Abstract
Loss of heterozygosity on chromosome 22q has been detected in approximately 60% of advanced nonsmall cell lung carcinoma (NSCLC) as well as small cell lung carcinoma (SCLC), suggesting the presence of a tumor suppressor gene on 22q that is involved in lung cancer progression. Here, we isolated a myosin family gene, MYO18B, located at chromosome 22q12.1 and found that it is frequently deleted, mutated, and hypermethylated in lung cancers. Somatic MYO18B mutations were detected in 19% (14/75) of lung cancer cell lines and 13% (6/46) of primary lung cancers of both SCLC and NSCLC types. MYO18B expression was reduced in 88% (30/34) of NSCLC and 47% (8/17) of SCLC cell lines. Its expression was restored by treatment with 5-aza-2′-deoxycytidine in 11 of 14 cell lines with reduced MYO18B expression, and the promoter CpG island of the MYO18B gene was methylated in 17% (8/47) of lung cancer cell lines and 35% (14/40) of primary lung cancers. Furthermore, restoration of MYO18B expression in lung carcinoma cells suppressed anchorage-independent growth. These results indicate that the MYO18B gene is a strong candidate for a novel tumor suppressor gene whose inactivation is involved in lung cancer progression.
Allelic losses on chromosome (chr) 22q have been frequently detected in human lung cancers by several methods, such as loss of heterozygosity (LOH) analysis (1–3), cytogenetic analysis (4, 5), and comparative genomic hybridization (6), indicating the presence of a tumor suppressor gene (TSG) on chr 22q, which is frequently inactivated in lung cancer. We previously reported that the incidence of LOH on 22q in advanced-stage non-small cell lung carcinoma (NSCLC) (≈60%) was significantly higher than that in early-stage NSCLC (≈30%) (1, 2). The incidence of LOH on chr 22q was also high in small cell lung carcinomas (SCLCs) (≈60%) irrespective of clinical stages (3). Thus, it is likely that inactivation of a TSG on chr 22q plays an important role in the progression of both SCLC and NSCLC types.
Three known TSGs, SNF5/INI1, NF2, and EP300, have been mapped to 22q11.2, 22q12.2, and 22q13, respectively. However, genetic and/or epigenetic alterations of these genes are infrequent in both SCLC and NSCLC (7–9). Therefore, a target gene(s) for 22q LOH in human lung cancer is still unknown. Recently, we identified a homozygous deletion on chr 22q12.1 in a SCLC cell line, Lu24 (10). The deleted region included the SEZ6L gene, whose structure had been partially characterized by the chr 22 sequencing project (11). Therefore, we determined the genomic structure of SEZ6L and performed a mutational analysis of this gene in lung cancers. However, SEZ6L was genetically altered only in a small subset (<10%) of lung cancers. Therefore, it was strongly suggested that another unknown gene(s) on chr 22q functioned as a major TSG in lung cancer progression. Subsequent analyses of the genomic sequence covering the Lu24 deletion identified a novel myosin heavy chain-like gene, bk125H2.1, whose structure also had been partially determined by the chr 22 sequencing project (11). It was recently proposed that the bk125H2.1 gene be named MYO18B based on the partial amino acid sequence deduced from the genomic sequence data (12). Thus, we use the name MYO18B for the bk125H2.1 gene in this article.
Here we isolated the full-length cDNA, determined the genomic structure of the MYO18B gene, and analyzed it for deletion, mutation, expression, and methylation in a large number of lung cancers. The MYO18B gene was frequently altered by several molecular mechanisms, including homozygous/hemizygous deletions, intragenic mutations, and hypermethylation of the CpG island. Restoration of MYO18B expression in lung cancer cells suppressed colony formation in soft agar. Thus, it was strongly suggested that the MYO18B gene is a target TSG of 22q LOH and is involved in the progression of lung cancer.
Materials and Methods
Samples.
Seventy-six lung cancer cell lines, consisting of 26 SCLCs and 50 NSCLCs, were used in this study (13). In 10 SCLCs and 15 NSCLCs, corresponding lymphoblast cells were available (14) (details are available on request). Forty-six surgical specimens (16 SCLCs and 30 NSCLCs) and adjacent noncancerous tissues were obtained at surgery. DNA was prepared as described (10). Allelic imbalance (AI) at two microsatellite loci, D22S429 and D22S538, in the MYO18B locus (Fig. 1A) was examined in these 46 cases by the method described (1). Forty cases were informative for either or both of the loci, and AI was detected in 25 (63%) of the cases. Noncancerous tissue DNA of 50 unrelated individuals was also used in this study. Randomly primed cDNAs for lung cancer cell lines, SAEC (Clonetics, Walkersville, MD), NHBE (Clonetics), WI-38, seven NSCLCs, and human normal tissues were prepared as described (10). Alveolar type II cells and bronchiolar epithelial cells were microdissected by using the LM200 LCM System (Arcturus Engineering, Mountain View, CA), and total RNA was prepared with a Micro RNA Isolation kit (Stratagene) (15). Randomly primed cDNA was reverse-transcribed from total RNA by using SensiScript reverse transcriptase (Qiagen, Tokyo).
