Abstract
Soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) mediate membrane fusion reactions in eukaryotic cells by assembling into complexes that link vesicle-associated SNAREs with SNAREs on target membranes (t-SNAREs). Many SNARE complexes contain two t-SNAREs that form a heterodimer, a putative intermediate in SNARE assembly. Individual t-SNAREs (e.g., syntaxin 1A) also regulate synaptic calcium channels and cystic fibrosis transmembrane conductance regulator (CFTR), the epithelial chloride channel that is defective in cystic fibrosis. Whether the regulation of ion channels by individual t-SNAREs is related to SNARE complex assembly and membrane fusion is unknown. Here we show that CFTR channels are coordinately regulated by two cognate t-SNAREs, SNAP-23 (synaptosome-associated protein of 23 kDa) and syntaxin 1A. SNAP-23 physically associates with CFTR by binding to its amino-terminal tail, a region that modulates channel gating. CFTR-mediated chloride currents are inhibited by introducing excess SNAP-23 into HT29-Cl.19A epithelial cells. Conversely, CFTR activity is stimulated by a SNAP-23 antibody that blocks the binding of this t-SNARE to the CFTR amino-terminal tail. The physical and functional interactions between SNAP-23 and CFTR depend on syntaxin 1A, which binds to both proteins. We conclude that CFTR channels are regulated by a t-SNARE complex that may tune CFTR activity to rates of membrane traffic in epithelial cells.
The cystic fibrosis transmembrane conductance regulator (CFTR) is a cAMP-activated chloride channel that localizes to the apical membranes of epithelial cells lining the airways, intestine, and exocrine glands (1). A major role of CFTR is to mediate salt and water secretion in submucosal glands and intestinal crypts, which lubricates the mucosal surface and helps deliver simultaneously secreted proteins out of the gland or crypt. Excessive activation of the CFTR chloride channel causes secretory diarrhea whereas defective synthesis or function leads to cystic fibrosis (2, 3).
The CFTR polypeptide contains two cytoplasmic tails, two membrane-spanning domains, two nucleotide binding domains (NBDs), and a large regulatory domain (R domain) that is phosphorylated by cAMP-dependent protein kinase (4). The NBDs and R domain are the major domains that control channel gating, although the amino-terminal tail modulates channel activity as well (5, 6). The amino-terminal tail also binds syntaxin 1A, a t-SNARE (t for target and SNARE for soluble N-ethylmaleimide-sensitive factor attachment protein receptor) that mediates membrane fusion reactions in cells (7, 8).
The role of syntaxin 1A in membrane fusion has been best characterized for neurons, where this t-SNARE localizes to the presynaptic plasma membrane. Syntaxin 1A forms a ternary complex with another t-SNARE at the plasma membrane, SNAP-25 (synaptosome-associated protein of 25 kDa), and with vesicle-associated membrane protein-2/synaptobrevin, a vesicle-associated SNARE on synaptic vesicles (9, 10). The ternary SNARE complex is an important mediator of synaptic vesicle fusion, a paradigm that applies to membrane fusion reactions in all types of eukaryotic cells. The t-SNAREs themselves are capable of forming heterodimers that have been argued to be intermediates in SNARE complex assembly and membrane fusion (11, 12).
In addition to regulating membrane fusion, syntaxin 1A binds to and modulates several ion channels including CFTR, voltage-gated calcium channels, and epithelial sodium channels (13–19). Syntaxin 1A inhibits CFTR-mediated chloride currents in heterologous expression systems (7, 8). Reagents that block the physical interaction between syntaxin 1A and the amino-terminal tail of CFTR potentiate CFTR-mediated currents in epithelial cells. The kinetic properties of voltage-gated calcium channels are modified by coexpression with syntaxin 1A or SNAP-25 in Xenopus oocytes or HEK293 cells (14, 15, 18, 20). For example, Zhong et al. (18) reported that SNAP-25 inhibited the activity of P/Q-type Ca2+ channels by shifting the voltage dependence of inactivation to more negative potentials. Coexpressing syntaxin 1A with SNAP-25 partially restored the normal voltage dependence of inactivation. Zhong et al. (18) interpreted these results to indicate that individual t-SNAREs bind to and inhibit voltage-gated calcium channels possibly as a means to couple calcium channels to the vesicle fusion machinery at sites of neurotransmitter release. The fact that syntaxin 1A reversed the effects of SNAP-25 on the gating of P/Q-type calcium channels implies that individual t-SNAREs rather than the t-SNARE heterodimer are more potent regulators of this ion channel.
Here we determined whether CFTR channels are regulated by a t-SNARE complex in epithelial cells, or alternatively, if this ion channel is reciprocally regulated by individual t-SNAREs as argued for P/Q-type calcium channels. We focused on SNAP-23, a SNAP-25 homologue, as a potential regulator of CFTR because: (i) this t-SNARE is expressed in many tissues including lung, intestine, and pancreas, tissues that also express CFTR (21, 22) and (ii) SNAP-23 binds to syntaxin 1A in vitro (21) and can be cross-linked to syntaxin 1A in epithelial cells in vivo (unpublished results). Our results indicate that epithelial CFTR channels are regulated by a t-SNARE complex composed of both SNAP-23 and syntaxin 1A. The interaction of CFTR with a putative intermediate in SNARE complex assembly may link its activity to protein secretion in epithelial tissues.
