Abstract
Xenopus laevis tadpole tails contain fast muscle fibers oriented in chevrons and two pairs of slow muscle “cords” along the length of the tail. When tail resorption is inhibited by a number of different treatments, fast muscle but not the slow cord muscle still is lost, demonstrating that the fast tail muscle is a direct target of the thyroid hormone-induced death program. Expression of a dominant negative form of the thyroid hormone receptor (TRDNα) was restricted to tadpole muscle by means of a muscle-specific promoter. Even though the transgene protects fast tail muscle from thyroid hormone (TH)-induced death, the tail shortens, and the distal muscle chevrons at the tail tip are degraded. This default pathway for muscle death is probably caused by the action of proteolytic enzymes secreted by neighboring fibroblasts. Non-muscle tissues that are sensitive to TH, such as the fibroblasts, are not protected by the transgene when it is expressed solely in muscle. If allowed to develop to metamorphosis, these transgenic animals die at the climax of metamorphosis before tail resorption has begun. Their limbs have very little muscle even though the rest of limb morphology is normal. Thus, fast tail muscle and limb muscle have their own cell autonomous death and growth programs, respectively, that are independent of the fate of the other neighboring cell types. In contrast, death of the slow muscle is controlled by the other cell types of the tail.
The majority of tadpole tissues and organs are targets of thyroid hormone (TH) during metamorphosis. Many experiments have demonstrated the organ autonomy of these TH-induced changes. For example, tail explants in organ culture respond to added TH by resorbing their fins (1). Tails transplanted to the lateral body wall of a tadpole resorb at the same time as the tadpole's normal tail (2), whereas eyes transplanted to the tip of the tail undergo their expected changes despite the resorption of the tail to which they are attached. Isolated hind limbs are reported to respond to TH induction (3), and the intestine begins to remodel in culture (4). Tadpole tails consist of epidermis, neural tissue, fast and slow muscle, and fibroblasts underlying the epidermis, in the myotendinous junctions, and surrounding the notochord. TH-induced genes in the tail fall into several groups based on their specific expression patterns (5). Some proteolytic enzymes are expressed mainly in fibroblasts, whereas several transcription factors are expressed in fibroblasts, epidermis, spinal cord, and the cells between muscle fibers (myotendinous junctions). One interpretation of these expression pattern data is that different cell types in the tail die by different programs even though death is the ultimate fate of all of the cells in the tail. Some cell types might be targets of the hormones themselves (cell autonomy), whereas others could be killed by the secreted products of neighboring cells. Clearly, the rapid resorption of the tail relies on the production of secreted proteolytic enzymes that are induced in the tail by TH (6).
The entire resorption of the tail in Xenopus laevis occurs in about 3 days at metamorphic climax. The dorsal fins are resorbed first, followed by fragmentation of muscle fibers. Shortening of the tail involves dissolution of the notochord lamella by the activated proteolytic enzymes and a contractive force that is aided by two pairs of parallel arrays of slow muscle fibers called “cords” that persist until the very end of tail resorption (7) after the fast muscle has been resorbed. Evidence to date suggests that muscle dies autonomously by a TH-induced pathway. Yaoita and Nakajima (8) cultured a cell line derived from tail muscle. These cells up-regulate and activate caspase-3 (CPP32/YAMA/apopain; ref. 9) and then die after a few days of TH treatment. Additional evidence for the cell autonomous death of fast tail muscle comes from transgenic tadpoles overexpressing the prolactin gene (10). These tadpoles metamorphose at the same time as control tadpoles, but they do not resorb their tails. Examination of these tails showed that they are almost completely devoid of fast muscle fibers. Their muscle cords remain intact. Tailed frogs created by overexpressing type III deiodinase (11) or treatment with the goitrogen methimazole (7) also have resorbed their fast muscle but not their cords. Therefore, the loss of fast but not slow muscle can be dissociated from dissolution of the other cell types in the tail.
Muscle is a prominent cell type in growth programs of metamorphosis. Limb development beyond a small dumbbell-shaped bud [Nieuwkoop and Faber (NF) stage 53; ref. 12] is entirely dependent on TH (12) and is one of the earliest TH-induced events of spontaneous metamorphosis. Hind limbs with well-developed musculature are formed as a result of TH induction before the climax of metamorphosis. The tadpole converts from tail swimming to leg swimming at the onset of climax (NF stage 59). Ossification of the long bones begins during premetamorphosis (NF stage 57) and continues after metamorphosis in the small frog. The addition of exogenous TH to young tadpoles results in visible limb bud growth that begins after 2 days. About 5 days after the addition of TH, the expression of terminally differentiated muscle genes is activated, and limb skeletal muscle begins to form (13). Therefore, these specialized muscle genes are not direct response genes. We estimated that there might be as many as four waves of gene expression that precede the activation of the muscle-specific genes (13).
By means of the transgenesis method (14), genes can be overexpressed throughout development of X. laevis in a tissue-specific manner. Multiple growth and death programs that are induced by TH can be inhibited by overexpressing deiodinase type III (D3; ref. 11) or a dominant negative form of the thyroid receptor (TRDNα) controlled by a broadly expressing promoter (15). In these experiments, we have used the TRDNα inhibitor of metamorphosis to analyze the cell autonomy of two different TH-induced muscle programs, the death of tail muscle and the growth of limb muscle. We also examine the effect of inhibiting the muscle program on neighboring non-muscle cell types.
