Abstract
It is well established that adrenal stress hormone-induced activation of the basolateral complex of the amygdala (BLA) influences memory consolidation. The present experiments investigated the involvement of corticotropin-releasing hormone (CRH) in the BLA in modulating memory consolidation. Bilateral infusions of the CRH receptor antagonist [9–41]-α-helical CRH (0.3, 1.0, or 3.0 μg in 0.2 μl) administered into the BLA of male Sprague–Dawley rats immediately after aversively motivated inhibitory avoidance training produced dose-dependent impairment of 48-h retention performance. Because the CRH receptor antagonist infusions did not impair retention when administered into the BLA 3 h after training, the retention impairment selectively was due to time-dependent influences on memory consolidation. Furthermore, because immediate posttraining infusions of [9–41]-α-helical CRH into the adjacent central nucleus of the amygdala (CEA) were ineffective, the effect selectively involved the BLA. Immunocytochemistry showed that the aversive training stimulus of a single, brief footshock increased CRH levels in the CEA. These findings indicate that activation of CRH receptors in the BLA, likely by training-induced release of endogenous peptide originating from the CEA, participates in mediating stress effects on memory consolidation.
Keywords: CRF‖CRH‖inhibitory avoidance‖memory storage‖neuropeptide
Enhanced long-term memory for stressful or emotionally arousing experiences is well documented. Likely mechanisms for acute stress effects on long-term memory include signaling processes induced by stressful challenges, including those of adrenal stress hormones and various neurotransmitters (1–3). It is well established that the stress-responsive neuropeptide corticotropin-releasing hormone (CRH) not only acts as a key neuroendocrine stress mediator, initiating activation of the hypothalamic–pituitary–adrenocortical axis (4, 5), but also directly modulates neuronal activity in several limbic regions (6, 7). CRH influences on learning and memory have been reported in studies of animal (8–15) and human subjects (16).
The basolateral complex of the amygdala (BLA, consisting of the basal, lateral, and accessory basal nuclei) is critically involved in mediating emotional arousal and stress hormone effects on memory consolidation (3, 17–19). The BLA contains a large population of projection neurons bearing CRH receptors (20, 21). The finding that infusions of CRH administered into the whole amygdala after training enhance inhibitory avoidance retention (22) clearly implicates the amygdala in mediating CRH effects on memory consolidation. However, studies have not yet determined whether such effects are due to activation of CRH receptors in the BLA. This is critical, because the central nucleus of the amygdala (CEA) also contains CRH receptors (20, 23) and is rich in CRH-expressing neuronal populations, many of which possess features of local-circuit neurons (24, 25). Activation of these neuronal populations in the CEA may alter anxiety, fear, or attentional processes (7, 26, 27), thereby indirectly affecting memory processes. Additionally, CRH-induced activation of neurons in the CEA may affect memory through influences on the hypothalamic–pituitary–adrenocortical axis (28–30). Also, importantly, it is not known from previous studies whether endogenous CRH, released in the amygdala during aversive training, is involved in mediating emotional arousal effects on memory consolidation.
The experiments reported here investigated the role of endogenous CRH in stress-influenced memory consolidation involving specific amygdala nuclei. In experiments in which microinfusions of the CRH receptor antagonist [9–41]-α-helical CRH were used, we investigated the effects of immediate posttraining blockade of CRH receptors in either the BLA or the CEA on retention of inhibitory avoidance training. Other groups of rats received delayed infusions (i.e., 3 h after training) of the CRH receptor antagonist into the BLA to examine whether CRH receptor antagonism specifically influenced memory consolidation. The CEA is a likely source of endogenous CRH activating CRH receptors in the BLA. In view of evidence that release of CRH from peptidergic neurons in the CEA is increased by single (31, 32) or repeated episodes of restraint stress (33), we also examined whether a single, brief aversive stimulus of mild footshock, used in the present experiments, increased CRH immunoreactivity in the CEA.
Methods
Animals.
Male Sprague–Dawley rats (n = 188; 270–300 g at time of surgery) from Charles River Breeding Laboratories were used. They were housed individually in a temperature-controlled (22°C) vivarium room and maintained on a standard 12-h light/12-h dark cycle (lights on: 7 a.m. to 7 p.m.), with food and water available ad libitum. Training and testing were performed between 10 a.m. and 3 p.m. All methods used were in compliance with National Institutes of Health guidelines and were approved by the University of California at Irvine's Institutional Animal Care and Use Committee.
Surgery.