Materials and Methods
Cell Culture and Transfections.
HT29-Cl.19A colonic epithelial cells and Cos-7 fibroblasts were cultured as described (7). L cells stably expressing human CFTR (23) were transfected with full-length human syntaxin 1A in pCDNA3 or mock-transfected (vector alone), and clones were selected in geneticin (GIBCO/BRL). One clone of each was analyzed by patch clamping and immunoprecipitation. CFTR, SNAP-23, and syntaxin 1A were transiently expressed in Cos-7 cells with a vaccinia-based expression system. Cells were infected for 30 min with vaccinia virus containing T7 polymerase at a multiplicity of infection of 10. Then 2–5 μg of DNA (pCDNA3 with T7 promoter) was mixed with 8–20 μg of 1,2-dioleyl-phosphatidyl ethanolamine (DOPE)/N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate (DOTAP) (DNA/DOPE–DOTAP, 1:4) in Opti-MEM (GIBCO/BRL) for 20 min and added to the cells. The cells were lysed 24–36 h later in 0.2% Triton X-100–PBS containing 2 μg/ml of leupeptin, aprotinin, and pepstatin A (Sigma), and the lysates were used for pull-down assays.
Coimmunoprecipitation Experiments.
HT29-Cl.19A and L cells were lysed in 1% Triton X-100–PBS. CFTR was immunoprecipitated by using 2 μg of a COOH-terminal mAb (C-CFTR mAb, which recognizes amino acids 1477–1480; R & D Systems 24–1) or an equivalent amount of nonimmune IgG cross-linked to protein A/G agarose beads. After incubating the lysates with cross-linked antibody at 4°C overnight or for 2 h for HT29-Cl.19A or L cells, respectively, the beads were pelleted and washed three times in 1% Triton X-100–PBS, and the samples were processed for Western blotting. The CFTR antibody used for Western blotting was a mouse mAb (GA1) raised against recombinant C-terminal tail of CFTR (amino acids 1382–1480) made by John Kearney and David Nelson at the University of Alabama, Birmingham. The SNAP-23 antibody was a rabbit polyclonal antibody raised against a synthetic peptide representing the last 14 aa of the human SNAP-23 protein (produced for us by Genemed Synthesis, South San Francisco, CA). Anti-SNAP-23 IgG was purified from serum on protein A agarose.
Direct Binding Assays and Pull-Down Assays.
These assays were performed as described (7, 8). For the antibody neutralization experiments, maltose binding protein (MBP)–SNAP-23 was preincubated with anti-SNAP-23 Fab fragment (produced by papain cleavage of anti-SNAP-23 IgG) for 15 min at room temperature. The binding assay was then performed with the GST–N-tail fusion protein for another 15 min after which excess glutathione beads were added for 1 h. The beads were then pelleted and washed extensively, and bound proteins were eluted and analyzed by immunoblotting as described (7).
Immunofluorescence.
HT29-Cl.19A cells were seeded on 24-mm filters (polyethylene terephtalate track-etched membrane, Becton Dickinson). After 10 days in culture when the cells develop a transepithelial resistance of at least 1,000 Ω/cm2, the cells were briefly rinsed in PBS and fixed in methanol for 30 min at −20°C. Then after blocking for 30 min with 1% BSA in PBS, the cells were incubated with anti-SNAP-23 IgG or preimmune IgG at 20 μg/ml for 1 h at 37°C. After several washings, the cells were exposed to secondary antibody at 40 μg/ml (Texas red goat anti-rabbit IgG). The monolayers were mounted and viewed in cross section by folding the filters as described (24).
Electrophysiology.
Whole-cell recordings were performed on HT29-Cl.19A cells and L cells by using methods and solutions as described (7, 25). Peptides and antibodies were introduced into the cells via the patch pipettes. CFTR was activated with 400 μM cpt-cAMP, 10 μM forskolin, and 1 mM 3-isobutyl-1-methylxanthine within 10 min of breaking into the cell. Simultaneous recordings of membrane capacitance (Cm) and conductance (Gm) were done as described (25). The holding potential in the capacitance experiments was −10 mV. All experiments were conducted at room temperature (22–24°C). Statistical significance of results was determined by using Student's t test.
Results
SNAP-23 Is Expressed in HT29-Cl.19A Colonic Epithelial Cells and Physically Interacts with CFTR.
