Abstract
The molecular-mimicry theory proposes that immune crossreactivity between microbial and self-antigen is the initiating event in the activation of autoaggressive immune responses leading to autoimmune disease. In support of this possibility, it is now accepted that T cell recognition of antigen is highly degenerate. However, it is to be expected that the immune system would have evolved mechanisms to counter such a potential danger. We studied the influence of CD4+CD25+ regulatory T cells (Treg) on the ability of suboptimal T cell receptor ligands to provoke autoimmunity. By using CD4+ T cell-driven experimental autoimmune encephalomyelitis as a model, it was found that depletion of CD4+CD25+Foxp3+ Treg allowed pathology to develop in response to suboptimal T cell stimulation. These data demonstrate the importance of Treg in raising the threshold of triggering of autoreactive T cell responses, thus limiting the risk of autoimmune disease due to molecular mimicry.
Keywords: experimental autoimmune encephalomyelitis (EAE), multiple sclerosis, regulation, tolerance
Extensive flexibility in T cell receptor (TCR) recognition of peptide-MHC (pMHC) complexes has been proposed to be essential to provide effective immune surveillance of all possible pathogen-derived pMHC complexes (1). The logical extension of this cross-reactivity is that the peripheral T cell repertoire should contain a sizeable population of cells that are capable of responding in a cross-reactive manner to both pathogen-derived antigens (Ags) and self-molecules. This concept is at the heart of the molecular-mimicry theory, in which self-reactive T cells, activated initially by infectious pathogens, subsequently provoke a self-destructive response in an organ expressing a crossreactive self-Ag (2). However, there is considerable debate over the validity of this theory as a general mechanism for the induction of autoimmune disease (3), and mechanisms that limit this potential risk are likely to exist. Naturally occurring regulatory T cells (Treg) may have a role by raising the activation threshold of T cell responses, potentially providing one mechanism by which weakly self-reactive T cells can be maintained in the T cell repertoire without inducing overt autoimmunity.
Treg activity is enriched in the subset of CD4+ T cells expressing CD25 in mice (4), rats (5), and humans (6), leading to the now widespread use of this marker to define a naturally occurring population of Treg. The influence of Treg on peripheral tolerance is shown most vividly by the widespread autoimmune and inflammatory lesions that are evident in humans and mice that lack these cells because of mutations in the Treg-specific transcription factor Foxp3 (7-10). Although conclusive evidence regarding the specificity of CD4+CD25+Foxp3+ Treg remains elusive, they are known to have a broad TCR repertoire (11), and there is evidence to suggest that CD25+ Treg developing in the thymus are selected to have high affinity for self-Ags expressed on thymic epithelium (12-14). Negative selection in the thymus should ensure that the only cells remaining in the mature T cell repertoire have low reactivity to self-Ag, but of these, Treg will be biased toward higher affinity to self than non-Treg, perhaps because of their greater resistance to agonist-induced clonal deletion (15). Also, it is known that the Ag dose that is required to activate suppressive function in TCR transgenic CD25+ Treg is lower than that required for the proliferation of CD25- T cells of identical specificity (11). Therefore, it is generally envisaged that Treg cells with high sensitivity for self would impose a dominant control on other potential autoaggressive cells with lower sensitivity for self while not preventing high-affinity responses to foreign Ags. In this regard, it is also known that high Ag dose and/or strong costimulation, as would be associated with the presence of infectious agents, can allow responder T cells to escape Treg-mediated suppression (16-18). This model predicts that a decrease in the number or function of Treg would increase the risk of self-reactive T cells becoming pathogenic and inducing autoimmune disease in response to suboptimal TCR ligation.
We tested this hypothesis in the setting of murine experimental autoimmune encephalomyelitis (EAE), induced in response to the I-Au-restricted Ac1-9 epitope of myelin basic protein (MBP). Altered peptide ligands (APLs) have been defined for which Ac1-9-reactive T cells have a range of sensitivities that are either higher or lower than the sensitivity shown for the WT Ac1-9 peptide (19), and they are referred to as superagonists and subagonists, respectively. Immunization with subagonist APL was used as a surrogate approach to activation of self-reactive T cells by infectious cross-reactive Ag. In vitro, Ac1-9-reactive Tg4 TCR transgenic CD25+ Treg had a purely quantitative effect on the proliferation of responder Tg4 CD25- cells, raising the dose-response curve of both subagonist and superagonist peptides by ≈100-fold. In effect, CD25+ Treg resulted in efficient suppression of CD25- responses to weak agonist APL over a wider concentration range compared with responses to WT or superagonist peptides. In vivo, depletion of CD25+ Treg prevented efficient recovery from Ac1-9-induced EAE in H-2u mice. Moreover, CD25+ Treg depletion allowed EAE to be induced with a subagonist Ac1-9 APL that normally resulted in only poor EAE induction. These data identify a role for CD25+ Treg in preventing the conversion of a suboptimal antigenic stimulation into an overt autoaggressive response. In this model, this effect was not associated with dramatic changes in the number or cytokine-producing capacity of transferred Ac1-9-specific TCR transgenic Tg4 T cells in vivo.