The animals were adapted to the vivarium for 1 week before surgery. They were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and given atropine sulfate (0.4 mg/kg, i.p.) to maintain respiration. They subsequently were injected with 3.0 ml of saline to facilitate clearance of these drugs and prevent dehydration. The skull was positioned in a stereotaxic frame (Kopf Instruments, Tujunga, CA), and stainless-steel guide cannulae (15 mm; 23 gauge) were implanted bilaterally with the cannula tips 2 mm above the BLA [coordinates: anteroposterior, −2.8 mm from bregma; mediolateral, ±5.0 mm from midline; dorsoventral, −6.5 mm from skull surface] or the CEA [coordinates: anteroposterior, −2.2 mm; mediolateral, ±4.3 mm; dorsoventral, −6.0 mm] according to the atlas of Paxinos and Watson (34). Stylets (15-mm-long 00 insect dissection pins) were inserted into the cannulae to maintain patency and were removed only for the infusion of drugs. Rats were allowed to recover 7 days before initiation of training and were handled three times for 1 min each during this recovery period.
Inhibitory Avoidance Apparatus and Procedure.
Rats were trained and tested in an inhibitory avoidance apparatus consisting of a trough-shaped alley (91 cm long, 15 cm deep, 20 cm wide at the top, 6.4 cm wide at the floor) divided into two compartments separated by a sliding door that opened by retracting into the floor. The starting compartment (31 cm) was made of opaque, white plastic and well lit; the shock compartment (60 cm) was made of dark, electrifiable metal plates and was not illuminated. Training and testing were conducted in a sound- and light-attenuated room.
The rat was placed in the starting compartment of the apparatus, facing away from the door, and was allowed to enter the dark (shock) compartment. As the rat stepped completely into the dark compartment, the door was closed and a single, inescapable footshock was delivered. For the first experiment, two footshock levels were used (i.e., 0.55 mA for 1.0 s or 0.60 mA for 1.5 s) to determine whether drug effects on retention performance were experience-dependent. For all other experiments, the higher footshock condition was used. The rat was removed from the shock compartment 15 s after shock termination and, after drug treatment, returned to its home cage. On the retention test 48 h after training, the rat was placed in the starting compartment, as in the training session, and the latency to reenter the dark compartment with all four paws (maximum latency of 600 s) was recorded (note: no shock was administered on the retention test). Longer latencies were interpreted as indicating better retention. Extensive evidence indicates that avoidance of the shock area indicates specific memory of the place where shock had been received (35, 36).
Drug and Infusion Procedures.
Rats received bilateral infusions of saline or the CRH receptor antagonist [9–41]-α-helical CRH (Bachem; 0.3, 1.0, or 3.0 μg) into either the BLA or the CEA. Experimental doses were selected based on previous experiments (33, 37). The infusions were made by using 30-gauge injection needles connected to a 10-μl Hamilton microsyringe by polyethylene tubing. The injection needle protruded 2 mm beyond the cannula tip to reach either the BLA or the CEA. A 0.2-μl injection volume per hemisphere was infused over a period of 25 s by an automated syringe pump (Sage Instruments, Boston). The injection needles were retained within the cannulae for an additional 20 s after drug infusion to maximize diffusion. The infusion volume was based on findings that drug infusions of this volume into either the BLA or the CEA induce differential effects on memory consolidation (38, 39).
Histology.
Rats were anesthetized with sodium pentobarbital (≈100 mg/kg, i.p.) and perfused intracardially with 0.9% saline followed by 4% formaldehyde. Brains were removed and placed in 4% formaldehyde, followed by cryoprotection by using a 20% sucrose solution. Sections of 40 μm were cut on a freezing microtome and stained with cresyl violet. Determination of the location of the infusion needle tips was made according to standardized atlas plates of Paxinos and Watson (34). Only rats with infusion needle tips within the boundaries of the targeted nucleus were included in the data analysis. Sixty animals were excluded from further analysis because of either cannula misplacement or extensive tissue damage.
Immunocytochemistry.
Brains were harvested 30 min after inhibitory avoidance training with the higher intensity footshock (i.e., 0.60 mA for 1.5 s). Control brains were harvested under relatively stress-free conditions (40, 41). Briefly, rats (n = 4 for each group) were left undisturbed for 24 h before experiments and then were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) within 45 s of entry into the animal facility. Anesthetized rats were removed to the laboratory and perfused by using 0.9% saline followed by fresh 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB; pH 7.4, 4°C). Brains were cryoprotected and stored as described (20, 41) and then sectioned coronally into 50-μm-thick slices by using a cryostat. For neuroanatomic orientation, adjacent sections were stained with cresyl violet. Adjacent series also were processed for immunocytochemistry for parvalbumin (1:10,000; Chemicon), because parvalbumin fibers are not found in the CEA.