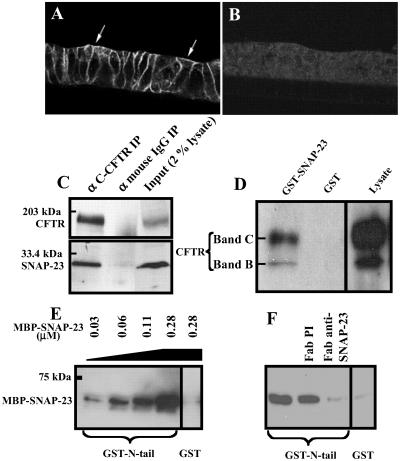
Pilot immunolocalization experiments and coimmunoprecipitation studies were performed to test for interactions between SNAP-23 and the apical CFTR channel. Fig. 1 A and B shows that SNAP-23 is distributed in part at the apical surface as well as at the lateral membranes in polarized monolayers of HT29-Cl.19A human colonic epithelial cells. This localization is similar to that reported for Madin–Darby canine kidney epithelial cells (26). In coimmunoprecipitation experiments SNAP-23 could be specifically and reproducibly precipitated from HT29-Cl.19A cell extracts by using two different CFTR antibodies including a mAb that recognizes the CFTR COOH-terminal tail (Fig. 1C). No SNAP-23 signal was detected with control mouse IgG. Attempts to perform reciprocal coimmunoprecipitations with two available antibodies raised against the COOH terminal region of SNAP-23 failed to precipitate CFTR (results not shown), apparently because this region of SNAP-23 participates in the CFTR interaction (see below). However, CFTR protein could be “pulled down” from extracts of Cos-7 cells expressing CFTR by using a GST–SNAP-23 fusion protein (Fig. 1D). The pull-down of CFTR by GST–SNAP-23 was specific (i.e., GST alone was negative) but inefficient. As will be shown below, the low efficiency is caused by the fact that, unlike HT29-Cl.19A cells, Cos-7 fibroblasts do not normally express syntaxin 1A. Syntaxin 1A substantially enhances the interaction between CFTR and SNAP-23 (see Fig. 3).
Fig 1.
SNAP-23 is present at the apical membranes of HT29-Cl.19A colonic epithelial cells and physically interacts with CFTR. (A) Immunofluorescence localization of SNAP-23 in HT29-Cl.19A cells. Arrows indicate the apical membrane. (B) Preimmune control. (C) Coimmunoprecipitation (IP) of SNAP-23 with CFTR from HT29-Cl.19A epithelial cells using C-CFTR mAb or an equivalent amount of nonimmune IgG. Immunoprecipitates were blotted with CFTR GA1 mAb or SNAP-23 polyclonal antibody. The right lane shows a Western blot of 2% of the lysate used for the immunoprecipitations. The SNAP-23 antibody recognized only a 25- to 30-kDa band in the cell lysates characteristic of SNAP-23 protein. (D) Pull-down of CFTR with GST–SNAP-23. CFTR was expressed in Cos-7 cells (see Materials and Methods). GST–SNAP-23 or GST (100 μg) was used to pull down CFTR from 1.2 ml of Cos-7 cell lysate. Twenty microliters lysate was loaded in the right lane. The blot was probed by using GA1 antibody. Bands B and C represent immature and mature CFTR, respectively. (E) SNAP-23 binding to the amino terminal tail (N-tail) of CFTR. GST–N-tail (amino acids 1–75) or GST (each at 0.15 μM) was used to pull down MBP–SNAP-23 at the indicated concentrations. (F) Binding of MBP–SNAP-23 (0.28 μM) to GST–N-tail (0.15 μM) in the presence of anti-SNAP-23 Fab fragment or preimmune Fab (each at 0.4 μM or 10 μg/ml). All experiments shown were repeated at least three times with similar results.
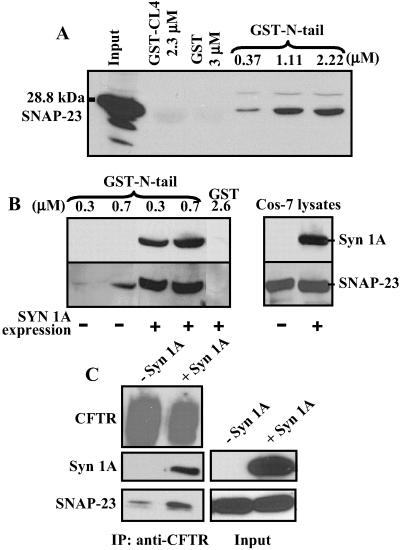
Fig 3.
SNAP-23 binding to CFTR is potentiated by syntaxin 1A. (A) SNAP-23 was pulled down from Cos-7 lysates with indicated amounts of GST–N-tail and detected by immunoblotting. Negative controls were GST alone and GST-CL4 (cytosolic loop 4 of CFTR: amino acids 1034–1103). (B) Binding of SNAP-23 to the CFTR N-tail is substantially enhanced by syntaxin 1A. Shown are the results of pull-down experiments in which different concentrations of GST–N-tail were added to lysates of Cos-7 cells that were expressing SNAP-23 with or without syntaxin 1A. GST alone served as a negative control (results repeated six times). (C) Syntaxin 1A expression enhances the physical association of full-length CFTR and SNAP-23 detected by coimmunoprecipitation (IP) in mouse L fibroblasts. CFTR was immunoprecipitated with C-CFTR mAb and the immunoprecipitates were probed for SNAP-23, syntaxin 1A, and CFTR by immunoblotting (results repeated three times). The CFTR signal was overexposed.