Materials and Methods
Constructs, Transgenesis, 3,5,3′-Triiodothyronine (T3) Treatment, and Animal Care.
Constructs were made in either pCS2+ or pCSGFP3-based vectors. A dominant negative thyroid receptor (TRDNα) was constructed by deleting 36 base pairs (12 aa) from the C terminus of the wild-type receptor TRα and fusing it by way of a 5-aa linker (GGGGS) to the N-terminal end of GFP. The fused TRDNα cassette was introduced into pCar/GFP2 as a HindIII + NotI fragment replacing the GFP.
Transgenic animals were generated by restriction enzyme-mediated integration (REMI) nuclear transplantation (14) with some modifications (11). The linearized pCar/TRDNα plasmid was always coinjected with a linearized γ-crystallin/GFP construct. Transgenic tadpoles were identified by nuclear localized GFP fluorescence in jaw and tail muscle on day 4 postfertilization by using a Leica (Deerfield, IL) MZ12 fluorescence stereo microscope. The tissue-specific expression of pCar/GFP2 was evaluated in intact animals and cryosections. Live anesthetized animals expressing GFP were photographed with the Leica fluorescence microscope and a Spot RT camera.
The early induction (“2-wk”) assay was described previously (11, 15). Briefly, 1-wk-old tadpoles were induced for either 4 days or 7 days in 10 nM T3 diluted in 0.1× MMR [10 mM NaCl/0.2 mM KCl/0.1 mM MgCl2/0.2 mM CaCl2/0.5 mM Hepes (pH 7.5)]. Transgenic and wild-type animals were treated in the same container and were distinguished by the γ-crystallin/GFP in the eyes of the transgenic tadpoles. The most obvious changes in tadpoles sensitive to T3 are complete resorption of their gill arches, protrusion of the lower jaw (Meckel's cartilage), and a widened brain with the nose closer to the anterior brain's olfactory bulb (see Fig. 2). Developmental stages are those described by Nieuwkoop and Faber (16).
Fig 2.
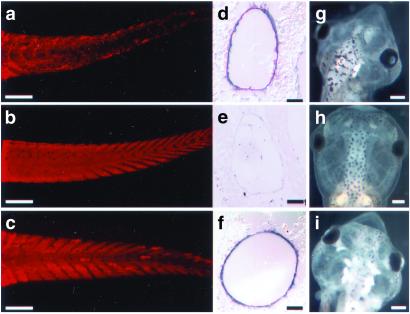
Assaying muscle loss by using a muscle-specific antibody (MF20) after the 2-wk assay of tadpoles with TH. (a) Tail of an animal treated with 10 nM T3 for 7 days. Note the loss of muscle fibers and disorganization of the chevron structures. (b) Tail of an untreated animal. (c) Tail of a pCar/TRDNα transgenic animal treated with 10 nM T3 for 7 days. (d–f) In situ hybridization of tail cross sections obtained from animals in a–c, respectively, with collagenase-3 antisense probe. (g–i) Changes in the head of the animals in a–c, respectively, after the 2-wk assay. [Bars = 500 μm (a–c and g–i), 100 μm (d and e), and 50 μm (f).]
Measuring Changes in Tail Length with TH Treatment.
To determine tail length (defined as the distance from the anus to the tail tip) changes caused by TH treatment, tail length of wild-type animals and those of pCar/TRDNα transgenics were measured both before and after 7 days treatment with TH. Data were analyzed by one-factor analysis of variance (ANOVA; statview, Abacus Concepts, Berkeley, CA), and pairwise post hoc comparisons using Fisher's probable least-squares difference were used. For all statistics, significance was accepted when P < 0.05.
Immunocytochemistry, in Situ Hybridization, and Bone-Cartilage Staining.
In situ hybridizations were done with a collagenase-3 digoxigenin-labeled probe as described (5). Whole mount immunocytochemistry of 2-wk-old tadpole tails was done as described (15). The primary antibodies were a mouse monoclonal against avian-striated muscle sarcomere myosin (MF20; Developmental Studies Hybridoma Bank, University of Iowa) diluted 1/100; a rabbit monoclonal antibody against the active form of caspase-3 (BD PharMingen) diluted 1/250, and a rabbit anti-GFP polyclonal antibody (Torrey Pines Biolabs, San Diego) diluted 1/800. The secondary antibodies were anti-mouse and anti-rabbit sheep polyclonal antibodies conjugated with Alexa Fluor 568 or 488 (Molecular Probes), which were diluted 1/400.
The number of activated caspase-3 immunoreactive muscle fibers for one side of a tail was counted by using the Leica MZ12 fluorescence stereo-microscope. Data were analyzed by one-factor ANOVA using a randomized complete block design (SuperANOVA, Abacus concepts, ref. 17) where the blocking factor was days of treatment. Pairwise post hoc comparisons using Fisher's probable least-squares difference were used after significance by ANOVA. Student's t test was used to compare numbers of caspase-3 immunoreactive fibers between 4 and 7 days treatment. Bone cartilage staining has been described (5).
