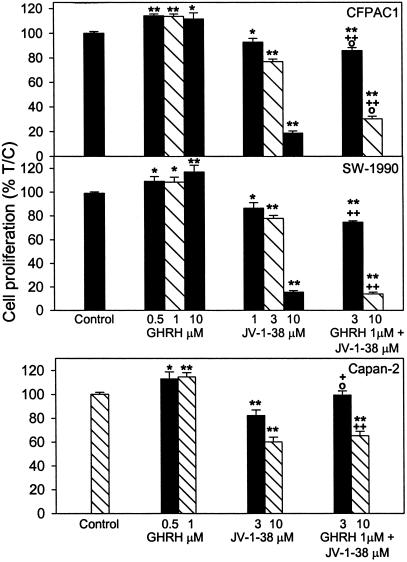
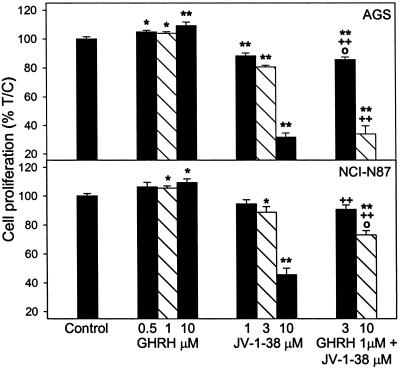
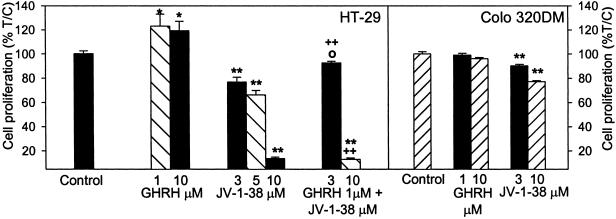
Cancer cells cultured in vitro were exposed to various concentrations of GHRH(1–29)NH2 and GHRH antagonist JV-1–38, and the rate of cell proliferation was measured by colorimetric tests. In the COLO 320DM cells that mostly float in culture, the thiazolyl-blue [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] test was conducted, but for the majority of cells that attach well to the surface of the wells, crystal violet assays were performed. As shown in Figs. 3–5, GHRH stimulated the growth of most of the cancer cell lines tested. The stimulation of the proliferation was significant at 0.5–10 μM doses of GHRH(1–29)NH2 in CFPAC1 and SW1990 pancreatic and AGS gastric cancers; at 0.5–1 μM concentration in Capan-2 pancreatic cancer; and at 1–10 μM doses in HT-29 colon and NCI-N87 gastric cancer cells. However, GHRH at concentrations of 1–10 μM did not affect the proliferation of COLO 320DM colon cancer cells (Fig. 4). The antagonistic analog of GHRH, JV-1–38, at 1–10 μM concentrations decreased the growth of the cancer cell lines tested. The reduction of the proliferation was significant at 3 and 10 μM doses of JV-1–38 in CFPAC1, SW1990, and Capan-2 pancreatic (Fig. 3), HT-29 and COLO 320DM colonic (Fig. 4), and AGS and NCI-N87 gastric cancer cells (Fig. 5). The growth of CFPAC-1, SW1990, and AGS cells was also inhibited significantly by 1 μM of JV-1–38. The inhibitory effect of 3–10 μM GHRH antagonist JV-1–38 on growth of the cancer cells was partially reversed by 1 μM GHRH(1–29)NH2 in the CFPAC-1 and Capan-2 pancreatic (Fig. 3), HT-29 colonic (Fig. 4), and AGS and NCI-N87 gastric cancers (Fig. 5). However, in each instance the antagonist in combination with GHRH had a significant inhibitory effect on cell proliferation, as compared with the cell growth in the presence of GHRH alone (Figs. 3–5).
Figure 3.