Methods
Mice and Peptides. B10.PL (H-2u), B10.PLxSJL (H-2uxs), Tg4 mice (expressing a transgenic Ac1-9-reactive TCR; ref. 20) and Tg4.Ly5.1 mice (backcrossed more than five generations onto to the Tg4 background) were bred and maintained in specific-pathogen-free conditions at the University of Edinburgh. The Ac1-9 peptide (Ac-ASQKRPSQR) and APL with substitutions at residues 3 or 4 were synthesized by Advanced Biotechnology Centre (Imperial College, London).
T Cell Purification. CD4+ T cells were enriched from spleen and lymph nodes (cervical, mesenteric, brachial, axillary, inguinal, and iliac) by negative selection involving incubation on ice with the following mixture of Abs (purified from hybridomas): RAB632 (anti-B220), 53-6.72 (anti-CD8), M1/70 (anti-Mac1), and M5/114.15.2 (anti-MHC class II), followed by washing and incubation with M450 sheep anti-rat IgG Dynabeads (Dynal, Bromborough, U.K.). Cells were then fractionated according to CD25 expression by using anti-CD25-phycoerythrin (PE) (clone 7D4, Miltenyi Biotec, Auburn, CA), followed by incubation with anti-PE microbeads (Miltenyi Biotec) and separation on columns according to the manufacturer's instructions. Highly pure (>95%) CD4+CD25+ cells from Tg4 mice were obtained after further cell sorting on a FACStar flow cytometer (BD Biosciences).
In Vitro Proliferation Assay. Cells were cultured in triplicate in U-bottomed 96-well plates in RPMI medium 1640 (GIBCO, Invitrogen) supplemented with 5% heat-inactivated FCS (Sigma), 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate, and 50 μM 2-mercaptoethanol (all obtained from GIBCO). Responder CD4+CD25- Tg4 T cells were added at 2.5 × 104 cells per well with or without the same number of CD4+CD25+ Tg4 cells and with 5 × 104 irradiated (30 Gy) B10.PL splenocytes as Ag-presenting cells and were stimulated with Ac1-9 WT or APL peptide. We added 0.5 μCi (1 Ci = 37 GBq) 3[H]thymidine (Amersham Biosciences) for the last 18 h of a 90-h culture period. Data are shown as mean ± SD of triplicate wells.
In Vivo Depletion of CD25+ Cells. Endogenous CD25+ cells were depleted from mice 2 or 3 days before induction of EAE by i.p. injection with 1 mg of the anti-CD25 Ab PC61(rat IgG1). Control mice received an i.p. injection of 1 mg of MAC49 (rat IgG1, anti-phytochrome). Confirmation of CD25+ cell depletion by PC61 was determined by staining peripheral blood of all mice 2 or 3 days after treatment with an Ab that recognizes a different epitope of CD25 (7D4), and it always resulted in >90% CD25+ cell depletion.
Induction of EAE. EAE was induced by s.c. immunization with 200 μg of either WT Ac1-9 or the indicated APL emulsified in complete Freund's adjuvant (Sigma) and injected in 50 μl per leg in each hind leg. Pertussis toxin (200 ng; Health Protection Agency, Porton Down, U.K.) was administered i.p. on the same day and 2 days later. In some experiments, 2.5 × 105 CD4+CD25- cells purified from Tg4.Ly5.1 mice were transferred i.v. to B10.PL mice 1 day before EAE induction. Mice were monitored daily for clinical signs of EAE by using the following scores: 0, healthy; 1, limp tail; 2, impaired gait/righting reflex; 3, partial hind limb paralysis; 4, total hind limb paralysis, or partial hind limb paralysis and front weakness; 5, total hind limb paralysis plus front leg weakness; and 6, moribund or dead. Comparison of disease incidence was performed by the Fisher's exact test, and overall disease burdens were compared by the Mann-Whitney U test.
Isolation of Spinal Cord Infiltrating Cells. Mice were killed by CO2 asphyxiation and perfused with cold PBS. Spinal cords were removed by intrathecal hydrostatic pressure, chopped into small pieces, and digested for 30 min at 37°C with 2.5 mg/ml collagenase (Worthington) and 1 mg/ml DNase (Sigma) before mechanical disaggregation. Cells were isolated from the interface of a 30:70% discontinuous Percoll gradient after centrifugation for 20 min at 2,000 × g.
