Abstract
1,25-Dihydroxyvitamin D3 [1,25(OH)2D3] is a principal regulator of calcium and phosphorus homeostasis through actions on intestine, kidney, and bone. 1,25(OH)2D3 is not considered to play a significant role in bone formation, except for its role in supporting mineralization. We report here on the properties of 2-methylene-19-nor-(20S)-1α,25(OH)2D3 (2MD), a highly potent analog of 1,25(OH)2D3 that induces bone formation both in vitro and in vivo. Selectivity for bone was first demonstrated through the observation that 2MD is at least 30-fold more effective than 1,25(OH)2D3 in stimulating osteoblast-mediated bone calcium mobilization while being only slightly more potent in supporting intestinal calcium transport. 2MD is also highly potent in promoting osteoblast-mediated osteoclast formation in vitro, a process essential to both bone resorption and formation. Most significantly, 2MD at concentrations as low as 10−12 M causes primary cultures of osteoblasts to produce bone in vitro. This effect is not found with 1,25(OH)2D3 even at 10−8 M, suggesting that 2MD might be osteogenic in vivo. Indeed, 2MD (7 pmol/day) causes a substantial increase (9%) in total body bone mass in ovariectomized rats over a 23-week period. 1,25(OH)2D3 (500 pmol three times a week) only prevented the bone loss associated with ovariectomy and did not increase bone mass. These results indicate that 2MD is a potent bone-selective analog of 1,25(OH)2D3 potentially effective in treating bone loss diseases.
Vitamin D-deficiency diseases such as rickets and osteomalacia represent a failure in bone formation primarily caused by a failure of mineralization (1–3). Indeed, early investigations into the underlying defect associated with rickets revealed that incubation of bone slices in media containing physiologic levels of calcium and phosphorus resulted in normal mineralization of rachitic matrix (3). Underwood and DeLuca (4) subsequently demonstrated that the mineralization defect associated with vitamin D deficiency is due directly to insufficient calcium and phosphorus in plasma at sites of mineralization. These early conclusions have been supported independently by recent studies that show that skeletal disease, which arises from deletion of the vitamin D receptor (VDR), the exclusive transcriptional mediator of vitamin D action in vivo, can be normalized in mice through lactose-containing diets high in calcium and phosphorus (5). Thus, there is little evidence that vitamin D plays a direct role in new bone formation apart from its action to maintain calcium and phosphorus levels in the blood.
We recently discovered a class of vitamin D compounds modified at the 2-carbon position of the A ring that is highly potent. The introduction of a methylene group at carbon 2, removal of a methylene group at carbon 10, and alteration of the stereochemistry of the 20-carbon to yield the 20S-derivative resulted in 2-methylene-19-nor-(20S)-1α,25(OH)2D3 (2MD), a compound that exhibits an affinity for the VDR equal to that of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (6). This analog is highly potent in inducing human HL-60 cell differentiation in vitro, and in vivo it seems to exhibit a preferential activity on bone relative to the intestine (6). In this article, we examine the actions of 2MD on bone formation both in vitro and in vivo and contrast these actions with those of 1,25(OH)2D3.
Materials and Methods
Compounds.
2MD and 1,25(OH)2D3 were synthesized by Tetrionics (Madison, WI) by methods as described (6, 7).
Bone Calcium Mobilization and Intestinal Calcium Transport Studies in Vivo.
Weanling male rats were obtained from the low-vitamin D colony of Harlan–Sprague–Dawley and fed a purified 0.47% calcium, 0.3% phosphorus vitamin D-deficient diet as described by Suda et al. (8) ad libitum for 1 week and then the same diet containing 0.02% calcium and 0.3% phosphorus diet for 3 weeks. During the final week, the indicated doses of compound were delivered in 0.1 ml propylene glycol/ethanol (95:5) i.p. each day for 7 days. Control animals received vehicle. Animals were housed in overhanging wire cages in a vivarium provided with a 12-h light/12-h dark cycle. The fluorescent lighting was shielded to block UV light. Twenty-four hours after the final dose, animals were killed by decapitation, and blood was taken to determine serum calcium levels. The first 10 cm of duodenum was used to determine intestinal calcium transport by the everted sac technique as described (9). Each group contained at least five animals, and the values represent the mean ± SEM.