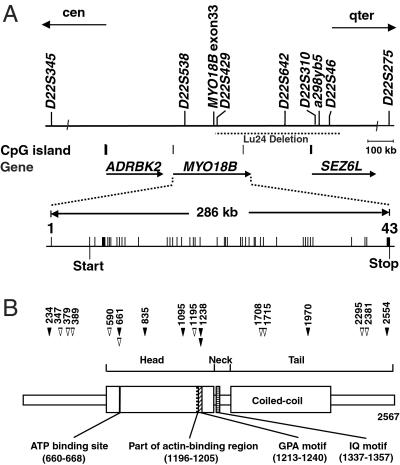
Fig 1.
Structures of the MYO18B gene and MYO18B protein. (A) Physical and transcriptional maps of the region containing the MYO18B gene. STS markers are marked on the top. Locations of CpG islands and three genes are indicated. The region of homozygous deletion in a SCLC cell line, Lu24, is indicated by a dashed horizontal line. Exon-intron organization of the MYO18B gene is depicted as vertical bars. Predicted transcriptional start and stop sites are indicated by vertical bars under exons 2 and 43, respectively. (B) Schematic diagram of a domain structure of MYO18B protein and positions of mutations in lung cancer cells. Numbers on the top indicate codon numbers. Closed arrowheads, somatic mutations; open arrowheads, base substitutions detected only in cell lines but not in 50 unrelated individuals. Putative functional domains are indicated at the bottom.
Isolation of Full-Length MYO18B cDNA.
Genome DNA sequences covering the Lu24 deletion region (GenBank accession nos. AL022329, AL080245, Z98949, AL079300, AL022337, AL080273, and AL023513) were used to identify exons by the genscan (16) and blast programs (17) after elimination of repetitive elements with the repeatmasker program (18). The algorithm at www.ebi.ac.uk/emboss/cpgplot/was used to detect potential CpG islands. Predicted exons were connected by exon-connection PCR with human skeletal muscle cDNA as a template with nine primer sets (primers and conditions are available on request). Amplified cDNA fragments were directly sequenced as described (10).
Real-Time Quantitative PCR (RTQ-PCR) Analysis.
Expression of MYO18B was measured by RTQ-PCR using ABI Prism 7900HT (Applied Biosystems). Probe and primers were designed by using primer express software (Applied Biosystems). Expression of MYO18B was normalized to RNA content for each sample by using GAPDH as an internal control (primers, probe, and conditions are available on request). The relative expression was calculated as the ratio of the average expression levels for all samples compared with adult human lung mRNA. Levels of relative expression of <0.5 were considered as being reduced.
Mutation Analysis.
For single-stranded conformation polymorphism (SSCP) and WAVE analyses, 42 coding exons of the MYO18B gene were amplified by PCR using 50 ng of genomic DNA in each of 51 sets of primers. SSCP analysis was performed as described (12). For WAVE analysis, the PCR products from tumor DNA were mixed with those from normal lung tissue DNA, denatured, reannealed, and analyzed by WAVE DNA Fragment Analysis and wavemaker software 4.0 (Transgenomic, Omaha, NE). PCR products with different mobilities in either SSCP or WAVE analysis were purified and directly sequenced. (Primers and conditions are available on request.)
5-Aza-2′-deoxycytidine (5-aza-dC) and/or Trichostatin A (TSA) Treatment.
For the first 48 h, cells were incubated with medium containing 1.0 μM 5-aza-dC (Sigma), and then for another 24 h with the addition of 1.0 μM TSA (Wako, Tokyo). Total RNA was then isolated with a RNeasy minikit (Qiagen), RTQ-PCR was performed as described above, and a relative expression of >2 compared with basal levels was considered as being induced.
Promoter Methylation Analysis.
Genomic DNA was treated with sodium bisulfite as described (19). PCR was performed by mixing DNA (<200 ng) with 50 pM of the CpG-F (5′-AAGGTATGTTTATATGTATT-3′) and CpG-R (5′-CAGGAAACAGCTACGACAACAAACAAAAAAATCAAAC-3′) primers (Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org) in a reaction mixture (40 μl) containing dNTPs (200 μM each) and 1 unit of HotstarTaq DNA polymerase (Qiagen) at 95°C (1 min), 50°C (1 min), and 72°C (4 min) for 40 cycles. The CpG-R primer contained the M13-RV sequence (underlined) as a site to initiate sequencing. PCR products were directly sequenced to obtain average methylation levels. PCR products of five cell lines and two normal lung tissues were subcloned into the pGEM T-Easy vector (Promega) and sequenced.
Definition of Methylation.
Plasmid DNA with or without converted cytosines at CpG sites 10 and 11 was amplified by the SP6 and T7 primers. Then, seven artificial heterozygote samples were prepared by mixing each of the PCR products in an equal ratio and sequenced by using the M13-RV primer. The percentage of methylation was calculated by the formula of the peak height of G divided by the sum of peak heights G and A. The mean values of the seven samples were 50.5% at both CpG sites 10 and 11, and small differences in the percentage of methylation were observed because of artificial signal variations (SD at sites 10 and 11 was 2.0% and 1.9%, respectively). When the percentage of methylation was higher than the mean plus 3 SD (56.5% and 56.2% at sites 10 and 11, respectively), we considered there to be cells with biallelic methylation.