Results
The Cardiac Actin Promoter Drives Expression in All Types of Muscle.
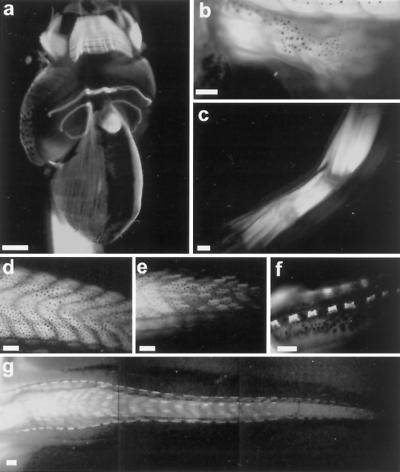
Transgenic tadpoles that express GFP controlled by the cardiac actin promoter (18) (pCar/GFP) were visualized at different stages of development. The transgene is detected first at NF stage 17 (14), and then it is expressed throughout the life of the animal. The ventral view of a 1-wk-old tadpole just beginning to feed (Fig. 1a) shows expression in jaw, abdominal fibers, cardiac and skeletal musculature, and eye muscles. The heart is strongly fluorescent as is the tail musculature. As limbs develop (Fig. 1 b and c), muscle is visualized as early as NF stage 54. The transgene is especially useful for illuminating the tail muscle and observing its resorption at the climax of metamorphosis (Fig. 1 d–g). The muscle fibers are stacked on either side of the notochord, forming chevron structures. The most dorsal and ventral pairs of muscle bundles in each chevron will give rise to the cords (7). These cords consist of slow muscle connected by collagen fibers, and they run the length of the tail. Near the completion of metamorphosis (NF stage 64), when the length of the tail is half of the length of the body, the cords are the only remaining muscle in the tail (Fig. 1f). The cord muscles contract, as the tail shortens, and ultimately disappear when tail resorption is complete. The GFP labeling of tail muscle graphically demonstrates the loss of fast muscle fibers during tail resorption and the shortening and persistence of the cords. Fiber loss occurs along the tail, but it is most obvious in the thinner distal end where there are the fewest fibers. Muscle loss can be seen best at the peak of climax (NF stage 63; Fig. 1g). The loss of muscle fibers occurs over about a 3-day period.
Fig 1.
The expression profile of tadpoles transgenic for pCar/GFP. (a) Ventral view of the head region of a 1-wk-old tadpole expressing GFP in cardiac and skeletal muscle. (b) GFP expression in the muscle of the developing limb at NF stage 54. (c) GFP expression in the limb muscle of a juvenile frog. (d) GFP expression in muscle fibers in the middle of the tail region of a stage 57 tadpole. (e) GFP expression in the mid-tail region of a stage 63 tadpole, demonstrating the loss of GFP fibers in the fast muscle chevrons. (f) GFP expression in the slow muscle cords of the tail in a stage 64 tadpole. (g) Loss of GFP-labeled muscle fibers in the tail of a stage 63 tadpole. [Bars = 500 μm (a, b, and f) and 1 mm (c–e and g).]
The cardiac actin promoter appears to drive expression in all types of muscle in the tadpole. Expression of the transgene is also strong in both the radial and longitudinal smooth muscle layers of the intestinal tract and vasculature throughout the body (data not shown).
The pCar/TRDNα Transgenic Construct Protects Tail Muscle Against TH-Induced Cell Death.
Previously, we have demonstrated that the overexpression of a dominant negative form of thyroid hormone receptor α (TRDNα) can protect a 1-wk-old tadpole against a wide range of TH-induced changes, including tail muscle death and tail resorption (15). In those experiments, two broadly expressing promoters were used to drive the mutant receptor. A major goal of the experiments in this paper has been to inhibit the TH-induced response of a single cell type, muscle, to determine whether it is a direct target of the hormone, and the extent that protecting muscle from TH-induced changes influences the response of neighboring cell types to the hormone. The GFP-fused TRDNα protein is localized in the nucleus (data not shown), and its direct action is cell autonomous. The specificity of the pCar promoter for muscle delimits expression to muscle cells. Transgenic tadpoles were prepared overexpressing pCar/TRDNα. At 1 wk after fertilization, they were induced for an additional week with 10 nM T3. We refer to this as the 2-wk assay (11). Many of the metamorphic programs can be simulated in this assay. In 100% of control tadpoles treated with 10 nM T3 for 7 days, the muscle is fragmented and degraded as seen by staining with the MF20 antibody (Fig. 2a). In contrast, 7 of 10 transgenic tadpoles selected on the basis of strong GFP fluorescence, and induced in the same container as the controls, have their muscle protected completely from TH-induced degradation (Fig. 2c). The other three transgenic tadpoles were partly protected (data not shown).