The immunocytochemistry procedure followed established protocols that use free-floating sections (20, 41). Briefly, after several washes with 0.01 M PBS containing 0.3% Triton X-100 (PBS-T) (pH 7.4), sections were treated for 30 min in 0.3% H2O2/PBS, followed by blockade of nonspecific sites with 2% normal goat serum in PBS for 30 min. After rinsing, sections were incubated for 36 h at 4°C with rabbit anti-CRH antiserum (1:60,000; a gift from W. W. Vale, The Salk Institute, La Jolla, CA) in 0.01 M PBS-T and 1% BSA and 2% normal goat serum and washed in 0.01 M PBS-T (3 × 5 min). Sections were incubated in biotinylated goat-anti-rabbit IgG (1:300; Vector Laboratories) in 0.01 M PBS-T for 1 h at room temperature. After washing (3 × 5 min), sections were incubated in the avidin–biotin–peroxidase complex solution (1:100; Vector Laboratories) for 2 h and rinsed (3 × 5 min), and the reaction product was visualized by incubating the sections in 0.04% 3,3′-diaminobenzidine containing 0.01% H2O2.
Statistics.
Inhibitory avoidance retention latencies were analyzed with one- or two-way ANOVAs, followed by Fisher's post hoc tests to determine the source of the significance. A probability level of less than 0.05 was accepted as statistical significance.
Results
Immediate Posttraining Infusions of a CRH Receptor Antagonist into the Basolateral Amygdala Impair Retention.
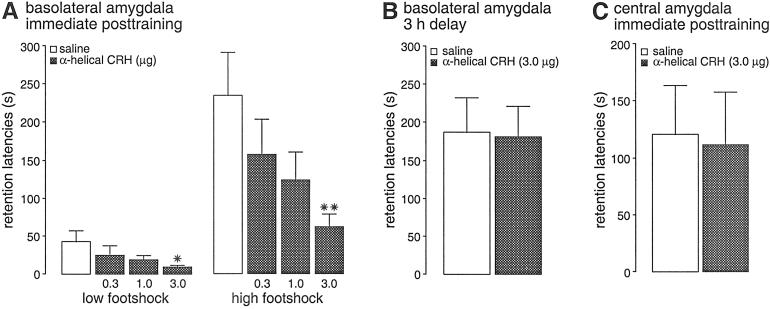
Immediate posttraining infusions of the CRH receptor antagonist [9–41]-α-helical CRH administered into the BLA impaired retention of inhibitory avoidance training by using two footshock levels. Average entrance latencies during training, before shock exposure, were 12.2 ± 0.7 s (mean ± SEM), and a two-way ANOVA indicated that these training latencies did not differ among the several posttraining treatment groups [F(3,80) = 0.57; P = 0.64; data not shown]. Latencies on the 48-h retention test of rats infused with saline were longer compared with their training latencies, in both the lower and higher footshock-level groups, indicating that the rats retained memory of the shock training (paired t tests: P = 0.05 and P < 0.005, respectively). As shown in Fig. 1A, immediate posttraining intra-BLA infusions of the CRH receptor antagonist [9–41]-α-helical CRH induced dose-dependent retention impairment in both the lower and higher footshock-level groups. Two-way ANOVA of retention latencies revealed a significant CRH receptor antagonist effect [F(3,80) = 3.83; P < 0.05], a significant footshock-level effect [F(1,80) = 30.67; P < 0.0001], and an insignificant interaction between both factors [F(3,80) = 1.75; P = 0.16]. As expected, retention latencies of saline-infused rats after training with the higher footshock level were significantly longer than those of saline-infused rats trained with the lower footshock (P < 0.005). Retention latencies of rats given the highest dose of [9–41]-α-helical CRH (3.0 μg) were impaired significantly compared with those of rats given saline infusions (lower footshock level, P < 0.05; higher footshock level, P < 0.005).
Figure 1.
Step-through latencies (mean + SEM) in seconds on a 48-h inhibitory avoidance retention test. (A) Rats given immediate posttraining infusions of saline or [9–41]-α-helical CRH (0.3, 1.0, or 3.0 μg in 0.2 μl) into the BLA after training with either a low (0.55 mA, 1.0 s) or high footshock level (0.60 mA, 1.5 s). (B) Rats given infusions of saline or [9–41]-α-helical CRH (3.0 μg in 0.2 μl) into the BLA 3 h after training (0.60 mA, 1.5 s). (C) Rats given immediate posttraining (0.60 mA, 1.5 s) infusions of saline or [9–41]-α-helical CRH (3.0 μg in 0.2 μl) into the CEA. *, P < 0.05; **, P < 0.01 as compared with the corresponding saline group (n = 7–13 per group).
To examine whether retention impairment induced by immediate posttraining administration of [9–41]-α-helical CRH was due to influences on consolidation processes, the antagonist (3.0 μg) was infused 3 h after inhibitory avoidance training in a separate group of rats. As shown in Fig. 1B, 48-h retention latencies of rats given [9–41]-α-helical CRH (3.0 μg) into the BLA 3 h after training (0.60 mA, 1.5 s) did not differ from those of rats given saline infusions 3 h after training (P = 0.92).