RNA Analysis.
Murine osteoblastic ST2 cells were cultured in αMEM (Earle's medium) supplemented with 10% FBS. Cells were plated at densities of 5 × 105/ml in 100-mm dishes and treated for 24 h with the indicated concentrations of dexamethasone, 1,25(OH)2D3, 2MD, or the indicated combinations. Total RNA was isolated by using Trizol reagent, and 20 μg was examined by Northern blot analysis. cDNA probes were end-labeled and used to detect receptor activator of NFκB ligand (RANKL), osteoprotegerin, vitamin D-24-hydroxylase (24OHase), and β-actin mRNA. Densitometric measurements were plotted as a function of ligand concentrations.
Osteoclast Formation Assays in Vitro.
Bone marrow cells were isolated under sterile conditions from the tibiae and femurs of 6-week-old C57B6 mice (Harlan–Sprague–Dawley) and plated at 1 × 106 cells/well in 48-well plates as described (10). Spleen cells were also isolated under sterile conditions, and 1 × 106 cells were plated together with 1.5 × 104 ST2 cells in 48-well plates as described (10). Cells were cultured in phenol red-free MEMα (MEM, α modification) with 10% charcoal–dextran-treated FBS in the presence or absence of compounds. Fresh medium containing the individual compounds was supplemented three times during the 8- to 10-day culture period. Cells were then fixed and stained for tartrate-resistant acid phosphatase (TRAP) activity as described (10). Osteoclast number was determined by counting the total number of multinucleated (>3 nuclei) TRAP-positive cells per well.
Osteoclastic Bone Resorption Assays in Vitro.
The ability of osteoclasts generated from murine bone marrow cells to resorb bone was assessed by first stimulating osteoclast formation with either 1,25(OH)2D3 or 2MD for 14 days on synthetic bone disks (BD Biosciences, Bedford, MA). Adherent cells were then removed by using 5% sodium hypochlorite and the discs examined for the presence of resorption lacunace or pits by using dark-field microscopy. Quantitation of the total resorbed surface area was carried out with Scion image software (Frederick, MD). Seven different fields on each disk were analyzed to determine total resorption area (measured in square millimeters). The two experimental conditions were carried out in triplicate.
Bone Formation Assays in Vitro.
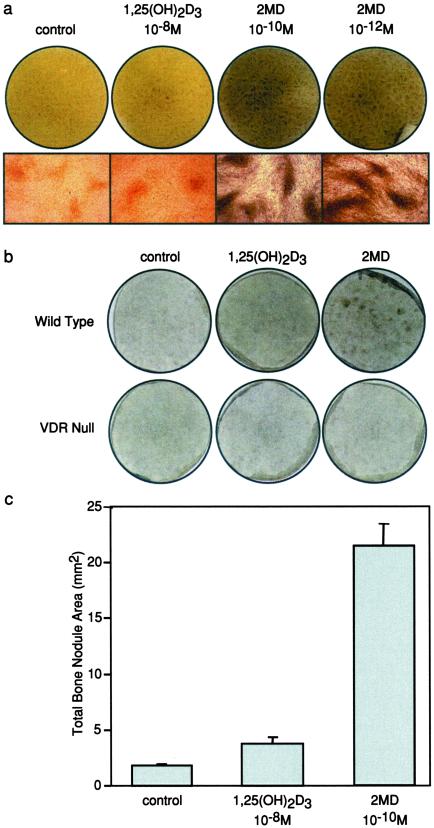
Primary human osteoblasts were isolated as described (11) and cultured in duplicate in 6-well plates at a density of 1 × 105 cells/ml in DMEM/F12 medium with 10% FBS. Primary fetal mouse calvarial cells were isolated as described (12) and cultured in αMEM containing 10% FBS. Cells were cultured for a period of 14 days with medium changes performed every 3 days. Cells were treated with 1,25(OH)2D3 or 2MD at the concentrations indicated in Fig. 3 on days 0, 3, and 6, followed by treatment with ascorbic acid (50 μg/ml) and β-glycerophosphate (10 mM) on days 9 and 12. Cells were stained on day 14 by using the Von Kossa staining technique to detect the presence of calcified matrix/bone (11). The dark brown to black stain is indicative of calcified bone nodules.