Flow Cytometry. Cells were stained with appropriately titrated Abs for 20 min on ice and washed with PBS plus 2% FCS. For intracellular cytokine staining, cells were restimulated ex vivo with phorbol 12-myristate 13-acetate (PMA; 10 ng/ml) and ionomycin (1 μg/ml) for 5 h in the presence of GolgiStop (BD Biosciences). Cells were surface-stained and then fixed and permeabilized by using BD Cytofix/Cytoperm according to the manufacturer's instructions before staining for intracellular cytokines by using the following mAbs: IL-2-PE, IL-10-PE, IFN-γ-PE, IL-17-PE, IL-4-PE, or appropriate isotype control mAbs (all purchased from BD Biosciences). Foxp3 staining was performed by using a Foxp3 staining set (eBioscience, San Diego; Insight Biotechnology, Wembley, U.K.) according to the manufacturer's instructions. Data were acquired on a FACSCaliber cytometer (BD Biosciences) and analyzed by using flowjo software (Treestar, San Carlos, CA).
Results
CD4+CD25+ Cells Increase the Threshold for Tg4 T Cell Proliferation in Vitro in Response to Ac1-9 APL. The molecular requirements for Tg4 T cell recognition of the Ac1-9-I-Au complex have been well characterized in previous studies. Briefly, residues 4 and 5 of this peptide interact with MHC class II Au (21), whereas positions 3 and 6 are important TCR contact residues (19, 22). Ac1-9 binds weakly to I-Au because the Lys at position 4 fits poorly with the large hydrophobic P4 pocket of Au (23, 24); consequently, most position-4 APL show stronger Au-binding affinities and act as superagonists for Ac1-9-reactive T cells (this characteristic is particularly true of the Tyr-4 and Ala-4 APLs) (19). Position-6 APLs cannot stimulate Tg4 T cells, whereas several position-3 APLs act as subagonist ligands for Tg4 cells, with the following hierarchy of responsiveness: Gln-3 > Met-3 > Phe-3 (22, 25). Studies have shown (22) that immunization of nontransgenic mice with these APL largely failed to induce EAE. Therefore, in this study, the Met-3 and Phe-3 APLs served as surrogate cross-reactive subagonists.
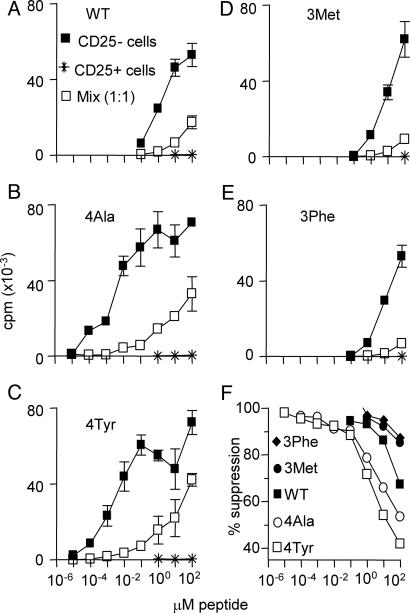
Our initial analysis of the impact of Treg on the strength of T cell stimulation was an in vitro assessment of the response of Tg4 T cells to WT Ac1-9 and various APLs (Fig. 1). Purified CD25- and CD25+ Tg4 T cells were cultured, either alone or in combination, with the peptide Ags. As shown in another TCR transgenic system (17), Tg4 CD25+ cells were able to inhibit responses to WT Ac1-9 at suboptimal doses, but as the dose increased, the level of suppression dropped, with only 50-75% suppression at saturating concentrations of Ac1-9 (100 μM) (Fig. 1 A). Two findings arose from the analysis of responses to superagonist APL (Ala-4 and Tyr-4). First, the purified CD25+ Tg4 cells remained hypoproliferative even under this intense TCR stimulation (Fig. 1 B and C). Second, Tg4 CD25+ Treg suppressed CD25- Tg4 cell activation by the superagonists, although, as with the WT Ac1-9 peptide, suppression was diminished at higher APL concentrations (Fig. 1 B and C). This result demonstrated that, even under conditions of supraoptimal TCR ligation, Treg cells could still suppress their CD25- counterparts. Stimulation of Tg4 cells with subagonist Met-3 and Phe-3 APLs resulted in essentially the same pattern of reduced responsiveness at lower peptide concentrations in the presence of CD25+ Treg (Fig. 1 D and E). Because CD25- T cell proliferation required higher concentrations of the subagonist APL compared with WT Ac1-9, good suppression was achieved by CD25+ Treg at most concentrations of subagonist APL (Fig. 1F). Therefore, the overall effect of CD25+ Treg under these culture conditions was to shift the activation requirements of the CD25- cells by 2-log (i.e., 100 times higher doses of peptide were required to give a similar level of proliferation in the presence of Treg cells). This effect was consistent whether the WT, superagonist, or subagonist peptides were used. Therefore, in this respect, the suppressive activity of Treg in this system appears to be entirely quantitative.