Immediate Posttraining Infusions of a CRH Receptor Antagonist into the Central Amygdala Do Not Affect Retention.
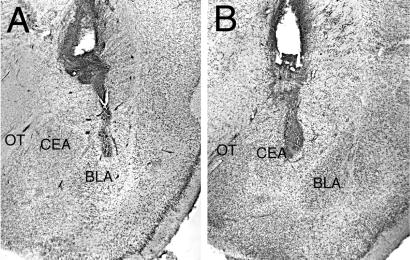
In view of the high density of CRH-producing neurons in the CEA (25), the precise site of action of endogenous CRH to enhance memory retention required elucidation. Therefore, we examined whether infusions of [9–41]-α-helical CRH administered into the CEA immediately after training (0.60 mA, 1.5 s) impaired inhibitory avoidance retention. As shown in Fig. 1C, retention latencies of rats given immediate posttraining infusions of [9–41]-α-helical CRH (3.0 μg) into the CEA did not differ from those of rats given saline infusions (P = 0.89). The precise neuroanatomic location of the CEA and BLA infusion sites, supporting the differential effects of the CRH receptor antagonist, are shown in Fig. 2.
Figure 2.
Representative photomicrographs illustrating placement of cannulae in the BLA (A) or the CEA (B). OT, optic tract.
Endogenous CRH Is Released During Inhibitory Avoidance Training.
Whereas the previous experiments clearly indicated that CRH does not act within the CEA to influence memory consolidation, the CEA contains a large group of CRH-expressing neurons. In addition, the proximity of the CEA and the BLA raised the possibility that CRH acting on receptors in the BLA originated in CEA peptidergic neurons and diffused relatively long distances (42). To examine whether inhibitory avoidance training with administration of a single, brief footshock (0.60 mA, 1.5 s) increases CRH levels in the CEA, we performed immunocytochemical analysis of CRH, focusing on cells, terminals, as well as “free” immunoreactive peptide in the neuropil of these two amygdala nuclei.
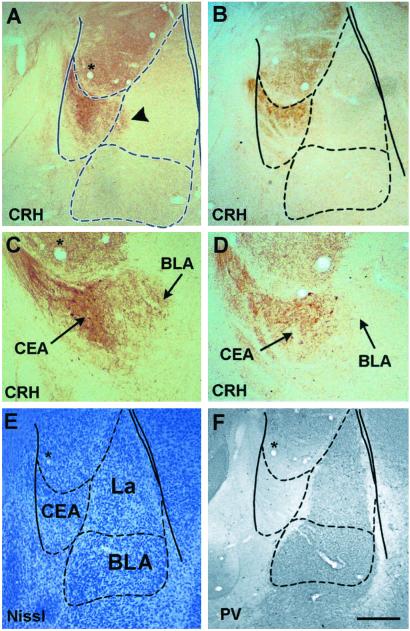
In stress-free animals (not subjected to training or footshock; see Methods), levels of “extracellular” immunoreactive CRH, not confined to cell bodies or processes, were low and contained within the neuroanatomical boundaries of the CEA (Fig. 3 B and D). In animals killed 30 min after the aversive training stimulus, an increase of immunoreactive extracellular CRH was found (Fig. 3 A and C). The BLA was delineated by using Nissl stain (Fig. 3E) and augmented by parvalbumin immunoreactivity (Fig. 3F and ref. 43).
Figure 3.
CRH is released from CEA neurons after footshock administration in an inhibitory avoidance task. Extracellular immunoreactive CRH in the CEA of rats killed 30 min after receiving the higher-intensity footshock (0.60 mA, 1.5 s) is enhanced (A and C) compared with stress-free controls (B and D) and appears to invade the BLA. Boundaries of the BLA are delineated using Nissl stain (E). Further illustration of the parvalbumin (PV)-free CEA is provided (F); note that only a portion of the lateral nucleus (La) expresses PV. Overlay is according to Paxinos and Watson (34). [Bar = 350 μm (A, B, E, and F) and 150 μm (C and D).]
Discussion
The major finding of these experiments is that immediate posttraining infusions of the CRH receptor antagonist [9–41]-α-helical CRH administered into the BLA, but not the adjacent CEA, induced dose-dependent inhibitory avoidance retention impairment. The use of posttraining infusion techniques excludes the possibility that the CRH receptor antagonist infusions altered retention by influences on anxiety, fear, locomotor activity, or attentional processes during acquisition (44, 45), effects of CRH that have been ascribed to the amygdala (26, 27, 46–48). The present study also demonstrates that infusions of [9–41]-α-helical CRH into the BLA impaired retention performance only when administered shortly after training. These findings strongly support the hypothesis that the antagonist infusions into the BLA interfered with the consolidation of long-term memory. A selective involvement of the BLA in mediating CRH receptor antagonist effects on memory consolidation is consistent with previous evidence indicating that posttraining infusions of drugs affecting several other hormonal or neurotransmitter systems, including adrenergic, glucocorticoid, and GABAergic systems, also affected memory only when administered into the BLA (39, 49, 50). Similarly, lesions of the BLA, but not the CEA, block memory modulation induced by systemic drug injections (51, 52).