Figure 3.
Effects of 1,25(OH)2D3 and 2MD on bone formation in osteoblast cultures in vitro. (a) Primary human osteoblasts were isolated as described (9) and cultured in 6-well plates at a density of 3 × 105 cells/ml in DMEM/F-12 medium with 10% FBS. Confluent cultures were treated with 1,25(OH)2D3 or 2MD at the concentrations indicated on days 0, 3, and 6 followed by treatment with ascorbic acid (50 μg/ml) and β−glycerophosphate (10 mM) on days 9 and 12. Cells were stained on day 14 by using the Von Kossa staining technique to detect the presence of calcified matrix/bone (11). The dark brown to black stain is indicative of calcified bone nodules. (Upper) Von Kossa-stained cultures are indicated. (Lower) Microscopic images (×10) are shown. (b) Primary fetal mouse calvarial cells were isolated from wild-type and VDR null mice as described (25). Confluent cultures were treated with vehicle, 1,25(OH)2D3 (10−8 M), or 2MD (10−10 M) and evaluated after 14 days as in a. (c) Bone nodule area (mm2) was quantitated in each culture as described in Materials and Methods. Numbers indicate mean ± SE.
Accumulation of Bone in Ovariectomized Rats in Vivo.
Retired breeders were sham-operated or ovariectomized by Harlan–Sprague–Dawley. The rats were fed a 0.47% calcium, 0.3% phosphorus diet supplemented with vitamins A, D, E, and K as described by Suda et al. (8), which provides 75 units (3 μg) of vitamin D2/week per animal. Before ovariectomy, the individual bone mineral densities (BMD) were assessed by using dual photon absorptiometry (DPXα General Electric, Lunar, Madison, WI). Three weeks after the initial BMD readings were made, oral administration of either 2MD or 1,25(OH)2D3 in 0.1 ml of Wesson oil was initiated at the doses and frequencies indicated. Ovariectomized and sham-operated control animals received 0.1 ml of Wesson oil. BMD/bone mineral content was then assessed at 13 weeks and again at 23 weeks after initiation of compound administration. Blood was collected frequently for total serum calcium determinations by atomic absorption spectrometry.
Results
2MD Exhibits Selectivity for Bone in Vivo.
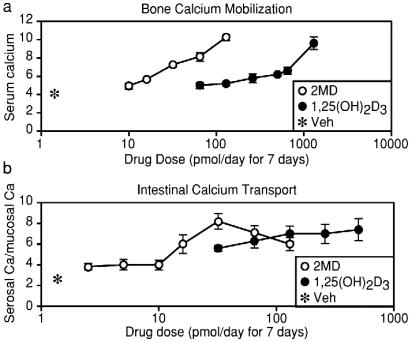
To compare the actions of 2MD on bone and intestine in vivo, we treated vitamin D-deficient rats fed a purified diet lacking calcium with increasing doses of 1,25(OH)2D3 or 2MD and assessed their ability to induce bone calcium mobilization as well as to stimulate intestinal calcium transport activity. Under these circumstances, the single source of calcium appearing in the blood is bone and probably results from osteoclast-mediated bone resorption (13, 14). 2MD is more than 30 times more potent than 1,25(OH)2D3 in stimulating bone calcium mobilization (Fig. 1a). A comparable assessment of the ligand's ability to stimulate intestinal calcium transport as assessed ex vivo revealed only a modest 2- to 3-fold separation (Fig. 1b). These data suggest that 2MD seems to possess a selectivity for calcium mobilization through presumed activation of the osteoclast.
Figure 1.