Effect of GHRH(1–29)NH2 (0.5–10 μM) and GHRH antagonist JV-1–38 (1–10 μM), alone or in combination with GHRH(1–29)NH2 at 1 μM concentration, on the proliferation of CFPAC1, SW1990, and Capan-2 pancreatic cancer cells. Data shown represent the mean ± SEM from three independent experiments performed in octuplicate. *, P < 0.05 and **, P < 0.001 vs. control; +, P < 0.05 and ++, P < 0.001 vs. 1 μM GHRH; ○, P < 0.05 vs. JV-1–38 alone at the corresponding concentration. Relative cell number in treated and control plates was determined by crystal violet staining and expressed as percent T/C values, where T = absorbance of treated cultures and C = absorbance of control cultures. Measured absorbance is proportionate to cell number.
Figure 5.
Effect of GHRH(1–29)NH2 (0.5–10 μM) and GHRH antagonist JV-1–38 (1–10 μM), alone or in combination with GHRH(1–29)NH2 at 1 μM concentration, on the proliferation of AGS and NCI-N87 gastric cancer cell lines. Data shown represent the mean ± SEM from three independent experiments performed in octuplicate. *, P < 0.05 and **, P < 0.001 vs. control; +, P < 0.05 and ++, P < 0.001 vs. 1 μM GHRH; ○, P < 0.05 vs. JV-1–38 alone at the corresponding concentration. Relative cell number in treated and control plates was determined by crystal violet staining.
Figure 4.
Effect of GHRH(1–29)NH2 (1–10 μM) and GHRH antagonist JV-1–38 (1–10 μM), alone or in combination with GHRH(1–29)NH2 at 1 μM concentration, on the proliferation of HT-29 and COLO 320DM colonic cancer cell lines in vitro. Data shown represent the mean ± SEM from three independent experiments performed in octuplicate. *, P < 0.05 and **, P < 0.001 vs. control; +, P < 0.05 and ++, P < 0.001 vs. 1 μM GHRH; ○, P < 0.05 vs. JV-1–38 alone at the corresponding concentration. Relative cell number in treated and control plates was determined by crystal violet staining (for HT-29) or 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay (for COLO 320DM).
Frequent Changes of Medium Prevent Autocrine Growth Stimulation of Pancreatic, Colonic, and Gastric Cancer Cell Lines.
Changes of the reduced-serum medium at every 24 h significantly decreased the proliferation of SW1990 and Capan-2 pancreatic cancer cells by 56% each (both P < 0.001); HT-29 colonic cancer cell line by 55% (P < 0.001); and AGS and NCI-N87 gastric cancer cells by 86% and 26% (both P < 0.001), respectively, as compared with the cells in 72-h continuous culture. The effect of frequent changes of medium on the proliferation of other cell lines was not investigated.
Effect of GHRH Antagonist JV-1–38 on cAMP Accumulation Induced by GHRH(1–29)NH2 or GHRH Agonist JI-38 in Pancreatic, Colonic, and Gastric Cancer Cell Lines.
As shown in Table 1, GHRH antagonist JV-1–38, used at 1 μM, blocked the cAMP response to 10 nM concentrations of GHRH or GHRH agonist JI-38 in Capan-2 and CFPAC1 pancreatic, HT-29 colonic, and NCI-N87 and AGS gastric cancer cell lines (P < 0.01 to < 0.001). GHRH antagonist JV-1–38 by itself at 1 μM had no effect on cAMP (data not shown).
Table 1.
Effect of GHRH antagonist JV-1–38 (1 μM) on cAMP production stimulated by 10 nM GHRH(1–29)NH2 or GHRH agonist JI-38 in pancreatic, colonic, and gastric cancer cell lines
| Cell line | GHRH (10 nM) + JV-1–38 (1 μM), % response | JI-38 (10 nM) + JV-1–38 (1 μM), % response |
|---|---|---|
| Capan-2 | 75.60 ± 1.46** | 63.50 ± 3.53** |
| CFPAC-1 | 67.91 ± 1.72** | 72.85 ± 2.69** |
| HT-29 | 34.12 ± 0.81** | 37.00 ± 1.02** |
| NCI-N87 | 62.86 ± 3.64* | 50.92 ± 2.41* |
| AGS | 75.57 ± 4.63* | 69.75 ± 0.90* |
cAMP response in the presence of GHRH antagonist JV-1–38 is expressed as percentage of the reference response of this cell line to 10 nM GHRH or 10 nM JI-38, respectively, arbitrarily accepted as 100% in each case. Data shown represent the mean (%) ± SEM from two separate experiments performed in triplicate.