We next tested for binding between SNAP-23 and the CFTR amino-terminal tail (N-tail), because this tail modulates CFTR channel gating (5, 6) and can bind to syntaxin 1A in vitro (8). Purified recombinant SNAP-23 fused to MBP-SNAP-23 bound specifically to a GST fusion protein containing the CFTR N-tail (GST–N-tail) in direct pairwise binding assays (Fig. 1E). The binding of SNAP-23 to the CFTR N-tail was blocked by an Fab fragment of a SNAP-23 COOH-terminal antibody (Fig. 1F). This antibody did not inhibit SNAP-23 binding to syntaxin 1A in similar pairwise binding assays (results not shown). In summary, with three different assays we could detect a physical interaction between SNAP-23 and CFTR that maps in part to the CFTR N-tail.
SNAP-23 Regulates CFTR-Mediated Chloride Currents in HT29-Cl.19A Epithelial Cells.
The effects of SNAP-23 on CFTR-mediated chloride currents were assessed in whole-cell patch-clamp studies of HT29-Cl.19A epithelial cells (Fig. 2). CFTR-mediated currents were operationally defined on the basis of the following criteria: activation by cAMP, time independence throughout the voltage activation range, current–voltage behavior consistent with Goldman rectification, insensitivity to 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), and block by diphenylamine-2,2′-dicarboxylic acid (DPC) (7, 27, 28). CFTR-mediated currents (i.e., cAMP-activated currents) were reduced by 70–80% when GST-SNAP-23 (0.8 μM) was included in the patch pipette. This inhibitory effect was specific in that neither GST alone (results not shown and refs. 7 and 27) nor a GST fusion protein containing the cytoplasmic domain (amino acids 1–95) of vesicle-associated membrane protein-2 (a vesicle-associated SNARE) affected CFTR-mediated currents (Fig. 2A). In addition, SNAP-23 protein had no effect on basal currents recorded in the absence of cAMP.
Fig 2.
SNAP-23 regulates CFTR-mediated chloride currents in HT29-Cl.19A epithelial cells. Whole-cell patch-clamp experiments and capacitance measurements were performed as described in Materials and Methods. (A) Inhibition of cAMP-dependent currents by GST–SNAP-23 (40 μg/ml or 0.8 μM) but not by GST–vesicle-associated membrane protein-2 (VAMP-2) (40 μg/ml or 1.1 μM). (B) Anti-SNAP-23 IgG or Fab fragment (20 μg/ml) increased cAMP-activated currents whereas preimmune IgG (20 μg/ml) had no effect. Note change in scale compared with A. Currents were recorded at +110 mV. (Inset) Current–voltage relationship for the Fab-potentiated currents in the presence and absence of 1 mM DPC. Data are expressed as mean ± SEM. Asterisk (*) indicates P < 0.05 relative to untreated control. (C) Whole-cell currents in the presence of anti-SNAP-23 IgG (20 μg/ml) showing time independence, DIDS insensitivity, and DPC sensitivity characteristic of CFTR-mediated currents. (D) Representative simultaneous recordings of cell capacitance (Cm) and membrane conductance (Gm). Each pair of traces derives from an individual HT29-Cl.19A cell. The anti-pan dynamin IgG (30) antibody and anti-SNAP-23 IgG were added at 100 and 20 μg/ml, respectively. (E) Mean changes in Cm. (F) Mean changes in Gm.
In contrast to the inhibitory effects of exogenous SNAP-23 on CFTR-mediated currents, a SNAP-23 COOH-terminal antibody markedly potentiated cAMP-dependent currents in HT29-Cl.19A cells (Fig. 2B). This stimulation was observed both for affinity-purified IgG and an Fab fragment. Preimmune IgG had no effect. The stimulated currents were mediated by CFTR on the basis of the forementioned criteria (Fig. 2 B Inset and C). Because the anti-SNAP-23 Fab inhibits the physical interaction between SNAP-23 and the CFTR N-tail (Fig. 1F), we interpret these data to indicate that endogenous SNAP-23 inhibits CFTR activity in epithelial cells and that CFTR can be rescued from this inhibition by an antibody that blocks this interaction. Native SNAP-23 appears to be partially limiting for the CFTR interaction as evidenced by the further inhibition observed upon the inclusion of exogenous SNAP-23 protein in the patch pipette (Fig. 2A).
Because SNAP-23 is a t-SNARE that can also modulate membrane traffic in cells (29), its effects on CFTR-mediated currents could be caused by generic effects on vesicle traffic with consequent alterations in the numbers of CFTR channels at the cell surface. Accordingly, we assayed the effects of the anti-SNAP-23 Fab fragment on cell capacitance (Cm) as a measure of membrane surface area (i.e., net endocytosis/exocytosis) (30) to determine whether this reagent dramatically altered vesicle traffic in HT29-Cl.19A colonic epithelial cells. We observed no significant effect of this antibody on Cm under the same conditions in which it potentiated CFTR currents and increased cell membrane conductance (Gm) (Fig. 2 D–F). As a positive control we observed that a dynamin antibody that blocks endocytosis (30) did markedly increase Cm when included in the patch pipette, which confirms that this method can detect alterations in net endocytosis/exocytosis in these cells. Syntaxin 1A peptides and the syntaxin binding protein Munc-18 also stimulate CFTR currents without affecting Cm (25). These findings are consistent with the idea that SNAP-23 regulates CFTR via specific protein–protein interactions rather than via global effects on membrane traffic.