In contrast to transgenics expressing the TRDNα in all cell types (15), these pCar/TRDNα tadpoles undergo the other visible TH-induced changes that characterize control tadpoles. Their brains enlarge, the gills resorb, limb buds appear, and Meckel's cartilage projects from the lower jaw just like controls (Fig. 2i). To assess the effect of protecting muscle on neighboring tail fibroblasts, sections of untreated and TH-treated control and transgenic tails were hybridized in situ with the TH-inducible gene collagenase-3 probe. The expression of this gene is especially high in fibroblasts lining the notochord sheath (5). Transgenic tadpoles for the TRDNα driven by a ubiquitous promoter are protected against TH-induced changes in all cell types of the tail, including the notochord fibroblasts (15), and TH-induced tail shortening does not occur in these transgenic tadpoles. However, up-regulation of collagenase-3 is unaffected in the pCar/TRDNα transgenics (Fig. 2f), and, despite the protection of tail muscle (Fig. 2c), the pCar/TRDNα tadpoles shorten their tails as much as TH-treated controls. After 7 days treatment with 10 nM T3, wild-type tail lengths (6.5 ± 0.9 mm, n = 5) shortened significantly (P < 0.05) compared with wild-type untreated animals (9.1 ± 0.6 mm, n = 5) or animals before the start of treatment (9.1 ± 0.3 mm, n = 3). After TH treatment, pCar/TRDNα tail lengths (7.0 ± 0.2 mm, n = 3) were also shortened and not significantly different from the treated wild-type animals (P > 0.05). Before the start of TH treatment, tail lengths of wild-type control and pCar/TRDNα animals were the same. Tail shortening can also be monitored by counting the muscle chevrons. Control tadpoles have 48 ± 1 (n = 5) muscle chevrons from the base of the skull to the tip of the tail. After a week in TH, the chevrons of a control tadpole are so fragmented that they can no longer be counted. The transgenics have preserved their muscle chevrons almost to the tail tip, yet the number of chevrons is reduced to 36–38 (n = 3). Therefore, tail shortening and muscle loss can occur even when the tail muscle death program is inhibited.
The antibody against human active caspase-3 labels the cytoplasm of dying cells (16). This heterologous antibody was found to be an excellent marker of dying tail muscle cells. The terminal deoxynucleotidyltransferase-mediated dUTP end labeling (TUNEL) assay can visualize nuclei of these dying muscle cells (data not shown), but identification of muscle by cytoplasmic staining with the anti-active caspase-3 antibody is definitive. We have assayed control and TRDNα transgenic tadpoles that were induced with TH in the 2-wk assay with this antibody. A detailed daily staging for active caspase-3 positive muscle fibers in whole tails of control wild-type tadpoles found that they peak in number at about 4 days after the onset of TH-induction, 1 day before any morphological changes occur. Untreated control animals had 1–3 active caspase-3 immunoreactive muscle fibers per tail side (n = 4; Fig. 3b; Table 1). The number of immunoreactive fibers per side in wild-type tadpoles increased significantly after treatment with TH for 7 or 4 days, respectively (Fig. 3a). In contrast, the number of active caspase-3 immunoreactive fibers in animals transgenic for pCar/TRDNα (Fig. 3c) treated with TH for either 7 or 4 days were not different from untreated wild-type basal values. TH induced the appearance of caspase-positive cells in skin and brain (data not shown) to about the same extent in TH-induced control and transgenic tadpoles. This assay supports the finding that pCar/TRDNα tadpoles are protected specifically in muscle cells from TH-induced cell death.
Fig 3.
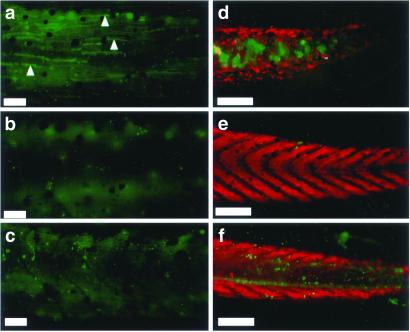
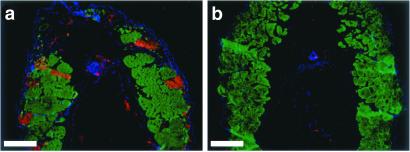
Caspase-3 activity in the tadpole tails in the 2-wk assay and retention of muscle in tail tip. (a) Tail from a wild-type animal treated with 10 nM T3 for 4 days. Note the caspase-3-positive muscle fibers (examples are shown with arrowheads). (b) Tail from an untreated control animal. (c) Tail from a pCar/TRDNα-expressing animal treated with 10 nM T3 for 4 days. Note the caspase-3-positive epidermal cells similar to a but the lack of caspase-positive muscle fibers. (d–f) Tips of the tail stained with MF20 (red) and anti-active caspase-3 antibody (green) of 10 nM T3-treated (7 days) wild-type, untreated wild-type, and 10 nM T3-treated (7 days) pCar/TRDNα tadpoles, respectively. [Bars = 100 μm (a–c) and 200 μm (d–f).]
Table 1.
Number of active caspase-3 immunoreactive tail muscle fibers after T3 treatment
| Genotype
|
Treatment
|
Days after treatment | ||
|---|---|---|---|---|
| 4
|
7 | |||
| Exp. 1 | Exp. 2 | |||
| Wild type | Control | 1.3 (1.0), 4 | 2.7 (1.5), 3 | 1.3 (1.0), 4 |
| Wild type | 10 nM T3 | 32.4 (5.0), 5 | 17.7 (7.5), 3 | 20.8 (4.7), 6 |
| pCar/TRDNα | 10 nM T3 | 2.4 (1.0), 5 | 0.3 (0.6), 3 | 2.0 (2.6), 3 |
One-week-old tadpoles were treated with 10 nM T3 for either 4 or 7 days. Data are scored as number of active caspase-3 immunoreactive muscle cells visible on one side of a tail [mean (SD), n].