Our finding that pharmacological inhibition of CRH receptors in the BLA impaired retention performance suggests that the antagonist prevented endogenous CRH from influencing memory consolidation. On the basis of the observation that the CRH receptor antagonist impaired retention performance irrespective of whether rats were trained on a low or high footshock level, it appears that CRH receptors in the BLA are stimulated either by mildly aversive training conditions or that they are tonically activated. Stress-induced increases in amygdalar CRH were found previously with more severe stressors such as single or repeated restraint (31, 32, 47). The present findings demonstrated that inhibitory avoidance training with a single, brief footshock markedly increased CRH immunoreactivity in the CEA, findings that support the hypothesis that CRH, released by the aversive training condition, participates in mediating stress effects on memory consolidation. It should be noted that within the amygdala, CRH mRNA-expressing cells and CRH-immunoreactive somata generally are confined to the CEA (25), where they comprise a large and heterogeneous neuronal population (24). Whereas the current experiments pinpoint the site of action (i.e., receptor activation) of endogenous amygdalar CRH, they do not distinguish between two possible release mechanisms of the peptide. CRH might be released within the CEA by the stressful training stimulus and travel to the BLA, consistent with the documented transport of exogenous peptide for long distances within the brain (42). The alternative possibility, that axon terminals of neurons located in the CEA might reach the BLA, releasing the peptide in close proximity to receptor-bearing target neurons, is not supported by anatomical studies (53).
If CRH diffuses from the CEA to the BLA, why was this not observed with the antagonist, which did not impair inhibitory avoidance retention when infused into the CEA? Findings have demonstrated that the [9–41]-α-helical CRH molecule is much less diffusible in aqueous tissues compared with CRH. Whereas ready diffusion of CRH from the lateral cerebral ventricle to the amygdala clearly has been demonstrated (42, 54), the less soluble antagonist would be far less diffusible (reviewed in ref. 55). In addition, the doses of antagonist infused into the CEA were sufficient to block all endogenous CRH: ratios of 6:1 to 12:1 of the CRH antagonist to the native peptide are required for fully antagonizing the central actions of CRH (56). Because the amounts of CRH in the CEA (0.62 ng/mg protein) are lower than 1 ng (57), the 0.3 μg of [9–41]-α-helical CRH infused into the CEA was ample to antagonize all local actions of native CRH.
Extensive evidence is consistent with a role of CRH in mediating stress effects on memory consolidation (refs. 8, 12, 15, 58, and 59; for a review, see ref. 60), but effects on memory retrieval also have been reported (37, 61). Furthermore, electrophysiological studies in hippocampal slices have shown that exogenous CRH application facilitates long-term potentiation (15, 62, 63) and that a CRH receptor antagonist blocks stress-induced facilitation of hippocampal long-term potentiation (15). Moreover, CRH produces a long-lasting enhancement of synaptic efficacy in the rat hippocampus in vivo (64, 65) and enhances protein synthesis (10). Previous evidence that posttraining infusions of a large volume of exogenous CRH into the amygdaloid complex enhanced memory consolidation for inhibitory avoidance training (22) is also compatible with our findings and suggests that CRH modulates amygdala influences on memory consolidation. Other studies have implicated CRH in the amygdala in reward-related learning. Local infusions of [9–41]-α-helical CRH or injections of a CRH antibody/toxin mixture into the amygdala reversed the negative motivational consequences of morphine withdrawal in a place-conditioning paradigm and in a conditioned operant suppression task in rats (66). Although that study did not aim specifically at the BLA, other studies reported that selective BLA lesions abolish the ability of drug-associated cues to reinstate responding during withdrawal from self-administered cocaine (67), supporting the view that the BLA may have been the site of action of CRH. Furthermore, Ambrosio et al. (68) reported a large reduction in CRF1-binding sites in the BLA after chronic cocaine administration. Several recent findings have suggested an involvement of CRF1 receptors in learning and memory functions (12). The BLA is particularly rich in CRF1 receptors (20, 69). Thus, although [9–41]-α-helical CRH is a nonspecific CRH receptor antagonist, which can bind to both CRF1 and CRF2 receptors, the memory impairments found in the present study may have been mediated selectively by a blockade of CRF1 receptors.