Weanling male rats were obtained from the low-vitamin D colony of Harlan–Sprague–Dawley and housed in overhanging wire cages in a vivarium provided with a 12-h light/12-h dark cycle. The fluorescent lighting was shielded to block UV light. Animals were fed a purified 0.47% calcium, 0.3% phosphorus vitamin D-deficient diet as described by Suda et al. (8) ad libitum for 1 week and then the same diet containing 0.02% calcium and 0.3% phosphorus diet for 3 weeks. During the final week, the indicated doses of either 1,25(OH)2D3 or 2MD (6) were delivered in 0.1 ml of propylene glycol/ethanol (95:5) i.p. each day for 7 days. Control animals received vehicle. (a) Twenty-four hours after the final dose, animals were killed by decapitation and blood was taken to determine serum calcium levels (mg/dl). (b) The first 10 cm of duodenum was used to determine intestinal calcium transport by the everted sac technique as described (8). Each group contained at least five animals, and the values represent the mean ± SEM.
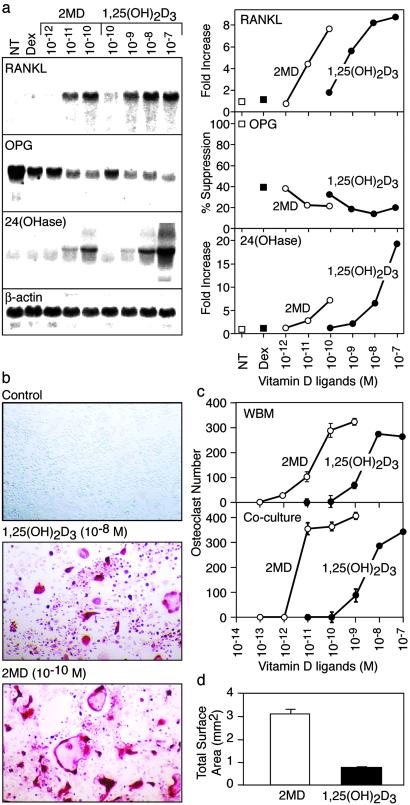
2MD Is a Potent Stimulator of RANKL Expression and Induces Osteoclast Formation in Vitro.
The primary cytokine regulator of osteoclast formation, activity, and survival is RANKL, a tumor necrosis factor-like molecule that is expressed primarily by stromal cells and osteoblasts in response to calciotropic hormones such as 1,25(OH)2D3 and parathyroid hormone (15, 16). Thus, the ability of vitamin D to induce bone resorption may be mediated through a direct action on the osteoblast. We assessed the ability of 1,25(OH)2D3 and 2MD to induce RANKL expression in murine osteoblastic ST2 cells and to stimulate osteoclast formation in vitro in both total bone marrow cultures and mixed cultures containing ST2 cells and spleen-derived, hematopoietic osteoclast precursors. Both compounds strongly induced RANKL as well as 24OHase mRNA expression, the latter a well-established target gene for 1,25(OH)2D3 (Fig. 2a, ref. 17). The compounds also suppressed the expression of osteoprotegerin, a soluble decoy receptor for RANKL that functions as an antagonist (Fig. 2a, ref. 18). 2MD is at least 100-fold more potent that 1,25(OH)2D3 in each of these responses. With respect to osteoclast formation, numerous osteoclast-like cells were induced in each of the culture systems by both 2MD and 1,25(OH)2D3, as determined by the appearance of large numbers of multinucleated, TRAP-positive cells typical of osteoclasts (Fig. 2b, ref. 10). Osteoclast formation is dose-dependent with 2MD again exhibiting a 2-log increase in potency relative to 1,25(OH)2D3 (Fig. 2c). The analog also produced osteoclasts that were larger than those with 1,25(OH)2D3. These differences resulted in an overall increase in osteoclastic bone-resorbing capability in response to 2MD in vitro, as assessed by measuring the total resorption area of osteoclasts produced in response to each ligand (Fig. 2d). These studies suggest a direct correlation between 1,25(OH)2D3 and 2MD's osteoclast-inducing activities in vitro and their relative bone calcium mobilizing activities in vivo.
Figure 2.