Cell Proliferation and Soft Agar Growth Assays.
FLAG-tagged MYO18B cDNA was ligated into the pcDNA3.1(+) plasmid vector (Invitrogen). The cDNA-containing or empty vector was transfected by using Lipofectamine 2000 (Invitrogen). G418-resistant colonies were picked up, and MYO18B expression was confirmed by Western blot analysis with anti-FLAG M2 Ab (Sigma). For cell proliferation assay, cells were seeded in a 96-well plate at a density of 1 × 104 per well, and the number of cells was estimated by using the TetraColor ONE Cell Proliferation Assay System (Seikagaku, Tokyo). Colony formation in soft agar was measured as described (20). Three weeks after plating, cells were stained with methylene blue, and the number of visible colonies was counted.
Sequence Analysis.
Sequence similarity and motif searches were performed by using blast and profilescan (www.ch.embnet.org/software/PFSCAN_form.html) programs. The coiled–coil region was predicted with MACSTRIPE 2.0 software (21).
Results and Discussion
The sequence data of chr 22 indicated that the Lu24 deletion contains the MYO18B gene, which encodes a novel myosin heavy chain-like protein, consisting of 25 exons (GenBank accession no. Z98949). However, the amino acid sequence of a deduced MYO18B protein contained only the C-terminal portion of a myosin head domain, a neck domain, and a tail domain with a short coiled–coil. Therefore, it was suggested that the size of the MYO18B gene is larger than predicted from the sequence data. Accordingly, we determined the structure of full-length MYO18B cDNA by genscan gene prediction program analysis of the genomic sequence covering the Lu24 deletion in conjunction with reverse transcription–PCR analysis. Then, the full-length cDNA sequence was compared with the genomic sequence of this locus. By this approach, 42 coding exons and a 5′ noncoding exon were identified (Fig. 1A). The 5′ noncoding exon was confirmed as being the initial exon (exon 1) by primer extension analysis (data not shown). The region of 424 bp including this exon met the mathematical criteria of a CpG island (22) (Fig. 5). The full-length MYO18B cDNA fragment was 8,051 bp in size, contained an ORF of 7,701 bp, and encoded 2,567 aa with a predicted Mr of 285,000. An ATG in exon 2, located 260 bp downstream of the predicted transcription start site, was speculated to be a translation start codon (Fig. 1A). The sequence context of this ATG was identical to the optimal translation initiation signal (23), whereas an in-frame termination codon was not found upstream of this ATG. Thus, the MYO18B gene consisted of 43 exons and was dispersed within a 286-kb region between the ADRBK2 gene and the SEZ6L gene on chr 22q (Fig. 1A).
Searches of public nucleotide and protein databases with the MYO18B cDNA sequence revealed that the amino acid sequence of MYO18B protein shows 92% homology with a mouse putative protein (GenBank accession no. AK016515) and shares 40% identity with human and mouse MysPDZ proteins (GenBank accession nos. D86970 and AB026497, respectively). A schematic diagram of the domain structure of MYO18B protein is shown in Fig. 1B. A head (motor) domain was identified from codons 573 to 1321. Alignments with other myosins revealed a consensus ATP binding site and part of an actin binding region (Fig. 1B). A GPA motif, which is very rich in Gly, Pro, and Ala residues and is believed to interact with F actin (24), was identified at the C terminus of the actin binding region. An IQ motif was found between the head and tail domains (Fig. 1B). To date, at least 18 myosin subfamilies have been identified (12). A phylogenetic analysis revealed that MYO18B, human and mouse MysPDZ, and Drosophila alt1 constituted a new class (XVIII) of myosin family proteins (data not shown), as described (12). MYO18B has an ATP binding site and a myosin light chain binding IQ motif, suggesting that this molecule acts as a motor protein regulated by light chains in the presence of ATP. MYO18B also contains a short coiled–coil domain, which probably allows for dimerization to form a two-headed structure and a globular structure at the end of the tail.