Several studies examining CRH effects in brain regions other than the BLA have indicated an intimate relationship with the noradrenergic system, affecting anxiety, arousal, and attention (70–74). These effects appear to be mediated by the CRF1 receptor (73). CRH–noradrenergic interactions also play a role in memory: the β-adrenoceptor antagonist propranolol or the noradrenergic toxin DSP-4 blocks memory enhancement induced by CRH infusions into the hippocampus (75). These findings would suggest that CRH, through a presynaptic facilitation mechanism, stimulates the release of norepinephrine in the hippocampus (76). Moreover, both the β-adrenoceptor and CRH receptor are coupled to the adenylate cyclase system (77–79), providing an additional potential locus of interaction at the postsynaptic level. These findings are highly relevant to CRH effects on memory modulation in the BLA because extensive evidence has demonstrated a central role for norepinephrine in the BLA in memory modulation. Stressful stimulation, including inhibitory avoidance training, induces the release of norepinephrine in the amygdala (80–82), whereas posttraining infusions of norepinephrine or β-adrenoceptor agonists administered into the BLA enhance memory consolidation (50, 83, 84). Furthermore, blockade of noradrenergic mechanisms in the BLA with a β-adrenoceptor antagonist prevents memory enhancement induced by systemic or intra-BLA administration of a glucocorticoid receptor agonist (84, 85). These findings lend support for the view that CRH, perhaps via an interaction with glucocorticoids (86, 87), may interact with the noradrenergic system of the BLA in influencing memory consolidation.
In summary, the present findings show that [9–41]-α-helical CRH impairs memory consolidation when administered into the BLA immediately after training. These findings suggest that acute stress induces rapid CRH release from CEA neurons and activation of CRH receptors on BLA neurons involved in modulating memory consolidation. This stress-activated mechanism serves the adaptive function of influencing the consolidation of strong, long-lasting memories of emotionally significant experiences.
Acknowledgments
We thank Christina Buranday, Emily Hahn, and Sheila Nathan for excellent technical assistance, and Dr. Yuncai Chen for his advice. This work was supported by U.S. Public Health Service Grants MH12526 (to J.L.M.), and NS28912 and NS39307 (to T.Z.B.).
Abbreviations
- BLA
basolateral complex of the amygdala
- CEA
central nucleus of the amygdala
- CRH
corticotropin-releasing hormone
References
- 1.De Wied D. In: Peripheral Signaling of the Brain. Frederickson R C A, McGaugh J L, Felten D L, editors. Toronto: Hogrefe and Huber; 1991. pp. 403–420. [Google Scholar]
- 2.Bohus B. In: The Memory Systems of the Brain. Delacour J, editor. Teaneck, NJ: World Scientific; 1994. pp. 337–364. [Google Scholar]
- 3.McGaugh J L, Roozendaal B. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 4.Spiess J, Rivier J, Rivier C, Vale W. Proc Natl Acad Sci USA. 1981;78:6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vale W, Spiess J, Rivier C, Rivier J. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 6.Chalmers D T, Lovenberg T W, De Souza E B. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koob G F. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 8.Sahgal A, Wright C, Edwardson J A, Keith A B. Neurosci Lett. 1983;36:81–86. doi: 10.1016/0304-3940(83)90490-1. [DOI] [PubMed] [Google Scholar]
- 9.Chen M F, Chui T H, Lee E H Y. Psychoneuroendocrinology. 1992;17:113–124. doi: 10.1016/0306-4530(92)90050-h. [DOI] [PubMed] [Google Scholar]
- 10.Lee E H, Hung H C, Lu K T, Chen W H, Chen H Y. Peptides. 1992;13:927–937. doi: 10.1016/0196-9781(92)90051-4. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y L, Chen K Y, Wei C L, Lee E H. Chin J Physiol. 1999;42:73–81. [PubMed] [Google Scholar]
- 12.Radulovic J, Rumhann A, Liepold T, Spiess J. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croiset G, Nijsen M J M A, Kamphuis P J G H. Eur J Pharmacol. 2000;405:225–234. doi: 10.1016/s0014-2999(00)00556-2. [DOI] [PubMed] [Google Scholar]