Fig. 1.
CD4+CD25+ Tg4 cells raise the threshold for CD4+CD25- Tg4 proliferation in vitro. (A-E) CD4+CD25- and CD4+CD25+ Tg4 cells were cultured either alone or in combination (at 1:1) in the presence of irradiated B10.PL Ag-presenting cells and the indicated concentration of WT Ac1-9 or APL. Proliferation was measured by 3[H] thymidine incorporation during the last 18 h of a 90-h culture period, and the results are shown as mean ± SD of triplicate wells. (F) Results in A-E are shown as the percentage of suppression in the presence of Treg.
CD4+CD25+Foxp3+ Cells Limit EAE Pathology. Our in vitro studies provided some important information; at high Ag loads (such as might be expected in draining lymph nodes after immunization), CD25+ Treg cell suppression was incomplete when the Ag was WT Ac1-9 but still intact when the Ag was a subagonist. This information led to the hypothesis that the reason that the subagonist APLs fail to induce EAE is because Treg function is dominant in this situation. This possibility was tested in vivo by depletion of CD25+ cells from B10.PL or B10.PLxSJL mice before immunization with either WT Ac1-9 or the Met-3 APL.
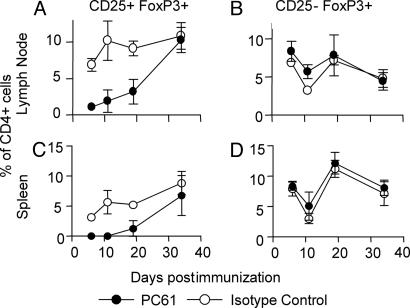
Administration of the PC61 anti-CD25 Ab resulted in the depletion of >90% of CD4+CD25+ T cells within 2 or 3 days (data not shown) and substantial reduction of their numbers for at least 3 weeks after treatment, with a gradual recovery to normal numbers by ≈5 weeks after treatment (Fig. 2). Analysis of Foxp3 expression (the transcription factor that is the only known unique identifier of Treg, both CD25+ and CD25-; ref. 26) demonstrated conclusively that the PC61 Ab does deplete CD25+ Treg, rather than simply inducing down-regulation of CD25 expression on these cells. As shown in Fig. 2, the number of CD25+Foxp3+ cells was greatly reduced after PC61 treatment, without resulting in a corresponding increase in CD25-Foxp3+ cells compared with control mice. Importantly, these results also demonstrate that as much as half of the total Treg pool, as defined by Foxp3 expression (26), remained undepleted after PC61 treatment because of the lack of CD25 expression (Fig. 2).
Fig. 2.
PC61 Ab depletes CD25+Foxp3+ cells in vivo. B10.PL mice were injected i.p. with 1 mg of either PC61 or isotype control Ab (MAC49), and EAE was induced 3 days later by using Ac1-9. CD25 and Foxp3 expression were examined on gated CD4+ lymphocytes from draining lymph nodes (A and B) and spleen (C and D) at various times after immunization. Results are shown as the mean ± SD of three to six mice per group at each time point.
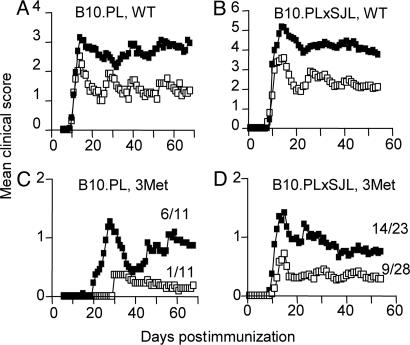
The in vivo depletion of CD25+ Treg cells resulted in EAE of substantially increased severity, both in terms of mean clinical scores and mortality, compared with control mice after immunization with WT Ac1-9. This finding was true for both B10.PL (Fig. 3A) and B10.PLxSJL (Fig. 3B) strains of mice, with B10.PLxSJL succumbing to more severe disease than B10.PL. CD25+ Treg depletion also resulted in impaired natural recovery from EAE, which was particularly noticeable in the B10.PL strain in which there was less mortality, but had no effect on the kinetics of disease onset (Fig. 3 A and B). These data reveal a clear role for endogenous CD25+ Treg in limiting autoimmune pathology induced with the Ac1-9 peptide.