, P < 0.01 and
, P < 0.001 vs. GHRH or JI-38.
Discussion
During the past 9 years we have been synthesizing potent antagonists of GHRH for the treatment of a wide range of neoplasms potentially dependent on IGF-I, IGF-II, and GHRH (1, 3). Numerous studied showed that these GHRH antagonists inhibit the growth of various human cancer cell lines, including SW1990 human pancreatic cancer, HT-29 human colon adenocarcinoma xenografted into nude mice, and the nitrosamine-induced pancreatic cancers in hamsters (10, 11). Accumulating evidence suggests that, in addition to inhibiting the pituitary GH/hepatic IGF-I axis, GHRH antagonists also exert direct antitumor effects on various cancers mediated through specific receptors (1, 3, 13). Earlier studies did not detect the expression of the pituitary form of GHRH receptors in the cancer models examined (9, 15, 17, 18, 24, 25). However, during the past 2 years, we established that SVs of GHRH receptors encoding alternate forms of GHRH receptor are expressed on various human cancer cell lines. Thus, we found SVs of GHRH receptors in bone sarcomas, renal, breast, ovarian, and prostatic cancers, as well as in specimens of primary human prostate tumors (18, 23–25, 27–30). Chopin and Herington (26) also found SVs of GHRH receptors in LNCaP prostate cancer. However, little is known about the nature and expression pattern of these tumoral receptors in gastroenteropancreatic tumors.
This study reports the expression of SVs of tumoral GHRH receptor in different models of human pancreatic, colon, and gastric cancers grown in nude mice. We were able to demonstrate that SV1, which exhibits the greatest similarity to the pituitary GHRH receptors, was present in all of the pancreatic and gastric cancer models tested, as well as in COLO 320DM and HT-29 colonic tumors. The presence of SV2 was shown in MIA PaCa-2, Capan-1, Capan-2, and CFPAC-1 pancreatic, NCI-N87 gastric tumors, as well as in HT-29 colonic cancers. No other SVs of GHRH receptors, such as SV3 or SV4, were found in our study. The present results are in agreement with a study that detected the expression of tumoral GHRH receptors in MIA PaCa-2 human pancreatic cancer cell line (25). SV1 differs from the pituitary GHRH receptor only in the first three exons, encoding a part of the extracellular domain of the receptor that in SV1 has been replaced by a fragment of intron 3, which has a new putative in-frame start codon (25). A major part of the nucleotide sequence of SV1 (exons 4–13) has more than 99% identity with the corresponding sequence of the pituitary GHRH receptor cDNA (25). However, the first 334 nucleotides of both SV1 and SV2 are completely different from those in the pituitary GHRH receptor (25, 41). The deduced protein sequences of SV1 and SV2 indicate the presence of a distinct 25-aa sequence at the N-terminal extracellular domain, which could serve as a signal peptide. The putative protein sequence encoded by SV1 is consistent with a functional receptor in which the large N-terminal extracellular tail, characteristic of the pituitary receptor, is truncated, but all of the transmembrane domains, intracellular and extracellular loops, and the C-terminal end necessary for signal transduction are present. SV2 encodes a GHRH receptor isoform truncated after the second transmembrane domain. The lack of any transmembrane domains in the other two GHRH receptor isoforms, SV3 and SV4, implies that they probably do not represent mature receptor proteins expressible on the cell surface (25). Structural changes in SV1, in comparison with the pituitary GHRH receptor, may result in a different ligand-binding affinity, but the ability to transmit the mitogenic and other effects of GHRH would be retained (24, 25, 42).