Syntaxin 1A Potentiates the Physical Interaction of SNAP-23 with CFTR.
Given that SNAP-23 physically and functionally interacts with CFTR, we tested the dependence of the SNAP-23 interaction on its partner t-SNARE, syntaxin 1A. Toward this end we exploited the fact that fibroblasts do not normally express syntaxin 1A. Fibroblasts do express other syntaxins but of those tested only syntaxin 1A physically and functionally interacts with CFTR (27). Recombinant SNAP-23 was expressed in Cos-7 fibroblasts that lack syntaxin 1A, and GST fusion proteins were examined for their abilities to pull down SNAP-23. Fig. 3A shows that SNAP-23 could be pulled down inefficiently from Cos-7 cell lysates by GST–N-tail (amino acids 1–75) in a dose-dependent manner but not by control GST fusion proteins. Cos-7 cells were then transfected with recombinant syntaxin 1A to determine whether syntaxin 1A influences the SNAP-23 interaction with the CFTR N-tail (Fig. 3B). The efficiency of capturing SNAP-23 with GST–N-tail was markedly improved in the presence of full-length syntaxin 1A, which also bound to the CFTR N-tail in these pull-down assays. This enhancement of the SNAP-23/N-tail binding interaction by syntaxin 1A occurred with no change in the total amount of SNAP-23 in the cell extracts (Fig. 3B Right).
To confirm this result we performed coimmunoprecipitation experiments in mouse L fibroblasts that were stably transfected with recombinant CFTR plus or minus recombinant syntaxin 1A (Fig. 3C). Again, we observed a weak interaction between SNAP-23 and CFTR in cells lacking syntaxin 1A and a stronger interaction in the presence of syntaxin 1A. Parallel experiments performed with nonimmune mouse IgG instead of the CFTR mAb verified that the SNAP-23 signal in both immunoprecipitations was specific, as we had observed for HT29-Cl.19A epithelial cells (Fig. 1C and results not shown). Both t-SNAREs coimmunoprecipitated with CFTR in L fibroblasts that expressed syntaxin 1A (Fig. 3C). These results indicate that CFTR channels interact more robustly with the t-SNARE complex than with SNAP-23 alone.
The Regulation of CFTR Activity by SNAP-23 Requires the Presence of Syntaxin 1A.
The fact that CFTR physically associates with the SNAP-23/syntaxin 1A complex raised the possibility that SNAP-23 regulates CFTR activity via its interaction with syntaxin 1A. To test this possibility whole-cell patch-clamp experiments were performed with the L fibroblasts that were stably transfected with CFTR plus or minus full-length syntaxin 1A. To validate the utility of this expression system we first confirmed that CFTR-mediated currents were reduced in the syntaxin 1A-expressing cells versus mock-transfected cells (Table 1), similar to that observed when CFTR is coexpressed with full-length syntaxin 1A in Xenopus oocytes (8, 27). Also, the introduction of soluble syntaxin 1A cytosolic domain lacking the membrane anchor increased the cAMP-activated currents in the syntaxin 1A-expressing cells but not in the cells lacking full-length syntaxin 1A (Table 1). As previously described, CFTR currents are inhibited only by the membrane-anchored form of syntaxin 1A and can be rescued from this inhibition by soluble syntaxin 1A peptides that contain the SNARE motif, or H3 domain (8). In addition, CFTR could be rescued from syntaxin 1A inhibition by the inclusion of GST–N-tail at 80 μg/ml in the patch pipette, as previously observed (8, 27), caused presumably by competition between GST–N-tail and CFTR for syntaxin 1A binding.
Table 1.
cAMP-activated currents (pA/pF) in L fibroblasts stably transfected with CFTR plus or minus syntaxin 1A
| Condition | − SYN 1A | + SYN 1A |
|---|---|---|
| Control | 37.4 ± 3.6 (5) | 18.0 ± 1.9 (7) |
| GST (80 μg/ml) | 33.1 ± 0.7 (3) | 19.3 ± 1.7 (4) |
| GST–Syn 1A (20 μg/ml) | 35.0 ± 3.3 (5) | 37.6 ± 3.5 (3) |
| GST–N-tail (20 μg/ml) | 32.6 ± 1.8 (5) | 21.1 ± 1.1 (4) |
| GST–N-tail (80 μg/ml) | 34.5 ± 5.6 (3) | 29.5 ± 2.2 (3) |
Currents were activated as described in Fig. 2 and measured in the presence of indicated GST fusion proteins in the patch pipette. GST–Syn 1A represents the cytosolic domain of syntaxin 1A (amino acids 1–266 fused to GST). Data are expressed as mean ± SEM. Asterisk (
) indicates P < 0.05 compared to control.