Fig. 3.
In vivo depletion of CD25+ Treg exacerbates clinical signs of EAE and potentiates EAE induction with a subagonist APL. B10PL (A and C) and B10PLxSJL (B and D) mice were injected i.p. with 1 mg of PC61 (filled symbols) or isotype control Ab (MAC49; open symbols). EAE was induced by immunization 2 or 3 days later with 200 μg of WT Ac1-9 (A and B) or Met-3 APL (C and D). For B10.PL mice (A and C), data were pooled from two experiments (n = 11). For B10.PLxSJL mice, data were pooled from three (n = 16; B) or four (n = 23-28; D) experiments. Mortality rates in response to WT peptide for PC61- and Mac49-treated mice, respectively, were 3/11 and 0/11 (A) and 9/16 and 4/16 (B) (with a disease incidence of 91-100% for each group). The numbers in C and D indicate the disease incidence in each group. PC61 treatment led to a significant increase in cumulative disease scores in each case (P < 0.0001, one-tailed Mann-Whitney U test), and it resulted in a significant increase in disease incidence in response to subagonist APL (C and D) (P < 0.05, one-tailed Fisher's exact test).
More importantly, CD25 depletion allowed mice to become susceptible to EAE development after immunization with the subagonist Ac1-9(Met-3) APL that normally induces EAE very poorly and with delayed onset. There was a significant increase in the incidence of EAE after CD25 depletion in both B10.PL and B10.PLxSJL mice (Fig. 3 C and D). This effect was most striking in the B10.PL mice, as these mice were less susceptible to Ac1-9(Met-3)-induced EAE than the B10XSJL strain. The lower mean clinical score of mice after subagonist peptide immunization compared with WT reflects this difference in EAE incidence, rather than a difference in EAE severity (i.e., mice that developed EAE reached the same maximum severity as those receiving WT peptide). These data show an important role of CD25+ Treg in preventing the occurrence of overt autoimmune disease after exposure to a weakly cross-reactive exogenous Ag.
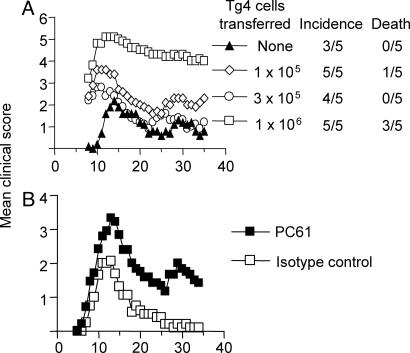
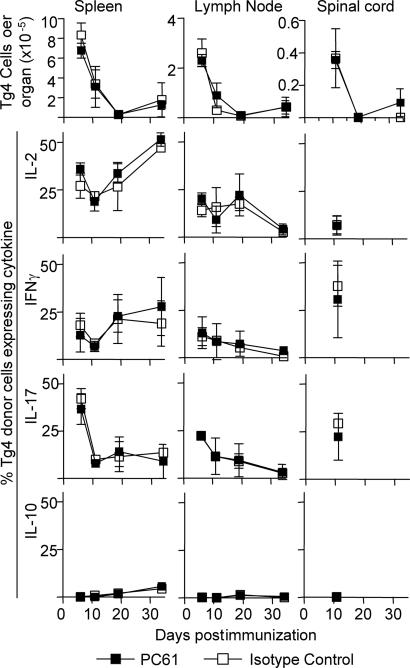
The Effects of CD25+ Cell Depletion on Transferred Ac1-9-Reactive Tg4 CD4+CD25- T Cells in Vivo. In an attempt to define the effects of CD25+ Treg on the expansion and differentiation of pathogenic Ac1-9-reactive CD4+ T cells in vivo, we transferred CD4+CD25- cells from Tg4Ly5.1+ mice into B10.PL (Ly5.1-) recipient mice. The Tg4 cells were confirmed to be playing an important pathogenic role in this transfer model as their presence at low cell doses (1-3 × 105) accelerated disease onset, with higher cell doses resulting in increased mortality (Fig. 4A). In this Tg4 transfer model, depletion of the host CD25+ Treg again resulted in more severe EAE after transfer of low Tg4 cell numbers (Fig. 4B). To examine the effects of host CD25+ Treg on Tg4 cells in vivo, cells from spleen and lymph nodes draining the site of immunization (inguinal and iliac), as well as the effector site (spinal cord), were obtained from PC61 or control Ab treated mice after immunization with WT peptide. A massive expansion of Tg4 cells was seen in the draining lymph nodes and spleen on day 6 after immunization, at a time before clinical signs of disease were apparent, which waned rapidly at later time points (Figs. 4B and 5). At the peak of EAE, there was a great enrichment of Tg4 cells in the spinal cord (Fig. 5). However, PC61 treatment did not significantly affect either the percentage (not shown) or the absolute number of Tg4 cells (Fig. 5) at any of the time points analyzed, either in the lymphoid tissue or the CNS. Tg4 cells produced enriched levels of IL-17, IL-2 and IFN-γ compared to host cells in the spleen, lymph nodes and spinal cord, and little IL-10 or IL-4 (Fig. 5 and data not shown). However, PC61 treatment was again found to have little effect on the differentiation of Tg4 cells with respect to their cytokine-producing capacity. These data using Tg4 cell function as the readout therefore argue against a simple effect of CD25+ Treg on the expansion, migration or differentiation of pathogenic T cells, despite their clear influence on pathology.