That SVs of GHRH receptors play a physiopathologic role in nonpituitary tissues remains to be proven. However, a recent study showed that SV1, encoding the main isoform of GHRH receptor expressed in NIH 3T3 mouse fibroblast cell line, can mediate the proliferative responses to GHRH and its analogs (42). Thus, the expression of SV1 in the transfected cells strongly augments both the mitogenic responses to GHRH(1–29)NH2 or GHRH agonist JI-38 and the antiproliferative effect of GHRH antagonist JV-1–38, as compared with the control cells transfected with an empty vector (42). In addition, the stimulation of 3T3 cells, transfected with SV1, by GHRH or its agonist JI-38, induces the production of cAMP, in contrast to the control cells that do not show a cAMP response (42). These results suggest that the GHRH receptor isoform encoded by SV1 could mediate the effect of GHRH and its antagonists on extrapituitary cells and various tumors.
Although it is known that various human neoplasms including pancreatic, gastric, and colorectal cancer can produce GHRH (31–33), the pathophysiological significance of this finding is, at present, only partially perceived. Therefore, in the present study, we investigated whether GHRH and its receptors are expressed in human gastroenteropancreatic cancer cell lines and thus if they could form together an autocrine/paracrine mitogenic loop. We detected mRNA for GHRH in pancreatic (SW1990, PANC-1, MIA PaCa-2, Capan-1, Capan-2, and CFPAC1), gastric (NCI-N87, HS746T, and AGS), and colonic (COLO 320DM and HT-29) cancer cell lines. In the proliferation studies in vitro with pancreatic (CFPAC1, SW1990, and Capan-2), colonic (HT-29), and gastric (NCI-N87 and AGS) cancer cell lines, we found that exogenously added GHRH(1–29)NH2 increased the rate of cell proliferation, even though autocrine GHRH was already present in these cells. It has been suggested that GHRH could function as an autocrine growth factor in breast and prostatic cancers, SCLC, and malignant bone tumors (21, 27–30). Thus, the inhibition of proliferation induced by GHRH antagonists in vitro could be exerted through competitive binding to the GHRH receptor isoforms on the pancreatic, colon, and gastric cancer cells and nullification of the mitogenic stimuli of autocrine GHRH. The inhibition of cell growth produced by 3 and 10 μM concentrations of JV-1–38 in the gastroenteropancreatic carcinomas was in some cases partially reversed by the addition of 1 μM GHRH(1–29)NH2, but the antagonist still retained a significant antiproliferative activity in the presence of GHRH. This finding is in accordance with the fact that the binding affinity of JV-1–38 to the tumoral and pituitary GHRH receptors is 20- to 40-fold higher than that of the GHRH (1, 2, 24, 30). GHRH seems to fulfill some of the parameters for an autocrine growth factor in gastric, colorectal, and pancreatic cancers, because it is apparently expressed by these malignancies and a tumoral GHRH receptor isoform is also present on tumors. When pancreatic, colonic, and gastric cancer cells were subjected to frequent changes of incubation medium, their growth rate was likewise decreased indicating that autocrine growth factors may actively promote their proliferation in vitro. GHRH could potentially be one of these growth factors.
GHRH receptors are coupled through G proteins to multiple signal transduction pathways. It is widely accepted that the pituitary GHRH receptor activates cAMP increase and extracellular Ca2+ entry (43). However, little is known about the functionality of the alternate forms of GHRH receptor. Our present results indicate that the adenylyl cyclase/cAMP signaling system could play a role in the action of GHRH and its antagonists on the gastric, pancreatic, and colorectal cancer cell lines investigated. The present report is in agreement with previous studies (40, 44). Other investigations in MIA PaCa-2 human pancreatic cancers indicated that GHRH antagonists may inhibit the proliferation of this cell line only in part by a cAMP-dependent mechanism, but other unidentified signaling pathways apparently play a more important role (12). Kineman (13) suggested that GHRH stimulates and GHRH antagonists inhibit human tumor cell proliferation mainly by a cAMP-independent mechanism. Recent studies in our laboratory have shown that the effects of GHRH and its antagonists on T47D breast cancer cells are partially mediated by adenylyl cyclase, Ca2+ channels, and protein kinase C-dependent pathways (29). Further investigations are needed for a more thorough elucidation of the signal transduction pathways involving tumoral GHRH receptors.