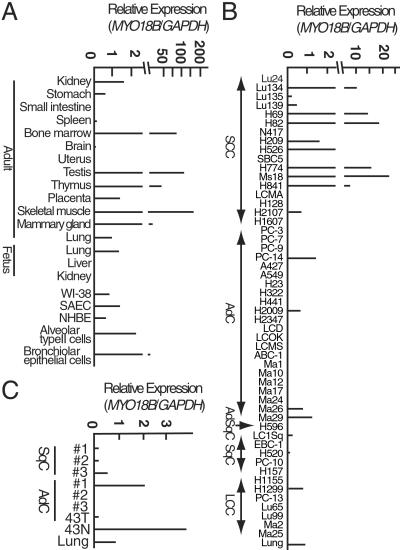
Northern blot analysis indicated that MYO18B transcripts of ≈8.0 kb in size were expressed in skeletal muscle and heart but not in other tissues, including lung (data not shown). However, RTQ-PCR analysis revealed that the MYO18B gene is expressed in diverse tissues, including adult and fetal lungs (Fig. 2A). Furthermore, MYO18B expression was also detected in two primary cultured bronchial epithelial cells, SAEC and NHBE, a lung fibroblast cell line, WI-38, and laser capture microdissected alveolar type II cells and bronchiolar epithelial cells (Fig. 2A). Thus, the MYO18B gene is expressed in both lung epithelial cells and fibroblasts. In particular, its expression was detected in possible precursor cells for lung adenocarcinoma and squamous cell carcinoma, alveolar type II cells, and bronchiolar epithelial cells, respectively. These results strongly indicated that MYO18B protein may have a common function in various types of human cells, and that MYO18B expression has some physiological function for lung epithelial cells.
Fig 2.
Expression of the MYO18B gene. SCC, small cell carcinoma; AdC, adenocarcinoma; AdSqC, adenosquamous carcinoma; SqC, squamous cell carcinoma; LCC, large cell carcinoma. (A and C) Expression in normal human tissues and cancerous and noncancerous lung cells. Noncancerous tissue (43N) and cancerous tissue (43T) were obtained from the same patient. (B) Expression in lung cancer cell lines.
Genomic PCR analysis revealed that exons 34–43 of the MYO18B gene were homozygously deleted, whereas exons 1–33 were retained, in the Lu24 cell line (Fig. 1A). To clarify whether the MYO18B gene is genetically altered in other lung cancers, 75 lung cancer cell lines were subjected to single-stranded conformation polymorphism and/or WAVE analyses followed by direct sequencing. No homozygous deletions were detected except in Lu24. However, 51 different types of nucleotide substitutions were detected among the 75 cell lines. Among them, six different types of substitutions detected in six cell lines, H209, H128, H2347, H2009, H2107, and H1607, were confirmed as being somatic mutations, because these substitutions were not detected in the corresponding lymphoblast cell lines (Table 1). Another 10 different types of substitutions detected in eight cell lines, H69, Ma29, Ma2, H23, N417, VMRC-LCD, H526, and A549, were likely to be somatic mutations and not genetic polymorphisms because these substitutions were not detected in 50 noncancerous tissues (Table 1). Fourteen of these 16 mutations were missense, and the remaining two were silent and intronic. Among the 14 cell lines with MYO18B mutations, four cell lines were homozygous for the mutant alleles, whereas the remaining 10 were heterozygous with retention of the wild-type allele (Table 1). The remaining 35 different types of substitutions were genetic polymorphisms, because these sequence variants were also detected in noncancerous tissues. In total, mutations of the MYO18B gene were detected in 14 of the 75 (19%) lung cancer cell lines. We next examined 46 primary lung cancers, and six (13%) were concluded as having somatic mutations, because these sequence variants were detected only in cancerous tissues but not in the corresponding noncancerous tissues. Four mutations were missense, and the remaining two were silent and intronic (Table 1; Fig. 1B). Two of the six cases showed loss of the wild-type allele.
Table 1.
Mutations of the MYO18B gene in human lung cancers
| Sample | Exon (nucleotide change) | Predicted effect | Exp./Met. |
|---|---|---|---|
| H209 (SCC) | 4 (G701T) | Gly-234–Val | +/U |
| H128 (SCC) | 9 (G2070A) | Silent | −/U |
| H2347 (AdC) | 18 (G3284T) | Arg-1095–Leu | −/U |
| H2009 (AdC) | 20 (C3712A) | Pro-1238–Thr | +/U |
| H2107 (SCC) | 38 (C5909A) | Ala-1970–Glu | +/U |
| H1607 (SCC) | IVS41 − 21 (C/T) | Unknown | −/U |
| S51T (SCC) | IVS4 + 26 (G/A) | Unknown | ND/M |
| N2111T (AdC) | 8 (C1981T) | Arg-661–Trp | ND/U |
| 1011T (SqC) | 12 (C2504G) | Ala-835–Gly | ND/U |
| 1551T (LCC) | 20 (C3713A) | Pro-1238–Gln | ND/U |
| 2231T (LCC) | 43 (C7662A) | Asp-2554–Glu | ND/U |
| N591T (SqC) | 43 (G7674T) | Silent | ND/U |
| H69 (SCC) | 4 (G1041T) | Lys-347–Asn | +/U |
| H69 (SCC) | 4 (G1136A) | Arg-379–Gln | +/U |
| Ma29 (AdC) | 4 (G1167T) | Trp-389–Cys | +/U |
| Ma2 (LCC) | 7 (C1769T) | Thr-590–Met | −/M |
| H23 (AdC) | 8 (C1981T) | Arg-661–Trp | −/U |
| N417 (SCC) | 20 (G3584A) | Arg-1195–Glu | −/U |
| H23 (AdC) | 31 (G5122A) | Glu-1708–Lys | −/U |
| VMRC-LCD (AdC) | 31 (G5145C) | Glu-1715–Asp | −/U |
| H526 (SCC) | 43 (G6883T) | Gly-2295–Cys | +/U |
| A549 (AdC) | 43 (G7142A) | Arg-2381–His | −/U |
SCC, small cell carcinoma; AdC, adenocarcinoma; SqC, squamous cell carcinoma; LCC, large cell carcinoma. Exp., expression; Met., methylation; −, reduced or absent; ND, not determined; M, methylated; U, unmethylated.