Mean values significantly different (P < 0.05) from other tadpoles of the same age within the same experiment.
Whole mount labeling with active caspase-3 antibody gives a staining pattern, in the notochord after 7 days of TH-induction, that is different from what it gives after 4 days (Fig. 3 d–f). In TH-induced TRDNα tadpoles, the number of caspase-positive cells in the fin epidermis is about the same as that of TH-treated controls. However, the distal end of the notochord is filled with large active caspase-3-positive cells in control but not transgenic tadpoles. This is the one instance where pCar/TRDNα appears to affect a cell type other than muscle from TH induction. It is important to emphasize here that the pCar/TRDNα transgene is not expressed in these notochord lumen cells. The muscle is protected, but so is the anti-active caspase-3 immunoreactivity of cells within the lumen of the notochord. The chevron-shaped muscle structures in the TH-induced pCar/TRDNα animals are retained almost to the tip of the tail. In contrast, the muscle and chevron structures are destroyed throughout the length of the wild-type tail.
Tadpoles Overexpressing the TRDNα in Muscle Do Not Activate Caspase-3 in Tail Muscle During Spontaneous Metamorphosis.
Tadpoles that strongly express the pCar/TRDNα transgene arrest at the climax of metamorphosis and die without completing metamorphosis. They do not advance beyond NF stage 63 (see Fig. 5). By this stage, tail muscle death and degradation have begun in control tadpoles. Although fin resorption is already advanced in the control and transgenic animals at this stage, the transgenic tail has few active caspase-3-positive muscle cells whereas many can be visualized in the control tail muscle (Fig. 4). The death of these transgenic tadpoles at stage 63 precluded observing the final stages of tail resorption in spontaneous metamorphosis.
Fig 5.
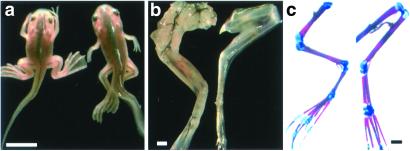
Inhibition of muscle formation in the limbs in the pCar/TRDNα animal. (a) Wild-type and pCar/TRDNα tadpoles at stage 63. The transgenic animal will die at this stage. (b) The skinned hind limbs of the animals shown in a, demonstrating the reduced muscle formation in the transgenic tadpole. (c) Bone (red) and cartilage (blue) staining of the same hind limbs. [Bars = 1 cm (a) and 500 μm (b and c).]
Fig 4.
Inhibition of activation of caspase-3 in tail muscle during spontaneous metamorphosis. (a) Cross section through the mid tail region of a wild-type tadpole at NF stage 62 stained with active caspase-3 antibody (red) and MF20 (green) and counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue). (b) Similarly stained cross section through the mid-tail region of a pCar/TRDNα tadpole. (Bars = 100 μm.)
Overexpression of the pCar/TRDNα Inhibits Limb Muscle Growth.
Transgenic tadpoles for pCar/TDRNα that express the transgene at high levels were screened and selected at 5 days postfertilization by their GFP fluorescence. Five of the 9 strongest expressing tadpoles showed the phenotype pictured in Fig. 5. These tadpoles arrested their development at the climax of metamorphosis (NF stage 63) and died. They have characteristically well developed but flaccid limbs. At this stage, normal tadpoles have converted from tail swimming to leg swimming. These transgenic tadpoles have uncoordinated leg movements at best. The forelimbs point posteriorly and hardly move. A closer examination of the hind limbs (Fig. 5b) shows much less muscle compared with a control. The bone has developed to the same extent of ossification as the control limb (Fig. 5c), even though the size of the femur and tibia-fibula bones are slightly longer in the transgenics. Clearly, the TRDNα has inhibited the growth program of muscle in the limb specifically.
Discussion
The Thyroid Hormone Receptors in X. laevis Development.
The discovery in chickens (19) and mammals (20) that thyroid hormone receptors are transcription factors has focused subsequent research on their roles controlling gene expression. The X. laevis genome encodes at least two receptor isoforms, TRα and TRβ (21), as has been described for other vertebrates (22). TRα in tadpoles is expressed constitutively and is present before the appearance of the thyroid gland (23). TRβ is a direct response gene of TH, and its expression follows the rise of TH concentration in the cell (23–25). Experiments done with a thyroid response element containing a reporter in a cultured cell line showed that overexpression of dominant negative forms of TRα or TRβ can inhibit either receptor if the TRDNα exceeds the wild-type receptor by as much as 5-fold (H.H., unpublished data). During the climax of spontaneous metamorphosis, TRβ is up-regulated in tails, and the TRβ protein is more abundant than TRα (23). However, limb development occurs very early at low levels of TH when TRα alone is expressed (25). Therefore, limb growth and differentiation are likely to be controlled completely by TRα whereas tail resorption requires TRβ to be up-regulated. Inhibition by the TRDNα is efficient in the 2-wk assay because of the low amounts of endogenous TR that can be competed effectively by the TRDNα.