Because GHRH antagonists can disrupt the endocrine as well as various autocrine/paracrine stimulatory loops of cell proliferation dependent on GHRH, GH, IGF-I, and IGF-II, they could be applicable for the therapy of a broad range of malignancies (1, 3). The relative importance of different inhibitory mechanisms depends on the characteristics of the particular tumor and seems to vary from cancer to cancer (1, 3, 8, 10, 11, 13–23). The inhibitory effect of GHRH antagonists on HT-29 colon and SW1990 pancreatic tumor growth in vivo is probably exerted mainly through the interaction with the tumoral GHRH receptors and abrogation of the mitogenic action of locally produced GHRH, and a subsequent decrease in the production and secretion of IGF-II by the cancer cells (10, 11).
In conclusion, this study reports the presence of GHRH and splice variants of GHRH receptor in human pancreatic, colorectal, and gastric cancers. Our findings suggest that GHRH and its tumoral receptor could form an autocrine/paracrine mitogenic loop, which might be involved in the control of the malignant growth. The inhibitory effect of GHRH antagonists on gastroenteropancreatic cancers could be based in part on the interference with the local stimulatory GHRH system.
Acknowledgments
We thank Dr. A. F. Parlow and the National Hormone and Pituitary Program, the National Institute of Diabetes and Digestive and Kidney Diseases (for RIA reagents), and Elena Glotser for technical assistance. During this study, M.O.G.-F. was on leave from the Department of Biochemistry and Molecular Biology, Alcala University School of Medicine, Madrid. This work was supported by the Medical Research Service of Veterans Affairs Department, and by a grant from Zentaris AG (Frankfurt am Main, Germany) to Tulane University School of Medicine (to A.V.S.). M.O.G.-F. is a recipient of a fellowship from Astra-Zeneca Pharmaceutic (Madrid).
Abbreviations
- GH
growth hormone
- GHRH
GH-releasing hormone
- IGF-I
insulin-like growth factor-I
- IGF-II
insulin-like growth factor-II
- SCLC
small-cell lung carcinoma
- SV
splice variant
- RT
reverse transcription
References
- 1.Schally A V, Varga J L. Trends Endocrinol Metab. 1999;10:383–391. doi: 10.1016/s1043-2760(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 2.Varga J L, Schally A V, Csernus V J, Zarandi M, Halmos G, Groot K, Rekasi Z. Proc Natl Acad Sci USA. 1999;96:692–697. doi: 10.1073/pnas.96.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schally A V, Comaru-Schally A M, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, Varga J L, Halmos G. Front Neuroendocrinol. 2001;22:248–291. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- 4.Pinski J, Schally A V, Groot K, Halmos G, Szepeshazi K, Zarandi M, Armatis P. J Natl Cancer Inst. 1995;87:1787–1794. doi: 10.1093/jnci/87.23.1787. [DOI] [PubMed] [Google Scholar]
- 5.Pinski J, Schally A V, Jungwirth A, Groot K, Halmos G, Armatis P, Zarandi M, Vadillo-Buenfil M. Int J Oncol. 1996;9:1099–1105. doi: 10.3892/ijo.9.6.1099. [DOI] [PubMed] [Google Scholar]