Whereas SNAP-23 protein and SNAP-23 antibody had marked and opposite effects on CFTR-mediated currents in HT29-Cl.19A epithelial cells (Fig. 2), neither reagent affected CFTR activity in L fibroblasts lacking syntaxin 1A (Fig. 4 A and B). However, the regulation of CFTR-mediated currents by SNAP-23 was restored by syntaxin 1A expression in these cells. The inclusion of GST–SNAP-23 in the patch pipette significantly decreased cAMP-activated currents but only in the syntaxin 1A-expressing cells. (Unlike syntaxin 1A, SNAP-23 is not an integral membrane protein and can be introduced into the cell in its entirety as a soluble protein.) To verify that the inhibitory effect of SNAP-23 protein was mediated through its interaction with the CFTR N-tail, SNAP-23 was premixed with GST–N-tail at a concentration at which the N-tail peptide had no effect on CFTR currents by itself (20 μg/ml; Table 1). The N-tail fusion protein partially neutralized the inhibitory effect of the SNAP-23 protein on CFTR currents (Fig. 4A). The introduction of anti-SNAP-23 (whole IgG or Fab fragment) increased significantly the cAMP-activated currents but, again, only in the syntaxin 1A-expressing cells (Fig. 4B). The antibody-stimulated currents were mediated by CFTR on the basis of the criteria mentioned above (time independence, block by DPC but not DIDS; Fig. 4 C and D). In summary, our functional data indicate that SNAP-23 regulates CFTR by associating with the amino-terminal tail of this ion channel, and that this regulation absolutely depends on the presence of its cognate t-SNARE, syntaxin 1A.
Fig 4.
CFTR regulation by SNAP-23 requires syntaxin 1A. Whole-cell patch-clamp experiments were performed on L fibroblasts expressing CFTR plus or minus syntaxin 1A. (A) Recombinant SNAP-23 (40 μg/ml) inhibited cAMP-activated currents only in syntaxin 1A-expressing cells. Premixing GST–SNAP-23 (40 μg/ml or 0.8 μM) with GST–N-tail (20 μg/ml or 0.6 μM) partially neutralized the inhibition of currents by SNAP-23. (B) Anti-SNAP-23 IgG or Fab fragment (20 μg/ml) significantly increased cAMP-activated currents only in syntaxin 1A-expressing cells. (C and D) Representative whole-cell currents and I–V curves without and with anti-SNAP-23 Fab (20 μg/ml) showing that the Fab-potentiated currents in the syntaxin 1A-expressing cells exhibit the features of CFTR-mediated currents: DIDS insensitive, DPC sensitive, and time independent. Data are expressed as mean ± SEM. Asterisk (*) indicates P < 0.05 relative to untreated control.
Discussion
Our results indicate that CFTR channels are regulated by a t-SNARE complex that includes SNAP-23 as a main component. SNAP-23 associates with the amino-terminal tail of CFTR, a region that modulates channel gating (6) and that also binds syntaxin 1A (8). CFTR currents were inhibited and stimulated by SNAP-23 protein and antibody, respectively, but only in cells that express syntaxin 1A. Thus, SNAP-23 by itself cannot regulate CFTR activity despite the fact that it can associate weakly with the channel in the absence of syntaxin 1A. At present we do not know whether the inhibition of CFTR activity by syntaxin 1A that we previously observed depends on SNAP-23, because this broadly expressed t-SNARE is likely present in all cell types in which we have studied the CFTR–syntaxin 1A interaction. The fact that addition of exogenous SNAP-23 further decreases CFTR activity in epithelial cells implies that these two t-SNAREs have additive (or possibly cooperative) effects on CFTR function.
SNAP-23 could regulate CFTR function by controlling the numbers of channels at the cell surface, the functional properties of individual channels, or both. A direct effect on the single channel properties of CFTR would be consistent with evidence that the N-tail, to which SNAP-23 and syntaxin 1A bind, modulates channel gating (5, 6). Also, the fact that we observed no effect of the SNAP-23 antibody on cell capacitance argues against a general effect of this reagent on membrane turnover in HT29-Cl.19A cells. Chang et al. (25) have shown that syntaxin 1A peptides and Munc-18 (a syntaxin binding protein) (7) also stimulate CFTR currents without affecting cell capacitance or FM1–43 exocytosis in epithelial cells, and have reported single-channel data that indicate that syntaxin 1A peptides can modulate CFTR gating in excised membrane patches. However, the latter single-channel experiments are difficult to perform and interpret because of considerable channel-to-channel variability in the peptide effects, which is caused conceivably by the dynamic nature of these interactions. Also, we cannot rule out the possibility that SNAP-23 and syntaxin 1A more specifically modulate the trafficking of a limited number of CFTR-containing vesicles whose fusion with the plasma membrane (or internalization from the cell surface) is below the detection limit of capacitance measurements. In this regard, Peters et al. (31) have argued that cAMP increases the numbers of CFTR channels at the plasma membrane of Xenopus oocytes, and that this effect is inhibited by coexpression with syntaxin 1A. The concept that cAMP increases CFTR channel number at the cell surface in oocytes or epithelial cells is controversial with several laboratories having reported no effect of cAMP on this parameter (32, 33). In our view further studies will be required to definitively establish whether syntaxin and SNAP-23 modulate CFTR activity primarily by altering channel gating, channel traffic, or both.