Fig. 4.
Ac1-9-reactive Tg4 cells are pathogenic in vivo. (A) Purified CD4+CD25- Tg4.Ly5.1+ cells were injected i.v. into B10.PL mice at the dose indicated 1 day before EAE induction with 100 μg of Ac1-9. (B) B10.PL mice were given either PC61 or isotype control Ab 2 days before receiving 2.5 × 105 CD4+CD25- Tg4 cells. EAE was induced 1 day later with 100 μg of Ac1-9 (16 mice per group). There was a significant increase in the cumulative disease score of PC61-treated mice (one-tailed Mann-Whitney U test; P < 0.0001).
Fig. 5.
Effects of CD25+ Treg depletion on Tg4 cell expansion and cytokine production in vivo. Tg4.Ly5.1+ cells from the experiment shown in Fig. 4B were enumerated by flow cytometry in the draining lymph nodes (inguinal and iliac), spleen, and spinal cord of mice on days 6, 11, 19, and 34 after EAE induction. Cytokine production by Tg4.Ly5.1+ cells was measured by intracellular staining for flow cytometry after 5 h of ex vivo stimulation of cells with phorbol 12-myristate 13-acetate (PMA) and Ionomycin. Results are shown as mean ± SD of three to six mice per group. Tg4 numbers in control mice that were immunized with CFA alone were <0.09 × 105 in spleen, <0.06 × 105 in lymph nodes, and <0.006 × 105 in the spinal cord at all time points (data not shown). Sufficient numbers of Tg4 cells for analysis of cytokine production were available from the spinal cord only on day 11.
Discussion
Flexibility in TCR recognition of pMHC complexes appears to be essential to provide immune surveillance against a universe of potential pathogens (1). This flexibility obviously carries the potential risk of autoaggression triggered by molecular mimicry. For the advantages of TCR crossreactivity to outweigh this hazard, the immune system must limit the scope for it to occur. CD25+ Treg have been shown to suppress effector cell activation under suboptimal conditions in vitro (17). Here, we report that this principle can be extended to a disease setting in vivo. By using a CD4+ T cell-mediated disease that targets the CNS, we found that CD25+ Treg cells limit progression to overt autoimmune disease after T cell triggering by suboptimal TCR ligands. Thus, with weakly cross-reactive initiating Ags, the presence or absence of CD25+ Treg is crucial in determining whether mice succumb to autoimmune disease.
Depletion of endogenous CD25+ Treg also impaired the recovery of H-2u mice from EAE induced with WT Ac1-9. This result confirms and extends observations from other models of EAE, in which endogenous CD25+ Treg were found to have an important role in limiting pathology induced with WT Ag (27-29). Note that the treatment of mice with PC61, which was confirmed in this study to genuinely deplete CD25+Foxp3+ Treg, removes only approximately half of the total Foxp3+ Treg pool, with a significant population of Foxp3+CD25- cells still remaining. Currently we have no way to deplete these CD25-Foxp3+ Treg, making their role in the regulation of EAE less amenable to investigation. Our assumption would be that the removal of all Foxp3+ cells would result in an even more profound effect on EAE development triggered by weak antigenic stimulation.
Our results conclusively show a role for CD25+ Treg in limiting autoimmune disease at the margins of T cell activation. However, it is not clear at what level this regulation is exerted (i.e., initial T cell activation, T cell differentiation and effector function, or T cell entry into the tissues). A functional role for Treg within the target organ is supported by our recent observation that CD25+ Treg accumulation in the CNS correlates with recovery from EAE (29). Alternatively, Treg may be active during the initial T cell priming events, and, in their absence, an altered and more virulent T cell population may expand. This alteration could be quantitative (either more T cells or cells with increased sensitivity for the self-Ag) or qualitative (increased production of inflammatory cytokines or altered expression of adhesion molecules or chemokine receptors). Studies in which TCR transgenic T cells have been tracked in vivo in the presence or absence of cotransferred Ag-specific CD25+ Treg have reported an influence of the CD25+ Treg on the later expansion but not the cytokine production of Ag-specific T cells (30), or an effect on cytokine production but not T cell expansion by responder T cells (31).