- 6.Jungwirth A, Schally A V, Pinski J, Halmos G, Groot K, Armatis P, Vadillo-Buenfil M. Br J Cancer. 1997;75:1585–1592. doi: 10.1038/bjc.1997.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jungwirth A, Schally A V, Pinski J, Groot K, Armatis P, Halmos G. Proc Natl Acad Sci USA. 1997;94:5810–5813. doi: 10.1073/pnas.94.11.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csernus V J, Schally A V, Kiaris H, Armatis P. Proc Natl Acad Sci USA. 1999;96:3098–3103. doi: 10.1073/pnas.96.6.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rekasi Z, Varga J L, Schally A V, Halmos G, Armatis P, Groot K, Czompoly T. Endocrinology. 2000;141:2120–2128. doi: 10.1210/endo.141.6.7511. [DOI] [PubMed] [Google Scholar]
- 10.Szepeshazi K, Schally A V, Groot K, Armatis P, Halmos G, Hebert F, Szende B, Varga J L, Zarandi M. Br J Cancer. 2000;82:1724–1731. doi: 10.1054/bjoc.2000.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szepeshazi K, Schally A V, Groot K, Armatis P, Hebert F, Halmos G. Eur J Cancer. 2000;36:128–136. doi: 10.1016/s0959-8049(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 12.Rekasi Z, Varga J L, Schally A V, Plonowski A, Halmos G, Csernus B, Armatis P, Groot K. Peptides (Tarrytown, NY) 2001;22:879–886. doi: 10.1016/s0196-9781(01)00413-2. [DOI] [PubMed] [Google Scholar]
- 13.Kineman R D. Proc Natl Acad Sci USA. 2000;97:532–534. doi: 10.1073/pnas.97.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamharzi N, Schally A V, Koppan M, Groot K. Proc Natl Acad Sci USA. 1998;95:8864–8868. doi: 10.1073/pnas.95.15.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahan Z, Varga J L, Schally A V, Rekasi Z, Armatis P, Chatzistamou I, Czompoly T, Halmos G. Breast Cancer Res Treat. 2000;60:71–79. doi: 10.1023/a:1006363230990. [DOI] [PubMed] [Google Scholar]
- 16.Kiaris H, Schally A V, Varga J L. Neoplasia. 2000;2:242–250. doi: 10.1038/sj.neo.7900074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plonowski A, Schally A V, Varga J L, Rekasi Z, Hebert F, Halmos G, Groot K. Prostate. 2000;44:172–180. doi: 10.1002/1097-0045(20000701)44:2<172::aid-pros10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 18.Chatzistamou I, Schally A V, Varga J L, Groot K, Armatis P, Busto R, Halmos G. J Clin Endocrinol Metab. 2001;86:2144–2152. doi: 10.1210/jcem.86.5.7487. [DOI] [PubMed] [Google Scholar]
- 19.Chatzistamou I, Schally A V, Varga J L, Groot K, Armatis P, Bajo A M. J Cancer Res Clin Oncol. 2001;127:645–652. doi: 10.1007/s004320100254. [DOI] [PubMed] [Google Scholar]
- 20. Braczkowski, R., Schally, A. V., Plonowski, A., Varga, J. L., Groot, K., Krupa, M. & Armatis, P. (2002) Cancer, in press. [DOI] [PubMed]
- 21.Kiaris H, Schally A V, Varga J L, Groot K, Armatis P. Proc Natl Acad Sci USA. 1999;96:14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiaris H, Schally A V, Varga J L. Cancer Lett. 2000;161:149–155. doi: 10.1016/s0304-3835(00)00580-2. [DOI] [PubMed] [Google Scholar]
- 23.Chatzistamou I, Schally A V, Varga J L, Groot K, Busto R, Armatis P, Halmos G. Anticancer Drugs. 2001;12:761–768. doi: 10.1097/00001813-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Halmos G, Schally A V, Varga J L, Plonowski A, Rekasi Z, Czompoly T. Proc Natl Acad Sci USA. 2000;97:10555–10560. doi: 10.1073/pnas.180313097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rekasi Z, Czompoly T, Schally A V, Halmos G. Proc Natl Acad Sci USA. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chopin L K, Herington A C. Prostate. 2001;49:116–121. doi: 10.1002/pros.1125. [DOI] [PubMed] [Google Scholar]