Surgical specimen.
Corresponding noncancerous tissue DNA was not available.
Homozygous base substitution.
Among the 18 missense mutations detected in lung cancers, mutations in codons 590, 835, and 1970 caused substitutions of evolutionarily conserved amino acids (Thr to Met, Ala to Gly, and Ala to Glu, respectively). Mutations in codon 661, detected as the same nucleotide substitution in H23 and N2111T, caused an amino acid change in the ATP binding site (Arg to Trp). Meanwhile, mutations in codon 1238, detected as two different types of nucleotide substitutions in H2009 and 1551T, caused different types of amino acid changes in the GPA motif (Pro to Thr or Gln). Thus, it is highly possible that these mutations have some effects on the physiological function of the MYO18B protein.
We next assessed expression of the MYO18B gene in 51 lung cancer cell lines and seven primary NSCLCs by RTQ-PCR analysis. MYO18B expression was reduced in 8/17 (47%) of SCLC cell lines, 30/34 (88%) of NSCLC cell lines, and 5/7 (71%) of primary NSCLCs (Fig. 2 B and C). Thus, expression of the MYO18B gene was reduced in approximately 85% of NSCLCs, including both cell lines and primary tumors, and 50% of SCLC cell lines.
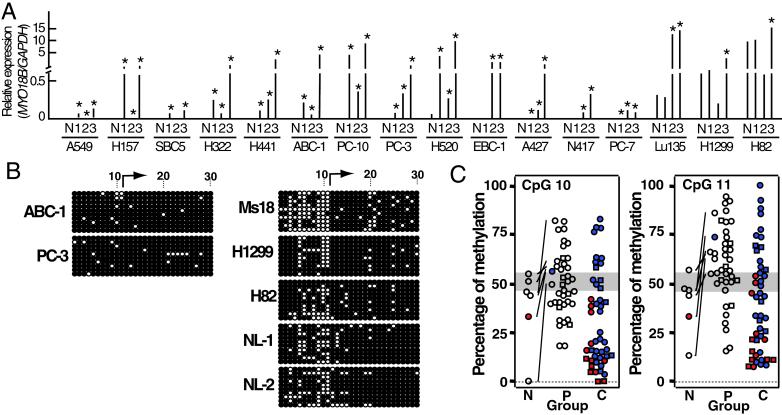
Aberrant promoter methylation is now acknowledged as a mechanism for gene inactivation in cancer cells (25, 26). Reduced MYO18B expression in lung cancer cells could be caused by transcriptional silencing of this gene by hypermethylation of the CpG island in the promoter region. To address this question, we first screened for a possible restoration of its expression by treatment of the cells with 5-aza-dC. Among 14 cell lines (three SCLCs and 11 NSCLCs) with reduced (or without) MYO18B expression, its expression was restored in 11 cell lines (79%) with 5-aza-dC. In contrast, in two cell lines (H1299 and H82) with relatively high MYO18B expression, its expression was not up-regulated by 5-aza-dC (Fig. 3A). This result strongly indicated that the MYO18B gene is frequently silenced by hypermethylation of the promoter region in lung cancer cells.
Fig 3.
Expression and methylation of the MYO18B gene in cancerous and noncancerous lung cells. (A) Restoration of MYO18B expression after treatment with 5-aza-dC and/or TSA in 14 cell lines with reduced (or without) MYO18B expression. N, no treatment; 1, 5-aza-dC treatment; 2, TSA treatment; 3, 5-aza-dC and TSA treatment. Each result is the average of two independent tests. * indicates expression levels were more than twice as much as basal levels. (B) Bisulfite sequencing of the 5′ CpG-rich region of the MYO18B gene in lung cancer cell lines without (ABC-1 and PC-3) or with (Ms18, H1299, and H82) MYO18B expression and two normal lung tissues (NL-1 and NL-2). Each circle indicates a CpG site in the primary DNA sequence, and each line of circles represents analysis of a single cloned allele. The numbers of the CpG site are indicated at the top and are identical to the numbers in Fig. 5. ○, unmethylated CpG sites; •, methylated CpG sites; arrow, transcription start site. (C) Methylation status at CpG 10/11 in normal lung tissues and lung cancer cells. The ranges of 50% methylation (n = 7, mean ± 3 SD) at CpG 10/11 are shaded. Paired cancerous and noncancerous lung tissues are connected with solid lines. Samples with MYO18B mutation, square; samples with or with reduced (or without) MYO18B expression, filled in red or blue, respectively. Open squares and circles indicate samples in which MYO18B expression was not determined. Groups N, P, and C indicate normal lung tissue (n = 6), primary tumor (n = 40), and cell line (n = 47), respectively.