Our data from Tg4 T cell transfers into CD25+ Treg-depleted mice revealed no difference in either the early or late expansion of Ac1-9-reactive cells after immunization in either the priming or effector site. Analysis of cytokine production by the transferred Tg4 cells also showed no significant qualitative or quantitative shifts as a result of CD25+ Treg depletion. Similarly, we lack evidence to show that PC61 treatment enabled Tg4 cells to respond to the subagonist peptide in vivo (data not shown). It still remains possible that Treg depletion may alter the ability of Tg4 cells to produce effector cytokines in vivo, despite the apparent lack of any effect ex vivo under the experimental conditions that we used. It is also possible that the suppression by Treg of other undefined effector mechanisms used by Tg4 cells is more important than the suppression of cytokine production in this model. Although Tg4 cells clearly enhance pathology in the transfer model, it is probable that polyclonal host CD4+ T cells also contribute to EAE pathogenesis. The Tg4 TCR is only one receptor from a heterogeneous Ac1-9-reactive repertoire. In particular, there are T cells bearing TCRs of higher affinity than Tg4 (32) and these would be predicted to have lower thresholds for activation than Tg4 cells. Therefore, Treg depletion may preferentially release these cells from normal regulatory control, allowing the development of overt disease that we see in response to subagonist immunization. In the absence of the appropriate TCR transgenic mouse, we have no way of tracking such cells. Alternatively, other cells of the immune system may also be an important target of Treg function, including cells of the innate immune system (33). Despite substantial research in many models, the exact mechanisms used by CD25+ Treg to mediate their suppressive effects remain enigmatic.
The immune system must achieve a difficult balance between the TCR crossreactivity necessary for effective immune surveillance and the risk of provoking a destructive response against self. The accumulating data point to a multilayered control. Negative selection deletes most (but not all) T cells bearing TCRs with high affinity for self-pMHC. Clearly, an absolute deletional mechanism for self-tolerance would be wasteful; T cells with self-reactivity below a certain sensitivity pose little threat. Moreover, such a mechanism would provide an extremely porous peripheral T cell repertoire if most T cells have some residual ability to recognize self-pMHC. The data presented here demonstrate a role for Treg in reducing the likelihood of an autoaggressive response as a consequence of crossreactivity with an infectious agent.
Acknowledgments
We thank Andrew Sanderson for flow cytometric cell sorting. This work was supported by the Wellcome Trust and the Medical Research Council (MRC). S.M.A. is an MRC Senior Research Fellow.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ag, antigen; Treg, regulatory T cell(s); pMHC, peptide-MHC; EAE, experimental autoimmune encephalomyelitis; APL, altered peptide ligand; TCR, T cell receptor; PE, phycoerythrin.
References
- 1.Mason, D. (1998) Immunol. Today 19, 395-404. [DOI] [PubMed] [Google Scholar]
- 2.Fujinami, R. S. & Oldstone, M. B. (1989) Immunol. Res. 8, 3-15. [DOI] [PubMed] [Google Scholar]
- 3.Benoist, C. & Mathis, D. (2001) Nat. Immunol. 2, 797-801. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. (1995) J. Immunol. 155, 1151-1164. [PubMed] [Google Scholar]
- 5.Stephens, L. A. & Mason, D. (2000) J. Immunol. 165, 3105-3110. [DOI] [PubMed] [Google Scholar]
- 6.Stephens, L. A., Mottet, C., Mason, D. & Powrie, F. (2001) Eur. J. Immunol. 31, 1247-1254. [DOI] [PubMed] [Google Scholar]
- 7.Hori, S., Nomura, T. & Sakaguchi, S. (2003) Science 299, 1057-1061.12522256 [Google Scholar]