The diverse developmental programs controlled by TH in the metamorphosis of anurans are mediated by these same receptors (15) because expression of the TRDNα driven by a constitutive promoter has a profound inhibitory effect on every program of metamorphosis that can be visualized in the 2-wk assay (15). However, the response of each tadpole organ involves multiple cell types, and the extent to which each component cell type is a target of the hormone is unknown. In these experiments, we have restricted expression of the transgene by placing it under the control of a specific muscle promoter (14, 18) and then studied the effect of the transgene on TH induction of the muscle growth and death programs. Attention has been paid to the TH-induced response of neighboring cells that do not express the transgene. We wish to determine whether muscle cell growth and death are direct targets of TH induction, and the extent to which the execution of their respective programs will influence neighboring cells not expressing the inhibitory transgene.
The Cardiac Actin Promoter and Muscle Cell Death Assays.
The specificity of the pCar promoter has been demonstrated in embryos and young tadpoles (14). We have extended these observations to the later tadpole stages that are relevant to metamorphosis. This promoter drives a GFP reporter in every kind of tadpole muscle. The loss of GFP fluorescence graphically documents the death of the tadpole tail muscle (Fig. 1). The rectangular fibers are oriented in an anterior to posterior direction, with the thick anterior multilayer muscle bundles tapering to a very few muscle bundles near the narrow tail tip. At the climax of metamorphosis (NF stage 62) after much of the dorsal fin has resorbed, individual muscle bundles begin to die all along the tail even though their loss is visualized most easily near the narrow tail tip (Fig. 1). The death of muscle cells can be monitored also by immunoreactivity to active caspase-3. During spontaneous metamorphosis the antibody detects dying cells in tissue sections (Fig. 4a). The antibody was also used for whole mount immunohistochemistry of young tadpoles subjected to the 2-wk assay, demonstrating almost complete protection of muscle fibers in transgenic tadpoles overexpressing the pCar/TRDNα (Fig. 2c). Protection by this transgene is very specific. Gill resorption, brain enlargement, limb bud induction, and Meckel's cartilage protrusion (Fig. 2 g–h), all programs that are protected when the TRDNα transgene is controlled by a ubiquitous promoter (15), change like those of control tadpoles under the influence of TH induction.
Tail Fast Muscle Is a Direct Target of TH.
Yaoita and Nakajima (8) provided the first evidence of the cell autonomous nature of TH-induced tail muscle cell death. They derived a myoblastic cell line from the tail muscle of X. laevis tadpoles that dies several days after TH induction. These investigators also cloned and characterized several X. laevis caspases (9). TH regulates the expression of some of these genes. The microscopic features of tail muscle cell death have been analogized with typical apoptosis (26). Nishikawa and Hayashi (27) describe “apoptotic bodies” in climax tadpole tail muscle. During climax, oligonucleosome–sized DNA is found in the tail. TH induces this same type of DNA degradation in tail (27). It has been suggested before, by expression profile of Bax mRNA and injecting DNA plasmid containing Bax promoter fused to luciferase reporter, that Bax is involved in the cell death pathway in tail muscle (28). The translocation of Bax into mitochondria from cytosol is an important step in releasing cytochrome c into the cytosol and initiating the caspase activation pathway (29).
In this study, we confirm that the fast muscle death program during tail resorption in Xenopus is cell autonomous. The pCar promoter driving the TRDNα protects the tadpole tail muscle from TH-induced cell death (Fig. 2). The fact that active caspase-3 is observed in muscle fibers (Figs. 3 and 4) suggests that the tail muscle death program proceeds by a caspase-dependent pathway. However, these experiments show that there is an alternative, default path for the loss of protected tail muscle fibers. Tails overexpressing the TRDNα in muscle begin to resorb in the 2-wk assay, as demonstrated by the shortened length and reduced number of muscle chevrons. These muscle fibers appear to have a completely different pattern of resorption than control fibers. Fast muscle fibers are protected except the most distal ones that are lost as the tail shortens. We have shown that these transgenic tadpoles can up-regulate collagenase-3 in the neighboring fibroblasts (Fig. 2f). The secretion of proteases by fibroblasts in the vicinity of muscle (5) suggests a mechanism to remove these otherwise protected muscle cells. The tail muscle chevrons are attached to myotendinous junctions that consist of fibroblasts and extra-cellular collagen. Digestion of these structures could activate a muscle fiber death pathway. Because these transgenic tadpoles die at the climax of metamorphosis before tail resorption, we could not follow their tail resorption during spontaneous metamorphosis.
An example of nonautonomous muscle death may be the parallel rows of slow muscle cords that never react positively for active caspase-3 antibody. They gradually become smaller as tail shortening occurs (Fig. 1) until they disappear entirely as the tail completes its resorption. The cords are resistant to the direct effects of TH induction and presumably are dissolved by the same secreted proteases that dissolve the bulk of the tail structures. Transgenic tadpoles that overexpress prolactin metamorphose normally but retain their tails. These tails have very large fins and a notochord, but all of the muscle except the cords has been lost (10). Therefore, the fast muscle resorption program can be independent of the rest of tail resorption whereas the fate of the cords depends on the loss of the rest of the tail.