Regulation of RANKL and osteoclast formation by 1,25(OH)2D3 and 2MD. (a) RNA analysis. Murine osteoblastic ST2 cells were cultured in MEMα supplemented with 10% FBS. Cells were plated at densities of 5 × 105/ml in 100-mm dishes and treated for 24 h with the indicated concentrations of vehicle, dexamethasone (10−7 M), dexamethasone (10−7 M) plus 1,25(OH)2D3, or dexamethasone (10−7 M) plus 2MD at the indicated concentrations. Total RNA was isolated by using the Trizol reagent, and 20 μg was used for Northern blot analysis, using end-labeled probes for RANKL, osteoprotegerin, 24OHase, or β-actin. Densitometric measurements were plotted as a function of ligand concentration. (Left) Autoradiograms. (Right) Fold quantitation of densitometric analyses. (b) Induction of osteoclast formation in whole bone marrow cultures treated with vehicle, 1,25(OH)2D3, or 2MD at the indicated concentrations. Bone marrow cells were isolated under sterile conditions from the tibiae and femurs of 6-week-old C57B6 mice (Harlan–Sprague–Dawley) and plated at 1 × 106 cells/well in 48-well plates as described (10). Cells were cultured in phenol red-free αMEM with 10% charcoal–dextran-treated FBS in the presence or absence of compounds. Fresh medium containing the individual compounds was supplemented three times during the 8- to 10-day culture period. Cells were then fixed and stained for TRAP activity as described (10). (c) Quantitation of osteoclast number/well induced by 1,25(OH)2D3 or 2MD at the indicated concentrations in either whole bone marrow or cocultures of ST2 and spleen cells. Spleen cells were also isolated under sterile conditions, and 1 × 106 cells were plated together with 5 × 104 ST2 cells/well in 48-well plates as described. Values represent the number of TRAP-positive, multinucleated (>3 nuclei) osteoclasts/well ± SE for triplicate determinations. (d) Quantitation of total resorption capacity of osteoclasts. The ability of osteoclasts generated from murine bone marrow cells to resorb bone was assessed by first stimulating osteoclast formation with either 1,25(OH)2D3 or 2MD for 14 days on synthetic bone disks (BD Biosciences). Adherent cells were removed by using 5% sodium hypochlorite, and the disks were examined for the presence of resorption lacunace or pits by using dark-field microscopy. Quantitation of the total resorbed surface area was carried out by using Scion image software and was indicated as total surface area resorbed (mm2/disk). Seven different fields on each disk were analyzed to determine total resorption area. The two experimental conditions were carried out in triplicate. Values represent mean ± SE.
2MD Induces Mineralization in Human Osteoblast Cultures in Vitro.
Osteoblast-induced osteoclastic bone resorption provides an important, if not essential, stimulus for new bone formation during skeletal remodeling (19). Thus, this regulatory feature of the osteoblast is an initial manifestation of the osteoblast's principal function, which is to synthesize bone. The striking activity of 2MD on the osteoblast prompted us to test whether 2MD could also stimulate new bone formation in vitro by using isolated primary human osteoblast precursors as target cells. As indicated earlier, 1,25(OH)2D3 is not known to induce mineralization through a direct action on the osteoblast either in vivo or in vitro (1, 3, 4). To our surprise, 2MD exhibited a remarkably potent ability to induce bone in the cultures, as evidenced by the detection of numerous mineralized bone nodules at concentrations of 2MD as low as 10−12 M (Fig. 3a). As expected, 1,25(OH)2D3 exhibited little or no activity even at 10−8 M. This activity of 2MD was also observed with fetal mouse calvarial osteoblasts, but not seen in cells derived from VDR-null mice (Fig. 3b). Quantitation of the total area of the bone nodules in the treated cultures revealed that 2MD caused a substantial 20-fold increase, whereas the activity of 1,25(OH)2D3 was limited (Fig. 3c). These results suggest that 2MD exerts a highly potent activity on the osteoblast that is not characteristic of 1,25(OH)2D3 and that could potentially lead to bone formation in vivo.
2MD Induces an Increase in Bone Mass in Ovariectomized Rats in Vivo.