- 14.Brunson K L, Eghbal-Ahmadi M, Bender R, Chen Y, Baram T Z. Proc Natl Acad Sci USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blank T, Nijholt I, Eckart K, Spiess J. J Neurosci. 2002;22:3788–3794. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behan D P, Heinrichs S C, Troncoso J C, Liu X J, Kawas C H, Ling N, De Souza E B. Nature. 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 17.McGaugh J L, Cahill L, Roozendaal B. Proc Natl Acad Sci USA. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGaugh J L. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 19.Roozendaal B. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Brunson K L, Muller M B, Cariaga W, Baram T Z. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Pett K, Viau V, Bittencourt J C, Chan R K, Li H Y, Arias C, Prins G S, Perrin M, Vale W, Sawchenko P E. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Liang K C, Lee E H Y. Psychopharmacology. 1988;96:232–236. doi: 10.1007/BF00177566. [DOI] [PubMed] [Google Scholar]
- 23.De Souza E B, Insel T R, Perrin M H, Rivier J, Vale W W, Kuhar M J. J Neurosci. 1985;5:3189–3203. doi: 10.1523/JNEUROSCI.05-12-03189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uryu K, Okumura T, Shibasaki T, Sakanaka M. Brain Res. 1992;577:175–179. doi: 10.1016/0006-8993(92)90554-m. [DOI] [PubMed] [Google Scholar]
- 25.Gray T S, Bingaman E W. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- 26.Liebsch G, Landgraf R, Gerstberger R, Probst J C, Wotjak C T, Engelmann M, Holsboer F, Montkowski A. Regul Peptides. 1995;59:229–239. doi: 10.1016/0167-0115(95)00099-w. [DOI] [PubMed] [Google Scholar]
- 27.Wiersma A, Baauw A D, Bohus B, Koolhaas J M. Psychoneuroendocrinology. 1995;20:423–432. doi: 10.1016/0306-4530(94)00074-3. [DOI] [PubMed] [Google Scholar]
- 28.Van de Kar L D, Piechowski R A, Rittenhouse P A, Gray T S. Neuroendocrinology. 1991;54:89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- 29.Schulkin J, McEwen B S, Gold P W. Neurosci Biobehav Rev. 1994;18:385–396. doi: 10.1016/0149-7634(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 30.Roozendaal B, Koolhaas J M, Bohus B. Acta Physiol Scand. 1997;640,Suppl.:51–54. [PubMed] [Google Scholar]
- 31.Kalin N H, Takahashi L K, Chen F-L. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- 32.Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. J Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatalski C G, Guirguis C, Baram T Z. J Neuroendocrinol. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd Ed. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- 35.Gold P E. Behav Neural Biol. 1986;46:87–98. doi: 10.1016/s0163-1047(86)90927-1. [DOI] [PubMed] [Google Scholar]
- 36.Liang K C. In: Memory Consolidation: Essays in Honor of James L. McGaugh. Gold P E, Greenough W T, editors. Washington, DC: Am. Psychol. Assoc.; 2001. pp. 165–183. [Google Scholar]
- 37.Kumar K B, Karanth K S. J Neural Transm. 1996;103:1117–1126. doi: 10.1007/BF01291796. [DOI] [PubMed] [Google Scholar]
- 38.Parent M B, McGaugh J L. Brain Res. 1994;661:97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- 39.Roozendaal B, McGaugh J L. Neurobiol Learn Mem. 1997;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- 40.Yan X X, Toth Z, Schultz L, Ribak C E, Baram T Z. Hippocampus. 1998;8:231–243. doi: 10.1002/(SICI)1098-1063(1998)8:3<231::AID-HIPO6>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Bender R A, Frotscher M, Baram T Z. J Neurosci. 2001;21:7171–7181. doi: 10.1523/JNEUROSCI.21-18-07171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bittencourt J C, Sawchenko P E. J Neurosci. 2000;20:1142–1156. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kemppainen S, Pitkanen A. J Comp Neurol. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 44.McGaugh J L. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- 45.McGaugh J L. Brain Res Bull. 1989;23:339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- 46.Liang K C, Melia K R, Campeau S, Falls W A, Miserendino M J, Davis M. J Neurosci. 1992;12:2313–2320. doi: 10.1523/JNEUROSCI.12-06-02313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swiergel A H, Takahashi L K, Kalin N H. Brain Res. 1993;623:229–234. doi: 10.1016/0006-8993(93)91432-r. [DOI] [PubMed] [Google Scholar]
- 48.Lee Y, Davis M. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Da Cunha C, Roozendaal B, Vazdarjanova A, McGaugh J L. Neurobiol Learn Mem. 1999;72:1–7. doi: 10.1006/nlme.1999.3912. [DOI] [PubMed] [Google Scholar]