An unexpected finding in this study is the inhibition of active caspase-3 in the distal notochord of the TRDNα transgenic tadpoles that becomes so prominent in control tails after 7 days of TH-induction (Fig. 3d). Muscle death in some way influences death of the neighboring notochord cells. Caspase-3 immunoreactivity in the notochord occurs several days after the muscle fibers are themselves immunoreactive. The location of these cells is in the lumen of the notochord where there are normally very few large vacuolated cells. A process of autophagic cell death of notochord lumen has been described where the macrophages invade the notochord and participate in phagocytosis (30). The autophagic cell death involves increased vacuolization and caspases (31, 32). Fibroblasts and macrophages that invade notochord most probably reside within the connective tissue between the muscle chevrons (33). So, it is quite possible that protection of muscle might inhibit the invasion of the notochord lumen by the macrophages. We see this same labeling of caspase-3 immunoreactivity in tail cross sections during normal metamorphosis (data not shown).
TH Control of Limb Muscle Growth Is Cell Autonomous.
The molecular basis of the TH-induced muscle death and growth programs is unknown. In both cases, thyroid receptors mediate the effects of TH. The subtractive screens that were applied to TH-induced limb (13) and tail (25, 34) identified very different sets of regulated genes. In both cases, up-regulation greatly exceeded down-regulation as a response to TH, but none of the genes found to be up-regulated by TH in the limb were activated in the tail. Limb development is one of the earliest responses to TH in metamorphosis. The limb buds develop to about NF stage 53 when the thyroid gland is completely inhibited by the goitrogen methimazole. This dumbbell-shaped limb bud has internal structure, but there is no muscle present. After TH addition, the first visible change is engorgement of the blood vessels in 48 h, followed by growth of the limb bud. The muscle-specific proteins are not synthesized for 4 to 5 days after the beginning of TH induction (13). The up-regulation of these terminally differentiated muscle proteins is a very delayed response to TH. In spontaneous development, muscle is identifiable by NF stage 54 (Fig. 1b). Tadpoles that strongly express the pCar/TRDNα transgene develop to the climax of metamorphosis and then die suddenly. This phenotype is very different from that seen in the transgenic tadpoles that overexpress deiodinase type III constitutively (11). These latter animals linger for many days at climax before finally dying. One possibility to explain the death of the pCar/TRDNα tadpoles might be inhibition of changes in the heart. Although this premature death precludes observing the effect of the transgene on tail resorption, the effect on limb development is dramatic. Muscle mass is greatly reduced whereas bone and cartilage development has progressed to the same extent as the controls (Fig. 5c). These tadpoles never develop the ability to move their legs. The molecular details of this inhibition are as yet unknown. An assessment of TH-induced genes in the limb (13) found a program of up-regulated genes, many of which had been implicated in cell division that had been studied in serum-starved cultured cells (35). T3 induced death in primary myoblast cultures from skeletal muscle of limb, body, and tail but didn't have any effect on the replication of those cells. Instead, T3 promoted differentiation of myoblasts into myotubes (36). The different response of limb and tail muscle to TH is reflected in the expression of some muscle proteins that are expressed differentially in these two organs. The fact that skeletal muscle in limb and tail behave differently in response to TH means their biochemical properties might be different. It has been suggested that some muscle-specific genes, myosin heavy chain isoforms and adult specific β-tropomyosin, are expressed specifically in the skeletal muscle of the limb and body but not in the tail muscle (37). These genes are expressed actively in the tail at climax, at low level in the growing limb, but not at all in the adult limb muscle. An adult isoform is activated at the climax in limb and in the cord muscle fibers in tail. It continues to be expressed in the adult limb.
By overexpressing the TRDNα transgene exclusively in muscle, we have identified three different TH-induced muscle programs. The fast muscle of tail that comprises the bulk of tail muscle has its own cell-autonomous response to TH that is independent of the other cell death events in the tail. The slow muscle cords share the fate of the other cell types in the tail, especially the fibroblasts that comprise the notochord and the fins. Finally, inhibition of the TH-induced response selectively in muscle blocks the growth program in the muscle of the limb.
Acknowledgments
We thank our colleagues for criticism of the manuscript. Heather Henry provided expert technical assistance. We thank Enrique Amaya for providing us with the pCar/GFP2 plasmid. This research was supported by a National Research Service Award (NRSA) fellowship (to A.M.S.), a National Institutes of Health grant (to D.D.B.), and a grant from the G. Harold and Leila Y. Mathers Foundation (to D.D.B.).