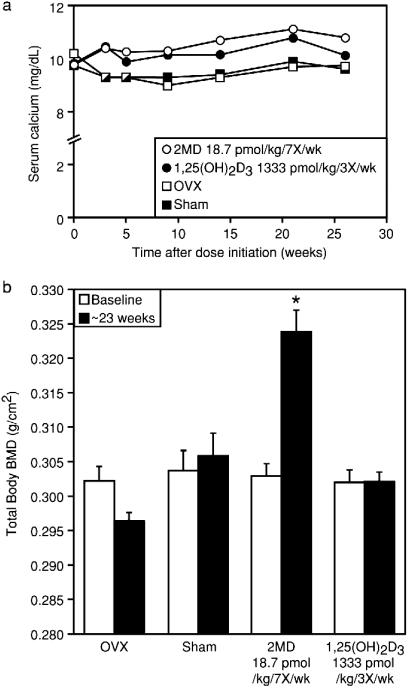
With these observations in hand, we tested whether 2MD could increase bone mass in rats in vivo. Retired female breeder rats were ovariectomized, placed on a normal purified diet, and treated with 2MD at 7 pmol/day for 23 weeks (18.7 pmol/kg, seven times per week). During the course of the study, BMD in the vehicle-treated ovariectomized animals decreased by 2% relative to sham-operated animals, although this did not reach significance. Serum calcium levels at this dose of 2MD were only slightly elevated (Fig. 4a). 2MD induced a marked rise in total bone mineral density that was evident at 13 weeks (not shown) and reached 7% above sham and 9% above ovariectomized controls at 23 weeks (Fig. 4b). A measurement of femur ash content confirmed the bone density measurement (data not shown). We also found that 32 pmol of 2MD given two times per week causes a significant increase in BMD (data not shown), whereas 1,25(OH)2D3 at 500 pmol three times per week (1,333 pmol/kg, three times per week) produced only a modest 2% rise in bone mass above ovariectomy, but this increase was not significant. When femurs of these rats were examined directly by bone-scanning techniques, there was a 25% increase in trabecular bone volume (data not shown). Our results both in cell culture and in vivo suggest that 2MD is selectively active on the osteoblast and capable of producing a marked increase in bone mineral density in ovariectomized rats. Histomorphometric analysis of tibia after tetracycline and calcein labeling revealed that 2MD increased bone mass by increasing bone formation and not by inhibiting resorption (data not shown).
Figure 4.
Induction of bone formation in ovariectomized rats by 2MD in vivo. Retired breeders received from Harlan–Sprague–Dawley were sham-operated or ovariectomized. The rats were fed a 0.47% calcium, 0.3% phosphorus diet supplemented with vitamins A, D, E, and K as described by Suda et al. (8), which provides 75 units (3 μg) of vitamin D2/week per animal. Before ovariectomy, the individual BMD were assessed by using dual photon absorptiometry (DPXα General Electric, Lunar). Five weeks after the initial BMD readings were made, oral administration of either 2MD or 1,25(OH)2D3 (6) in 0.1 ml of Wesson oil was initiated at the doses and frequencies indicated. Ovariectomized and sham-operated control animals received 0.1 ml of Wesson oil. (a) Serum calcium levels were determined by atomic absorption spectrometry at the indicated times for vehicle-treated sham-operated rats and ovariectomized rats treated with vehicle, 2MD, or 1,25(OH)2D3 at the indicated dose. (b) Total body bone mass was assessed in each group at 13 weeks and again at 23 weeks as above. *, Significance at P < 0.001.
Discussion
There has been little evidence to suggest that vitamin D functions in bone synthesis beyond its actions to ensure plasma levels of calcium and phosphorus sufficient for the mineralization process (4). This fact is substantiated by the modest efficacy of 1,25(OH)2D3 in the treatment of osteoporosis. 1,25(OH)2D3 does play a role in the expression of osteoblast matrix proteins such as osteopontin and osteocalcin (20, 21) that may in part account for the improved bone strength and reduced fracture rate found in 1,25(OH)2D3 or 1α-hydroxyvitamin D3-treated osteoporotic patients (20, 23). Our results suggest that 2MD exhibits at very low concentrations a marked and unexpected activity in stimulating the synthesis of new bone. This activity is at best only weakly observed with 1,25(OH)2D3 and then only at very high concentrations. Regardless, 2MD seems to possess preferential activity in the stimulation of osteoblastic bone formation in vitro that seems to result in net bone formation in vivo.