- 50.Ferry B, Roozendaal B, McGaugh J L. J Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomaz C, Dickinson-Anson H, McGaugh J L. Proc Natl Acad Sci USA. 1992;89:3615–3619. doi: 10.1073/pnas.89.8.3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roozendaal B, McGaugh J L. Neurobiol Learn Mem. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- 53.Pitkanen A, Savander V, LeDoux J E. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 54.Dube C, Brunson K, Nehlig A, Baram T Z. J Cereb Blood Metab. 2000;20:1414–1424. doi: 10.1097/00004647-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hauger R L, Dauzenberg F M. In: Neuroendocrinology in Physiology and Medicine. Conn P M, Freeman E, editors. Clifton, NJ: Humana; 2000. pp. 261–286. [Google Scholar]
- 56.Fisher L, Rivier C, Rivier J, Brown M. Endocrinology. 1991;129:312–316. doi: 10.1210/endo-129-3-1312. [DOI] [PubMed] [Google Scholar]
- 57.Palkovits M, Brownstein M J, Vale W. Fed Proc. 1985;44:215–219. [PubMed] [Google Scholar]
- 58.Koob G F, Bloom F E. Fed Proc. 1985;44:259–263. [PubMed] [Google Scholar]
- 59.Heinrichs S C, Vale E A, Lapsansky J, Behan D P, McClure L V, Ling N, De Souza E B, Schulteis G. Peptides. 1997;18:711–716. doi: 10.1016/s0196-9781(97)00120-4. [DOI] [PubMed] [Google Scholar]
- 60.Steckler T, Holsboer F. Biol Psychiatry. 1999;46:1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 61.Kikusui T, Takeuchi Y, Mori Y. Physiol Behav. 2000;71:323–328. doi: 10.1016/s0031-9384(00)00352-8. [DOI] [PubMed] [Google Scholar]
- 62.Aldenhoff J B, Gruol D L, Rivier J, Vale W, Siggins G R. Science. 1983;22:875–877. doi: 10.1126/science.6603658. [DOI] [PubMed] [Google Scholar]
- 63.Hollrigel G S, Chen K, Baram T Z, Soltesz I. Neuroscience. 1998;84:71–79. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H L, Wayner M J, Chai C Y, Lee E H Y. Eur J Neurosci. 1998;10:3428–3437. doi: 10.1046/j.1460-9568.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- 65.Wang H L, Tsai L Y, Lee E H Y. J Neurophysiol. 2000;83:343–349. doi: 10.1152/jn.2000.83.1.343. [DOI] [PubMed] [Google Scholar]
- 66.Heinrichs S C, Menzaghi F, Schulteis G, Koob G F, Stinus L. Behav Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- 67.Meil W M, See R E. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- 68.Ambrosio E, Sharpe L G, Pilotte N S. Synapse. 1997;25:272–276. doi: 10.1002/(SICI)1098-2396(199703)25:3<272::AID-SYN6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 69.Baram T Z, Hatalski C G. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lavicky J, Dunn A J. J Neurochem. 1993;60:602–612. doi: 10.1111/j.1471-4159.1993.tb03191.x. [DOI] [PubMed] [Google Scholar]
- 71.Pan J T, Lookingland K J, Moore K E. J Biomed Sci. 1995;2:50–56. doi: 10.1007/BF02257925. [DOI] [PubMed] [Google Scholar]
- 72.Liang K C, Chen H C, Chen D Y. Chin J Physiol. 2001;44:33–43. [PubMed] [Google Scholar]
- 73.Sauvage M, Steckler T. Neuroscience. 2001;104:643–652. doi: 10.1016/s0306-4522(01)00137-3. [DOI] [PubMed] [Google Scholar]
- 74.Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand J-P, Soubrié P. J Pharmacol Exp Ther. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- 75.Lee E H Y, Lee C P, Wang H I, Lin W R. Synapse. 1993;14:144–153. doi: 10.1002/syn.890140207. [DOI] [PubMed] [Google Scholar]
- 76.Lee E H Y, Chang S Y, Chen A Y. Neurosci Res. 1994;19:327–330. doi: 10.1016/0168-0102(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 77.Perkins J P, Moore M M. J Pharmacol Exp Ther. 1973;185:371–378. [PubMed] [Google Scholar]
- 78.Daly J W, Padgett W, Creveling D, Cantacuzene D, Kirk K L. J Neurosci. 1981;1:49–59. doi: 10.1523/JNEUROSCI.01-01-00049.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kapcala L P, Aguilera G. Brain Res. 1995;678:207–212. doi: 10.1016/0006-8993(95)00184-r. [DOI] [PubMed] [Google Scholar]
- 80.Galvez R, Mesches M H, McGaugh J L. Neurobiol Learn Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- 81.Quirarte G L, Galvez R, Roozendaal B, McGaugh J L. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- 82. McIntyre, C. K., Hatfield, T. & McGaugh, J. L. (2002) Eur. J. Neurosci., in press. [DOI] [PubMed]
- 83.Hatfield T, McGaugh J L. Neurobiol Learn Mem. 1999;71:232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- 84.Roozendaal B, Quirarte G L, McGaugh J L. Eur J Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- 85.Quirarte G L, Roozendaal B, McGaugh J L. Proc Natl Acad Sci USA. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chappell P B, Smith M A, Kilts C D, Bissette G, Ritchie J, Anderson C, Nemeroff C M. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Owens M J, Bartolome J, Schanberg S M, Nemeroff C B. Neuroendocrinology. 1990;52:626–631. doi: 10.1159/000125655. [DOI] [PubMed] [Google Scholar]