Abbreviations
TH, thyroid hormone
NF, Nieuwkoop and Faber
TRDNα, dominant negative form of the thyroid hormone receptor α
T3, 3,5,3′-triiodothyronine
References
- 1.Weber R. (1969) Gen. Comp. Endocrinol. Suppl. 2, 408-416. [Google Scholar]
- 2.Schwind J. L. (1933) J. Exp. Zool. 66, 1-14. [Google Scholar]
- 3.Tata J. R., Kawahara, A. & Baker, B. S. (1991) Dev. Biol. 146, 72-80. [DOI] [PubMed] [Google Scholar]
- 4.Ishizuya-Oka A., Ueda, S., Damjanovski, S., Li, Q., Liang, V. C. & Shi, Y. B. (1997) Dev. Biol. 192, 149-161. [DOI] [PubMed] [Google Scholar]
- 5.Berry D. L., Schwartzman, R. A. & Brown, D. D. (1998) Dev. Biol. 203, 12-23. [DOI] [PubMed] [Google Scholar]
- 6.Gross J. & Lapiere, C. M. (1962) Proc. Natl. Acad. Sci. USA 48, 1014-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elinson R. P., Remo, B. & Brown, D. D. (1999) Dev. Biol. 215, 243-252. [DOI] [PubMed] [Google Scholar]
- 8.Yaoita Y. & Nakajima, K. (1997) J. Biol. Chem. 272, 5122-5127. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima K., Takahashi, A. & Yaoita, Y. (2000) J. Biol. Chem. 275, 10484-10491. [DOI] [PubMed] [Google Scholar]
- 10.Huang H. & Brown, D. D. (2000) Proc. Natl. Acad. Sci. USA 97, 195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang H., Marsh-Armstrong, N. & Brown, D. D. (1999) Proc. Natl. Acad. Sci. USA 96, 962-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodd M. H. I. & Dodd, J. M. (1976) in Physiology of the Amphibia, ed. Lofts, B. (Academic, New York), Vol. 2, pp. 467–599. [Google Scholar]
- 13.Buckbinder L. & Brown, D. D. (1992) J. Biol. Chem. 267, 25786-25791. [PubMed] [Google Scholar]
- 14.Kroll K. L. & Amaya, E. (1996) Development (Cambridge, U.K.) 122, 3173-3183. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber A. M., Das, B., Huang, H., Marsh-Armstrong, N. & Brown, D. D. (2001) Proc. Natl. Acad. Sci. USA 98, 10739-10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieuwkoop P. D. & Faber, J., (1956) Normal Table of Xenopus laevis (Daudin) (Elsevier Biomedical, Amsterdam).
- 17.Zar J. H., (1999) Biostatistical Analysis (Prentice–Hall, Englewood Cliffs, NJ).
- 18.Mohun T. J., Garrett, N. & Gurdon, J. B. (1986) EMBO J. 5, 3185-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sap J., Munoz, A., Damm, K., Goldberg, Y., Ghysdael, J., Leutz, A., Beug, H. & Vennstrom, B. (1986) Nature (London) 324, 635-640. [DOI] [PubMed] [Google Scholar]
- 20.Weinberger C., Thompson, C. C., Ong, E. S., Lebo, R., Gruol, D. J. & Evans, R. M. (1986) Nature (London) 324, 641-646. [DOI] [PubMed] [Google Scholar]
- 21.Yaoita Y., Shi, Y. B. & Brown, D. D. (1990) Proc. Natl. Acad. Sci. USA 87, 7090-7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrest D., Munoz, A., Raynoschek, C., Vennstrom, B. & Beug, H. (1990) Oncogene 5, 309-316. [PubMed] [Google Scholar]
- 23.Eliceiri B. P. & Brown, D. D. (1994) J. Biol. Chem. 269, 24459-24465. [PubMed] [Google Scholar]
- 24.Yaoita Y. & Brown, D. D. (1990) Genes Dev. 4, 1917-1924. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z. & Brown, D. D. (1993) J. Biol. Chem. 268, 16270-16278. [PubMed] [Google Scholar]
- 26.Kerr J. F., Harmon, B. & Searle, J. (1974) J. Cell Sci. 14, 571-585. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa A. & Hayashi, H. (1995) Differentiation (Berlin) 59, 207-214. [DOI] [PubMed] [Google Scholar]
- 28.Sachs L. M., Lebrun, J. J., de Luze, A., Kelly, P. A. & Demeneix, B. A. (1997) Mol. Cell. Endocrinol. 131, 211-219. [DOI] [PubMed] [Google Scholar]
- 29.Vander Heiden M. G., Chandel, N. S., Schumacker, P. T. & Thompson, C. B. (1999) Mol. Cell 3, 159-167. [DOI] [PubMed] [Google Scholar]
- 30.Fox H. (1973) Z. Zellforsch. Mikrosk. Anat. 138, 371-386. [DOI] [PubMed] [Google Scholar]
- 31.Lee C. Y. & Baehrecke, E. H. (2001) Development (Cambridge, U.K.) 128, 1443-1455. [DOI] [PubMed] [Google Scholar]
- 32.Kim J. & Klionsky, D. J. (2000) Annu. Rev. Biochem. 69, 303-342. [DOI] [PubMed] [Google Scholar]
- 33.Weber R. (1964) J. Cell Biol. 22, 481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown D. D., Wang, Z., Furlow, J. D., Kanamori, A., Schwartzman, R. A., Remo, B. F. & Pinder, A. (1996) Proc. Natl. Acad. Sci. USA 93, 1924-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau L. F. & Nathans, D. (1991) in Molecular Aspects of Cellular Regulation, eds. Cohen, P. & Foulkes, J. G. (Elsevier, London), Vol. 6, pp. 257–293. [Google Scholar]
- 36.Shibota Y., Kaneko, Y., Kuroda, M. & Nishikawa, A. (2000) Differentiation (Berlin) 66, 227-238. [DOI] [PubMed] [Google Scholar]
- 37.Nishikawa A. & Hayashi, H. (1994) Dev. Biol. 165, 86-94. [DOI] [PubMed] [Google Scholar]