We next undertook bisulfite-sequencing analysis of a CpG-rich region containing the CpG island with the transcription start sites of this gene (Fig. 5). DNAs from two NSCLC cell lines without MYO18B expression, those from one NSCLC cell line and two SCLC cell lines with MYO18B expression, and those from two normal lung tissues were used for the analysis. PCR products of 748 bp containing 30 CpG sites surrounding the first exon were then subcloned into a TA-cloning vector, and 10 independent clones were sequenced. Several specific CpG sites were hypermethylated in the two cell lines without MYO18B expression but hypomethylated in the three cell lines with expression and in the normal lung tissues (Fig. 3B). In particular, CpG sites 10 and 11 (CpG 10/11) in the CpG island appeared to be consistently hypermethylated in cell lines without MYO18B expression but hypomethylated in cell lines with MYO18B expression. Namely, CpG 10/11 were methylated in >60% of the clones analyzed for cell lines without MYO18B expression, but in <50% of the clones analyzed for those with MYO18B expression. This finding indicates that methylation-associated MYO18B silencing occurs, at least in part, through methylation of CpG 10/11. To further assess the effect of methylation at CpG 10/11, genomic DNAs from 42 other lung cancer cell lines were treated with sodium bisulfite, amplified by PCR with the same primers, and analyzed by direct sequencing. Thus, in total, 47 cell lines were analyzed for the methylation status of CpG 10/11. Among 34 cell lines with reduced (or without) MYO18B expression, eight showed hypermethylation at both CpG 10/11, and 26 showed low methylation levels at these sites (Fig. 3C; Table 2). In particular, none of 13 cell lines with MYO18B expression showed hypermethylation at CpG 10/11. This result indicated that, in approximately 17% (8/47) of cell lines, the MYO18B gene is silenced by methylation of CpG 10/11. We next analyzed 40 primary lung cancers for CpG 10/11 methylation by the same method. Fourteen (35%) of the 40 primary cancers showed hypermethylation, but the remaining 26 showed hypomethylation (Fig. 3C; Table 2). In all six pairs of primary cancer and the corresponding normal tissue, methylation levels were higher in cancers than in normal tissues. In one case, the tumor (43T) with hypermethylation at CpG 10/11 showed reduced MYO18B expression, but the corresponding normal lung tissue (43N) with hypomethylation at both sites showed MYO18B expression (Figs. 2C and 3C). Thus, it was suggested that CpG 10/11 are hypermethylated in a subset of primary lung cancers, and hypermethylation of those CpG sites leads to MYO18B gene silencing in cancer cells.
Table 2.
Incidence of hypermethylation at CpG sites 10 and 11 in lung cancers
| CpG site
|
Cell lines (%) | Surgical specimens (%) | ||||
|---|---|---|---|---|---|---|
| SCLC (n = 15) | NSCLC (n = 32) | Total (n = 47) | SCLC (n = 11) | NSCLC (n = 29) | Total (n = 40) | |
| 10 | 1 (7) | 8 (25) | 9 (19) | 5 (45) | 10 (34) | 15 (37) |
| 11 | 1 (7) | 12 (37) | 13 (27) | 7 (63) | 16 (55) | 23 (57) |
| 10 and 11 | 1 (7) | 7 (22) | 8 (17) | 5 (45) | 9 (31) | 14 (35) |
5-aza-dC failed to restore MYO18B gene expression in three of the 14 cell lines, suggesting that mechanisms other than hypermethylation, such as histone deacetylation, may also be involved in the repression of the MYO18B gene. To address this question, we screened for a possible restoration of its expression by treatment of the cells with a histone deacetylase inhibitor, TSA (Fig. 3A). By TSA treatment, MYO18B expression was restored in all three cell lines, which showed no response to 5-aza-dC. Thus, histone deacetylation is likely to be another mechanism of MYO18B gene silencing in lung cancer cells. Restoration of MYO18B expression by TSA in a total of 13 of the 14 cell lines indicates that deacetylation contributes to MYO18B gene silencing as frequently as DNA methylation, and that histone deacetylation cooperates with DNA methylation for the gene silencing. Among the 11 cell lines with restoration of MYO18B expression by 5-aza-dC, hypermethylation at CpG 10/11 was detected only in four cell lines (ABC1, PC10, PC3, and H520). Thus, repression of MYO18B expression could also be caused by methylation of other CpG sites in the MYO18B gene or methylation of a transactivating regulator for the MYO18B gene.