Related to this point is the fact that we observed no effect of an anti-pan dynamin antibody that blocks endocytosis on CFTR-mediated currents in HT29-Cl.19A cells. This antibody also had no effect on CFTR-mediated currents in 16HBE14o- airway cells (25). It has been argued that CFTR channels can recycle between the cell surface and endosomes (34), although there is no evidence that dynamin participates in this process. If CFTR channels do appreciably recycle in epithelial cells, they would appear to do so via a pathway that does not involve conventional dynamin isoforms. Otherwise, one might have expected an increase in CFTR-mediated currents subsequent to the block of endocytosis by the dynamin antibody.
The coordinate regulation of CFTR channels by two interacting t-SNAREs seems different from the situation for P/Q-type calcium channels reported by Zhong et al. (18). They reported that an individual t-SNARE (SNAP-25) could affect the gating of P/Q-type channels, but that the coexpression of syntaxin 1A with SNAP-25 partially reversed this effect. This observation suggests that t-SNAREs reciprocally regulate rather than cooperatively modulate the gating of P/Q-type calcium channels. Irrespective of this apparent difference, SNARE interactions with either channel may help tune its activity to SNARE complex assembly and membrane traffic. CFTR mediates the secretion of salt and fluid in glands and intestinal crypts (2), which probably aids in the delivery of simultaneously secreted proteins to the mucosal surface (35). Because t-SNAREs form heterodimers that may function as intermediates in membrane fusion reactions (11, 12), the association of CFTR with a t-SNARE complex may link its channel activity to rates of protein secretion. Other functional roles are also possible; e.g., CFTR might regulate SNARE function and macromolecule secretion by modulating SNARE complex assembly in certain membrane microdomains. In this regard, CFTR has been reported to regulate the secretion of glycoconjugates by airway epithelial cells (36). It also seems prudent to consider the possibility that syntaxin 1A or SNAP-23/25 could be general regulators of ion transport independently of their participation in membrane traffic, because these SNAREs have been reported to interact with a number of ion channels (13–16, 18). Defining the physiologic role of these interactions would be helped by the identification of conditions or factors that regulate the association of t-SNAREs with these channels.
Acknowledgments
We thank John F. Kearney and David Nelson for producing the GA1 CFTR mAb and Emily Hodges and Holly Gentry for excellent technical assistance. This work was supported by National Institutes of Health Grants HL58341 and DK56796.
Abbreviations
CFTR, cystic fibrosis transmembrane conductance regulator
DIDS, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid
DPC, diphenylamine 2,2′-dicarboxylic acid
MBP, maltose binding protein
SNAP-23/25, synaptosome-associated protein of 23/25 kDa, respectively
SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor
t-SNARE, target-SNARE
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Denning G. M., Ostedgaard, L. S., Cheng, S. H., Smith, A. E. & Welsh, M. J. (1992) J. Clin. Invest. 89, 339-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabriel S. E., Brigman, K. N., Koller, B. H., Boucher, R. C. & Stutts, M. J. (1994) Science 266, 107-109. [DOI] [PubMed] [Google Scholar]
- 3.Welsh M. J. & Smith, A. E. (1993) Cell 73, 1251-1254. [DOI] [PubMed] [Google Scholar]
- 4.Riordan J. R., Rommens, J. M., Kerem, B., Alon, N., Rozmahel, R., Grzelczak, Z., Zielenski, J., Lok, S., Plavsic, N., Chou, J. L., et al. (1989) Science 245, 1066-1073. [DOI] [PubMed] [Google Scholar]
- 5.Naren A. P., Cormet-Boyaka, E., Fu, J., Villain, M., Blalock, J. E., Quick, M. W. & Kirk, K. L. (1999) Science 286, 544-548. [DOI] [PubMed] [Google Scholar]
- 6.Fu J. & Kirk, K. L. (2001) J. Biol. Chem. 276, 35660-35668. [DOI] [PubMed] [Google Scholar]
- 7.Naren A. P., Nelson, D. J., Xie, W., Jovov, B., Pevsner, J., Bennett, M. K., Benos, D. J., Quick, M. W. & Kirk, K. L. (1997) Nature (London) 390, 302-305. [DOI] [PubMed] [Google Scholar]