- 8.Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. (2003) Nat. Immunol. 4, 330-336. [DOI] [PubMed] [Google Scholar]
- 9.Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. (2003) Nat. Immunol. 4, 337-342. [DOI] [PubMed] [Google Scholar]
- 10.Gambineri, E., Torgerson, T. R. & Ochs, H. D. (2003) Curr. Opin. Rheumatol. 15, 430-435. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi, T., Kuniyasu, Y., Toda, M., Sakaguchi, N., Itoh, M., Iwata, M., Shimizu, J. & Sakaguchi, S. (1998) Int. Immunol. 10, 1969-1980. [DOI] [PubMed] [Google Scholar]
- 12.Jordan, M. S., Boesteanu, A., Reed, A. J., Petrone, A. L., Holenbeck, A. E., Lerman, M. A., Naji, A. & Caton, A. J. (2001) Nat. Immunol. 2, 301-306. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh, C.-S., Liang, Y., Tyznik, A. J., Self, S. G., Liggitt, D. & Rudensky, A. Y. (2004) Immunity 21, 267-277. [DOI] [PubMed] [Google Scholar]
- 14.Bensinger, S. J., Bandeira, A., Jordan, M. S., Caton, A. J. & Laufer, T. M. (2001) J. Exp. Med. 194, 427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Santen, H. M., Benoist, C. & Mathis, D. (2004) J. Exp. Med. 200, 1221-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ermann, J., Szanya, V., Ford, G. S., Paragas, V., Fathman, C. G. & Lejon, K. (2001) J. Immunol. 167, 4271-4275. [DOI] [PubMed] [Google Scholar]
- 17.George, T. C., Bilsborough, J., Viney, J. L. & Norment, A. M. (2003) Eur. J. Immunol. 33, 502-511. [DOI] [PubMed] [Google Scholar]
- 18.Pasare, C. & Medzhitov, R. (2003) Science 299, 1033-1036. [DOI] [PubMed] [Google Scholar]
- 19.Anderton, S., Burkhart, C., Metzler, B. & Wraith, D. (1999) Immunol. Rev. 169, 123-137. [DOI] [PubMed] [Google Scholar]
- 20.Liu, G. Y., Fairchild, P. J., Smith, R. M., Prowle, J. R., Kioussis, D. & Wraith, D. C. (1995) Immunity 3, 407-415. [DOI] [PubMed] [Google Scholar]
- 21.Wraith, D. C., Smilek, D. E., Mitchell, D. J., Steinman, L. & McDevitt, H. O. (1990) Int. Rev. Immunol. 6, 37-47. [DOI] [PubMed] [Google Scholar]
- 22.Wraith, D. C., Bruun, B. & Fairchild, P. J. (1992) J. Immunol. 149, 3765-3770. [PubMed] [Google Scholar]
- 23.Pearson, C. I., Gautam, A. M., Rulifson, I. C., Liblau, R. S. & McDevitt, H. O. (1999) Proc. Natl. Acad. Sci. USA 96, 197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, C., Liang, M. N., Tate, K. M., Rabinowitz, J. D., Beeson, C., Jones, P. P. & McConnell, H. M. (1998) J. Exp. Med. 187, 1505-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderton, S. M., Manickasingham, S. P., Burkhart, C., Luckcuck, T. A., Holland, S. J., Lamont, A. G. & Wraith, D. C. (1998) J. Immunol. 161, 3357-3364. [PubMed] [Google Scholar]
- 26.Fontenot, J. D., Rasmussen, J. P., Williams, L. M., Dooley, J. L., Farr, A. G. & Rudensky, A. Y. (2005) Immunity 22, 329-341. [DOI] [PubMed] [Google Scholar]
- 27.Montero, E., Nussbaum, G., Kaye, J. F., Perez, R., Lage, A., Ben-Nun, A. & Cohen, I. R. (2004) J. Autoimmun. 23, 1-7. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, X., Koldzic, D. N., Izikson, L., Reddy, J., Nazareno, R. F., Sakaguchi, S., Kuchroo, V. K. & Weiner, H. L. (2004) Int. Immunol. 16, 249-256. [DOI] [PubMed] [Google Scholar]
- 29.McGeachy, M. J., Stephens, L. A. & Anderton, S. A. (2005) J. Immunol. 175, 3025-3032. [DOI] [PubMed] [Google Scholar]
- 30.Klein, L., Khazaie, K. & von Boehmer, H. (2003) Proc. Natl. Acad. Sci. USA 100, 8886-8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarween, N., Chodos, A., Raykundalia, C., Khan, M., Abbas, A. K. & Walker, L. S. (2004) J. Immunol. 173, 2942-2951. [DOI] [PubMed] [Google Scholar]
- 32.McCue, D., Ryan, K. R., Wraith, D. C. & Anderton, S. M. (2004) J. Neuroimmunol. 156, 96-106. [DOI] [PubMed] [Google Scholar]
- 33.Maloy, K. J., Salaun, L., Cahill, R., Dougan, G., Saunders, N. J. & Powrie, F. (2003) J. Exp. Med. 197, 111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]