The finding that 2MD is much more potent than 1,25(OH)2D3 in stimulating the expression of genes such as the 24OHase and RANKL, suppressing osteoprotegerin, and inducing osteoclast formation is curious in view of the fact that both ligands exhibit similar equilibrium dissociation constants for the VDR (6). Some evidence exists that certain analogs of vitamin D3 are able to induce unique conformations in the receptor that are postulated to result in enhanced comodulator recruitment that favor enhanced transcriptional activity (23, 24). Alternative possibilities to account for the difference in potency of 2MD in vitro as well as in vivo include a difference in the relative affinity of the two ligands for vitamin D binding protein, a serum carrier for vitamin D metabolites, or a difference in the stability and/or degradation of the two ligands. Additional studies should help determine the underlying molecular basis for the increased biological potency of 2MD.
Perhaps the most interesting observation we have made is at the cellular level, where there is the finding that 2MD exerts a highly potent action on the osteoblast to induce bone formation in vitro. This activity is at best weakly observed when the cells are treated with concentrations of 1,25(OH)2D3 as high as 10−8 and even 10−7 M. This finding suggests that 2MD is not only more potent than 1,25(OH)2D3 in osteoblasts, but it may also possess a unique capacity to induce genes capable of orchestrating the processes leading to the formation of bone nodules. The molecular basis for this action is similarly unclear, although one possibility is that 2MD may induce a unique conformation within the VDR that is somehow manifested through selective anabolic activity.
Of great interest is that the in vivo data are in complete accord with the in vitro data. When calcium is removed from the diet of vitamin D-deficient rats, serum calcium reflects the availability of bone calcium. When a vitamin D compound is then provided to these animals, serum calcium rises at the expense of bone. This is believed to result from the stimulation of RANKL that causes osteoclastogenesis and activates existing osteoclasts to resorb bone. In this test, 2MD is 30–100 times more potent than 1,25(OH)2D3 in harmony with the in vitro osteoclastogenesis system. Similarly, 2MD is effective in stimulating new bone formation in vivo in ovariectomized rats and is uniquely active in vitro on bone nodule formation by primary osteoblast cultures. In contrast, 1,25(OH)2D3 has little activity either in vivo or in vitro on bone formation. These results are further supported by histomorphometry data in vivo. We suggest that 2MD magnifies a modest anabolic activity of 1,25(OH)2D3 on bone by an obscure mechanism.
In summary, we report on the biological properties of 2MD, a vitamin D analog that exhibits a unique ability to induce bone formation both in vitro and in vivo. We suggest that 2MD might be a useful anabolic agent for the treatment of bone loss diseases caused by estrogen deficiency, age, or steroid therapy.
Acknowledgments
We thank C. Smith and X. Ma for excellent technical assistance in the in vivo rat studies. We are also grateful to Dr. S. Kato for the VDR null mutant mice. This work was supported by funds from the Wisconsin Alumni Research Foundation (to H.F.D.), Deltanoid Pharmaceuticals (to H.F.D., M.C.-D., and L.A.P.), and National Institutes of Health Grant DK 52453-06 (to J.W.P.).