- 27. Busto, R., Schally, A. V., Braczkowski, R., Plonowski, A., Krupa, M., Groot, K., Armatis, P. & Varga, J. L. (2002) Regul. Pept., in press. [DOI] [PubMed]
- 28.Plonowski A, Schally A V, Busto R, Krupa M, Varga J L, Halmos G. Peptides (Tarrytown, NY) 2002;23:1127–1133. doi: 10.1016/s0196-9781(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 29. Garcia-Fernandez, M. O., Schally, A. V., Varga, J. L., Groot, K. & Busto, R. (2002) Breast Cancer Res. Treat., in press. [DOI] [PubMed]
- 30. Halmos, G., Schally, A. V., Czompoly, T., Krupa, M., Varga, J. L. & Rekasi, Z. (2002) J. Clin. Endocrinol. Metab., in press. [DOI] [PubMed]
- 31.Christofides N D, Stephanou A, Suzuki H, Yiangou Y, Bloom S R. J Clin Endocrinol Metab. 1984;59:747–751. doi: 10.1210/jcem-59-4-747. [DOI] [PubMed] [Google Scholar]
- 32.Asa S L, Kovacs K, Thorner M O, Leong D A, Rivier J, Vale W. J Clin Endocrinol Metab. 1985;60:423–427. doi: 10.1210/jcem-60-3-423. [DOI] [PubMed] [Google Scholar]
- 33.Losa M, Wolfram G, Mojto J, Schopohl J, Spiess Y, Huber R, Muller O A, von Werder K. J Clin Endocrinol Metab. 1990;70:62–67. doi: 10.1210/jcem-70-1-62. [DOI] [PubMed] [Google Scholar]
- 34.Benlot C, Levy L, Fontanaud P, Roche A, Rouannet P, Joubert D. J Clin Endocrinol Metab. 1997;82:690–696. doi: 10.1210/jcem.82.2.3754. [DOI] [PubMed] [Google Scholar]
- 35.Kahan Z, Arencibia J, Csernus V, Groot K, Kineman R, Robinson W R, Schally A V. J Clin Endocrinol Metab. 1999;84:582–589. doi: 10.1210/jcem.84.2.5487. [DOI] [PubMed] [Google Scholar]
- 36.Khorram O, Garthwaiter M, Grosen E, Golos T. Fertil Steril. 2001;75:174–179. doi: 10.1016/s0015-0282(00)01658-7. [DOI] [PubMed] [Google Scholar]
- 37.Izdebski J, Pinski J, Horvath J E, Halmos G, Groot K, Schally A V. Proc Natl Acad Sci USA. 1995;92:4872–4876. doi: 10.1073/pnas.92.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reile H, Birnbock H, Bernhardt G, Spruss T, Schonenberger H. Anal Biochem. 1990;187:262–267. doi: 10.1016/0003-2697(90)90454-h. [DOI] [PubMed] [Google Scholar]
- 39.Plumb J A, Milroy R, Kaye S B. Cancer Res. 1989;49:4435–4440. [PubMed] [Google Scholar]
- 40.Csernus V, Schally A V, Groot K. Peptides (Tarrytown, NY) 1999;20:843–850. doi: 10.1016/s0196-9781(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 41.Gaylinn B D, Harrison J K, Zysk J R, Lyons C E, Lynch K R, Thorner M O. Mol Endocrinol. 1993;7:77–84. doi: 10.1210/mend.7.1.7680413. [DOI] [PubMed] [Google Scholar]
- 42.Kiaris H, Schally A V, Busto R, Halmos G, Artavanis-Tsakonas S, Varga J L. Proc Natl Acad Sci USA. 2002;99:196–200. doi: 10.1073/pnas.012590999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Müller E E, Locatelli V, Cocchi D. Physiol Rev. 1999;79:511–607. doi: 10.1152/physrev.1999.79.2.511. [DOI] [PubMed] [Google Scholar]
- 44.Csernus V, Schally A V, Groot K. J Endocrinol. 1999;163:269–280. doi: 10.1677/joe.0.1630269. [DOI] [PubMed] [Google Scholar]