We next assessed the expression and methylation as well as deletions of the wild-type allele of the MYO18B gene in 14 cell lines with mutated MYO18B genes (Table 1). Expression was reduced or absent in eight of them, thus, repression of MYO18B expression seemed to occur irrespectively of the mutation status in this gene. Methylation at CpG 10/11 was detected only in one of the eight cell lines, Ma2, thus, repression of MYO18B expression in the other seven cell lines might have occurred by several other mechanisms, as described above. Among the six cell lines with MYO18B expression, the wild-type allele was deleted only in the Ma29 cell line, suggesting that the MYO18B gene is inactivated by mutation accompanied by loss of the wild-type allele in this cell line. Two different mutations were detected in another cell line with MYO18B expression, H69. The mutations might have occurred in both alleles in this cell line. In the four other cell lines with MYO18B expression, the wild-type allele was retained. Thus, it was unclear whether the MYO18B gene is inactivated in these cell lines. However, it is possible that some of the mutated MYO18B proteins have dominant negative effects to the wild-type MYO18B protein. Although we could not assess the expression status in six primary lung cancers with mutated MYO18B genes, the wild-type allele was deleted in two cases, and methylation at CpG 10/11 was detected in one case (Table 1).
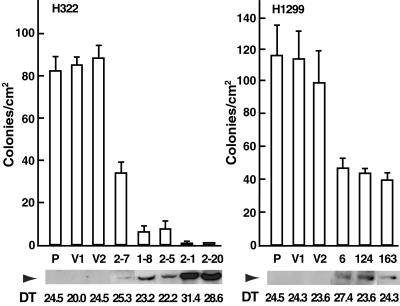
Molecular analyses of the MYO18B gene in a large number of lung cancers suggested that this gene could play an important role as a tumor suppressor in the development of lung cancer. Thus, we further assessed whether the restoration of wild-type MYO18B expression suppresses the growth of lung cancer cells. A FLAG-tagged MYO18B expression vector was transfected into the H322 cell line, in which endogenous MYO18B expression was undetectable and restored by 5-aza-dC. Compared with the parent (H322) or empty vector transfectants (V1 and V2), the anchorage-independent growth was markedly suppressed in clones with MYO18B expression in an expression-dependent manner (Fig. 4, and Fig. 6, which is published as supporting information on the PNAS web site). Furthermore, the population doubling time was >10% prolonged in clones with strong MYO18B expression, 2–1 and 2–20. Expression of exogenous MYO18B also inhibited the anchorage-independent growth of the H1299 cell line, in which endogenous MYO18B expression was detected and not up-regulated by 5-aza-dC (Figs. 4 and 6). These results suggest that overexpressed MYO18B has a growth suppressor activity in lung cancer cells.
Fig 4.
Suppression of proliferation and anchorage-independent growth by exogenous MYO18B expression. H322 (P), vector-transfected controls (V1, V2), and MYO18B-transfected clones (2–7, 1–8, 2–5, 2–1, 2–20) were analyzed for population doubling time and colony formation in soft agar (Left). H1299 (P), vector-transfected controls (V1, V2), and MYO18B-transfected clones (6, 124, 163) were also analyzed (Right). The arrowhead indicates the expression of FLAG-tagged MYO18B protein (285 kDa) by Western blot analysis. Twenty micrograms of whole-cell lysate was loaded on each lane. The doubling time [DT (hr)] was calculated from the logarithmic phase of the growth curve. The ability to form colonies in soft agar was measured by counting the number of visible colonies/cm2 in triplicate of a 6-well plate. Error bars indicate SD.
We provide here the evidence that MYO18B is a strong candidate for TSG at chr 22q12.1, which is inactivated in approximately 50% of lung cancer by deletion, mutation, and promoter methylation. The activity of anchorage-independent growth suppression in lung cancer cells supports the fact that MYO18B acts as a TSG in lung carcinogenesis. Further functional and biological studies of the MYO18B gene will help us understand the molecular mechanisms of human lung cancer progression.
Supplementary Material
Acknowledgments
We thank T. Kamigaito, M. Yoshizumi, and T. Nakano for technical assistance and T. Akiyama and M. Shiraishi for helpful discussion. We also thank the following scientists for providing cell lines: Drs. Y. Hayata, T. Terasaki, S. Hirohashi, M. Takada, and A. F. Gazdar. Cell lines were also obtained from the American Type Culture Collection and the Japanese Collection of Research Bioresources. This work was supported in part by grants-in-aid from the Ministry of Health, Labor, and Welfare for the 2nd-term Comprehensive 10-year Strategy for Cancer Control, the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research, the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and the G. Harold and Leila Y. Mathers Charitable Foundation and National Cancer Institute Specialized Programs of Research Excellence Grant P50 CA70907. K.K. is a recipient of the Research Resident Fellowship from the Foundation for Promotion of Cancer Research.
Abbreviations
LOH, loss of heterozygosity
TSG, tumor suppressor gene
chr, chromosome
SCLC, small cell lung carcinoma
NSCLC, non-small cell lung carcinoma
5-aza-dC, 5-aza-2′-deoxycytidine
TSA, trichostatin A
RTQ-PCR, real-time quantitative PCR
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB075376).