Abbreviations
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- 2MD
2-methylene-19-nor-(20S)-1α,25(OH)2D3
- VDR
vitamin D receptor
- RANKL
receptor activator of NFκB ligand
- 24OHase
vitamin D-24-hydroxylase
- TRAP
tartrate-resistant acid phosphatase
- BMD
bone mineral density
References
- 1.DeLuca H F. Harvey Lect. 1981;75:333–379. [PubMed] [Google Scholar]
- 2.Lamm M, Neuman W F. Arch Pathol. 1958;66:204–209. [PubMed] [Google Scholar]
- 3.Shipley P G, Kramer B, Howland J. Am J Dis Child. 1925;30:37–39. [Google Scholar]
- 4.Underwood J L, DeLuca H F. Am J Physiol. 1984;246:E493–E498. doi: 10.1152/ajpendo.1984.246.6.E493. [DOI] [PubMed] [Google Scholar]
- 5.Li Y C, Amling M, Pirro A E, Priemel M, Meuse J, Baron R, Delling G, Demay M B. Endocrinology. 1998;139:4391–4396. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- 6.Sicinski R R, Prahl J M, Smith C M, DeLuca H F. J Med Chem. 1998;41:4662–4674. doi: 10.1021/jm9802618. [DOI] [PubMed] [Google Scholar]
- 7. Paaren, H. E., Schnoes, H. K. & DeLuca, H. F. (1977) J. Chem. Soc. Chem. Commun., 890–892.
- 8.Suda T, DeLuca H F, Tanaka Y. J Nutr. 1970;100:1049–1052. doi: 10.1093/jn/100.9.1049. [DOI] [PubMed] [Google Scholar]
- 9.Perlman K, Kutner A, Prahl J, Smith C, Inaba M, Schnoes H K, DeLuca H F. Biochemistry. 1990;29:190–196. doi: 10.1021/bi00453a026. [DOI] [PubMed] [Google Scholar]
- 10.Shevde N K, Bendixen A C, Maruyama M, Li B L, Billmire D A. Cleft Palate Craniofac J. 2001;38:606–614. doi: 10.1597/1545-1569_2001_038_0606_eaoodf_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 11.Shevde N K, Bendixen A C, Dienger K M, Pike J W. Proc Natl Acad Sci USA. 2000;97:7829–7834. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley J M, Martin T J, Suda T. Endocrinology. 1988;123:2600–2612. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson A. Acta Physiol Scand. 1952;26:212–220. doi: 10.1111/j.1748-1716.1952.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 14.Blunt J W, Tanaka Y, DeLuca H F. Proc Natl Acad Sci USA. 1968;61:1503–1506. doi: 10.1073/pnas.61.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong Y Y, Yoshida H, Sarosi I, Tan H L, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos A J, Van G, Itie A, et al. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 16.Morony S, Capparelli C, Lee R, Shimamoto G, Boone T, Lacey D L, Dunstan C R. J Bone Miner Res. 1999;14:1478–1485. doi: 10.1359/jbmr.1999.14.9.1478. [DOI] [PubMed] [Google Scholar]
- 17.Zierold C, Darwish H M, DeLuca H F. Proc Natl Acad Sci USA. 1994;91:900–902. doi: 10.1073/pnas.91.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonet W S, Lacey D L, Dunstan C R, Kelley M, Chang M S, Luthy R, Nguyen H Q, Wooden S, Bennett L, Boone T, et al. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 19.Rodan G A. In: Osteoporosis. Marcus R, Feldman D, Kelsey J, editors. San Diego: Academic; 1996. pp. 289–299. [Google Scholar]
- 20.Denhardt D T, Noda M. J Cell Biochem. 1998;30–31,Suppl.:92–102. [PubMed] [Google Scholar]
- 21.Shen J, Montecino M A, Lian J B, Stein G S, van Wijnen A J, Stein J L. J Biol Chem. 2002;277:20284–20292. doi: 10.1074/jbc.M112440200. [DOI] [PubMed] [Google Scholar]
- 22.Orimo H, Shiraki M, Hayashi T, Nakamura T. Bone Miner. 1987;3:47–52. [PubMed] [Google Scholar]
- 23.Tilyard M W, Sprars G F S, Thomson J, Dovey S. N Engl J Med. 1992;326:357–362. doi: 10.1056/NEJM199202063260601. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y Y, Nguyen C, Peleg S. Mol Endocrinol. 2000;14:1776–1787. doi: 10.1210/mend.14.11.0560. [DOI] [PubMed] [Google Scholar]
- 25.Yang W, Freedman L P. J Biol Chem. 1999;274:16838–16845. doi: 10.1074/jbc.274.24.16838. [DOI] [PubMed] [Google Scholar]