- 8.Naren A. P., Quick, M. W., Collawn, J. F., Nelson, D. J. & Kirk, K. L. (1998) Proc. Natl. Acad. Sci. USA 95, 10972-10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothman J. E. (1994) Nature (London) 372, 55-63. [DOI] [PubMed] [Google Scholar]
- 10.Sutton R. B., Fasshauer, D., Jahn, R. & Brunger, A. T. (1998) Nature (London) 395, 347-353. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson K. L., Munson, M., Miller, R. B., Filip, T. J., Fairman, R. & Hughson, F. M. (1998) Nat. Struct. Biol. 5, 793-802. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence G. W. & Dolly, J. O. (2002) J. Cell Sci. 115, 667-673. [DOI] [PubMed] [Google Scholar]
- 13.Bezprozvanny I., Scheller, R. H. & Tsien, R. W. (1995) Nature (London) 378, 623-626. [DOI] [PubMed] [Google Scholar]
- 14.Rettig J., Sheng, Z. H., Kim, D. K., Hodson, C. D., Snutch, T. P. & Catterall, W. A. (1996) Proc. Natl. Acad. Sci. USA 93, 7363-7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiser O., Bennett, M. K. & Atlas, D. (1996) EMBO J. 15, 4100-4110. [PMC free article] [PubMed] [Google Scholar]
- 16.Qi J., Peters, K. W., Liu, C., Wang, J. M., Edinger, R. S., Johnson, J. P., Watkins, S. C. & Frizzell, R. A. (1999) J. Biol. Chem. 274, 30345-30348. [DOI] [PubMed] [Google Scholar]
- 17.Saxena S., Quick, M. W., Tousson, A., Oh, Y. & Warnock, D. G. (1999) J. Biol. Chem. 274, 20812-20817. [DOI] [PubMed] [Google Scholar]
- 18.Zhong H., Yokoyama, C. T., Scheuer, T. & Catterall, W. A. (1999) Nat. Neurosci. 2, 939-941. [DOI] [PubMed] [Google Scholar]
- 19.Fili O., Michaelevski, I., Bledi, Y., Chikvashvili, D., Singer-Lahat, D., Boshwitz, H., Linial, M. & Lotan, I. (2001) J. Neurosci. 15, 1964-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutton K. G., McRory, J. E., Guthrie, H., Murphy, T. H. & Snutch, T. P. (1999) Nature (London) 401, 800-804. [DOI] [PubMed] [Google Scholar]
- 21.Ravichandran V., Chawla, A. & Roche, P. A. (1996) J. Biol. Chem. 271, 13300-13303. [DOI] [PubMed] [Google Scholar]
- 22.Wong P. P., Daneman, N., Volchuk, A., Lassam, N., Wilson, M. C., Klip, A. & Trimble, W. S. (1997) Biochem. Biophys. Res. Commun. 230, 64-68. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y., Devor, D. C., Engelhardt, J. F., Ernst, S. A., Strong, T. V., Collins, F. S., Cohn, J. A., Frizzell, R. A. & Wilson, J. M. (1993) Hum. Mol. Genet. 2, 1253-1261. [DOI] [PubMed] [Google Scholar]
- 24.Tousson A., Alley, C. D., Sorscher, E. J., Brinkley, B. R. & Benos, D. J. (1989) J. Cell Sci. 93, 349-362. [DOI] [PubMed] [Google Scholar]
- 25.Chang S. Y., Di, A., Naren, A. P., Palfrey, H. C., Kirk, K. L. & Nelson, D. J. (2002) J. Cell Sci. 115, 783-791. [DOI] [PubMed] [Google Scholar]
- 26.Low S. H., Roche, P. A., Anderson, H. A., van Ijzendoorn, S. C., Zhang, M., Mostov, K. E. & Weimbs, T. (1998) J. Biol. Chem. 273, 3422-3430. [DOI] [PubMed] [Google Scholar]
- 27.Naren A. P., Di, A., Cormet-Boyaka, E., Boyaka, P. N., McGhee, J. R., Zhou, W., Akagawa, K., Fujiwara, T., Thome, U., Engelhardt, J. F., et al. (2000) J. Clin. Invest. 105, 377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheppard D. N. & Welsh, M. J. (1999) Physiol. Rev. 79, S23-S45. [DOI] [PubMed] [Google Scholar]
- 29.Cheatham B., Volchuk, A., Kahn, C. R., Wang, L., Rhodes, C. J. & Klip, A. (1996) Proc. Natl. Acad. Sci. USA 93, 15169-15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elhamdani A., Brown, M. E., Artalejo, C. R. & Palfrey, H. C. (2000) J. Neurosci. 20, 2495-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters K. W., Qi, J., Watkins, S. C. & Frizzell, R. A. (1999) Am. J. Physiol. 277, C174-C180. [DOI] [PubMed] [Google Scholar]
- 32.Liu X., Smith, S. S., Sun, F. & Dawson, D. C. (2001) J. Gen. Physiol. 118, 433-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng P., Hwang, T. C. & Gillis, K. D. (2001) J. Gen. Physiol. 118, 135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prince L. S., Workman, R. B., Jr. & Marchase, R. B. (1994) Proc. Natl. Acad. Sci. USA 91, 5192-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jilling T. & Kirk, K. L. (1996) J. Biol. Chem. 271, 4381-4387. [DOI] [PubMed] [Google Scholar]
- 36.Mergey M., Lemnaouar, M., Veissiere, D., Perricaudet, M., Gruenert, D. C., Picard, J., Capeau, J., Brahimi-Horn, M. C. & Paul, A. (1995) Am. J. Physiol. 269, L855-L864. [DOI] [PubMed] [Google Scholar]