References
- 1.Shiseki M., Kohno, T., Nishikawa, R., Sameshima, Y., Mizoguchi, H. & Yokota, J. (1994) Cancer Res. 54, 5643-5648. [PubMed] [Google Scholar]
- 2.Kawanishi M., Kohno, T., Otsuka, T., Adachi, J., Sone, S., Noguchi, M., Hirohashi, S. & Yokota, J. (1997) Carcinogenesis 18, 2057-2062. [DOI] [PubMed] [Google Scholar]
- 3.Virmani A. K., Fong, K. M., Kodagoda, D., McIntire, D., Hung, J., Tonk, V., Minna, J. D. & Gazdar, A. F. (1998) Genes Chromosomes Cancer 21, 308-319. [DOI] [PubMed] [Google Scholar]
- 4.Testa J. R., Siegfried, J. M., Liu, Z., Hunt, J. D., Feder, M. M., Litwin, S., Zhou, J. Y., Taguchi, T. & Keller, S. M. (1994) Genes Chromosomes Cancer 11, 178-194. [DOI] [PubMed] [Google Scholar]
- 5.Mertens F., Johansson, B., Hoglund, M. & Mitelman, F. (1997) Cancer Res. 57, 2765-2780. [PubMed] [Google Scholar]
- 6.Levin N. A., Brzoska, P., Gupta, N., Minna, J. D., Gray, J. W. & Christman, M. F. (1994) Cancer Res. 54, 5086-5091. [PubMed] [Google Scholar]
- 7.Sekido Y., Pass, H. I., Bader, S., Mew, D. J., Christman, M. F., Gazdar, A. F. & Minna, J. D. (1995) Cancer Res. 55, 1227-1231. [PubMed] [Google Scholar]
- 8.Manda R., Kohno, T., Hamada, K., Takenoshita, S., Kuwano, H. & Yokota, J. (2000) Cancer Lett. 153, 57-61. [DOI] [PubMed] [Google Scholar]
- 9.Gayther S. A., Batley, S. J., Linger, L., Bannister, A., Thorpe, K., Chin, S. F., Daigo, Y., Russell, P., Wilson, A., Sowter, H. M., et al. (2000) Nat. Genet. 24, 300-303. [DOI] [PubMed] [Google Scholar]
- 10.Nishioka M., Kohno, T., Takahashi, M., Niki, T., Yamada, T., Sone, S. & Yokota, J. (2000) Oncogene 19, 6251-6260. [DOI] [PubMed] [Google Scholar]
- 11.Dunham I., Shimizu, N., Roe, B. A., Chissoe, S., Hunt, A. R., Collins, J. E., Bruskiewich, R., Beare, D. M., Clamp, M., Smink, L. J., et al. (1999) Nature (London) 402, 489-495. [DOI] [PubMed] [Google Scholar]
- 12.Berg J. S., Powell, B. C. & Cheney, R. E. (2001) Mol. Biol. Cell 12, 780-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamada K., Kohno, T., Takahashi, M., Yamazaki, M., Yamazaki, M., Tashiro, H., Sugawara, C., Ohwada, S., Sekido, Y., Minna, J. D., et al. (2000) Genes Chromosomes Cancer. 27, 308-318. [PubMed] [Google Scholar]
- 14.Phelps R. M., Johnson, B. E., Ihde, D. C., Gazdar, A. F., Carbone, D. P., McClintock, P. R., Linnoila, R. I., Matthews, M. J., Bunn, P. A., Jr., Carney, D., et al. (1996) J. Cell Biochem. Suppl. 24,, 32-91. [DOI] [PubMed] [Google Scholar]
- 15.Fend F., Emmert-Buck, M. R., Chuaqui, R., Cole, K., Lee, J., Liotta, L. A. & Raffeld, M. (1999) Am. J. Pathol. 154, 61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burge C. & Karlin, S. (1997) J. Mol. Biol. 268, 78-94. [DOI] [PubMed] [Google Scholar]
- 17.Altschul S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. (1990) J. Mol. Biol. 215, 403-410. [DOI] [PubMed] [Google Scholar]
- 18.Smit A. F. (1996) Curr. Opin. Genet. Dev. 6, 743-748. [DOI] [PubMed] [Google Scholar]
- 19.Clark S. J., Harrison, J., Paul, C. L. & Frommer, M. (1994) Nucleic Acids Res. 22, 2990-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolligs F. T., Kolligs, B., Hajra, M. H., Hu, G., Tani, M., Cho, K. R. & Fearon, E. R. (2000) Genes Dev. 14, 1319-1331. [PMC free article] [PubMed] [Google Scholar]
- 21.Lupas A., Van-Dyke, M. & Stock, J. (1991) Science 252, 1162-1164. [DOI] [PubMed] [Google Scholar]
- 22.Gardiner-Garden M. & Frommer, M. (1987) J. Mol. Biol. 196, 261-282. [DOI] [PubMed] [Google Scholar]
- 23.Kozak M. (1987) Nucleic Acids Res. 15, 8125-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urrutia R. A., Jung, G. & Hammer, J. A., III (1993) Biochim. Biophys. Acta 28, 225-229. [DOI] [PubMed] [Google Scholar]
- 25.Jones P. A. & Laird, P. W. (1999) Nat. Genet. 21, 163-167. [DOI] [PubMed] [Google Scholar]
- 26.Baylin S. B., Herman, J. G., Graff, J. R., Vertino, P. M. & Issa, J. P. (1998) Adv. Cancer Res. 72, 141-196. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.