Abstract
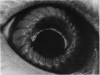
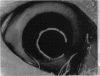
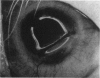
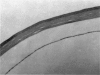
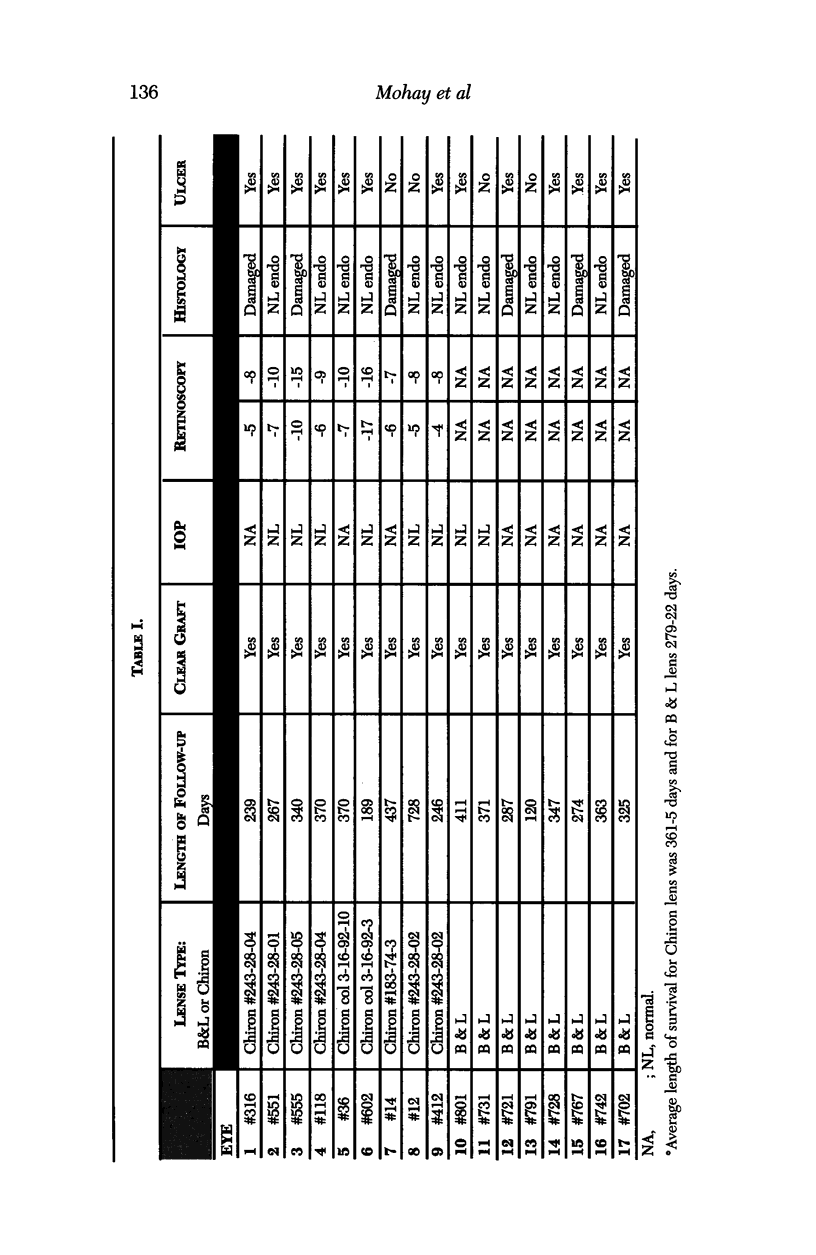
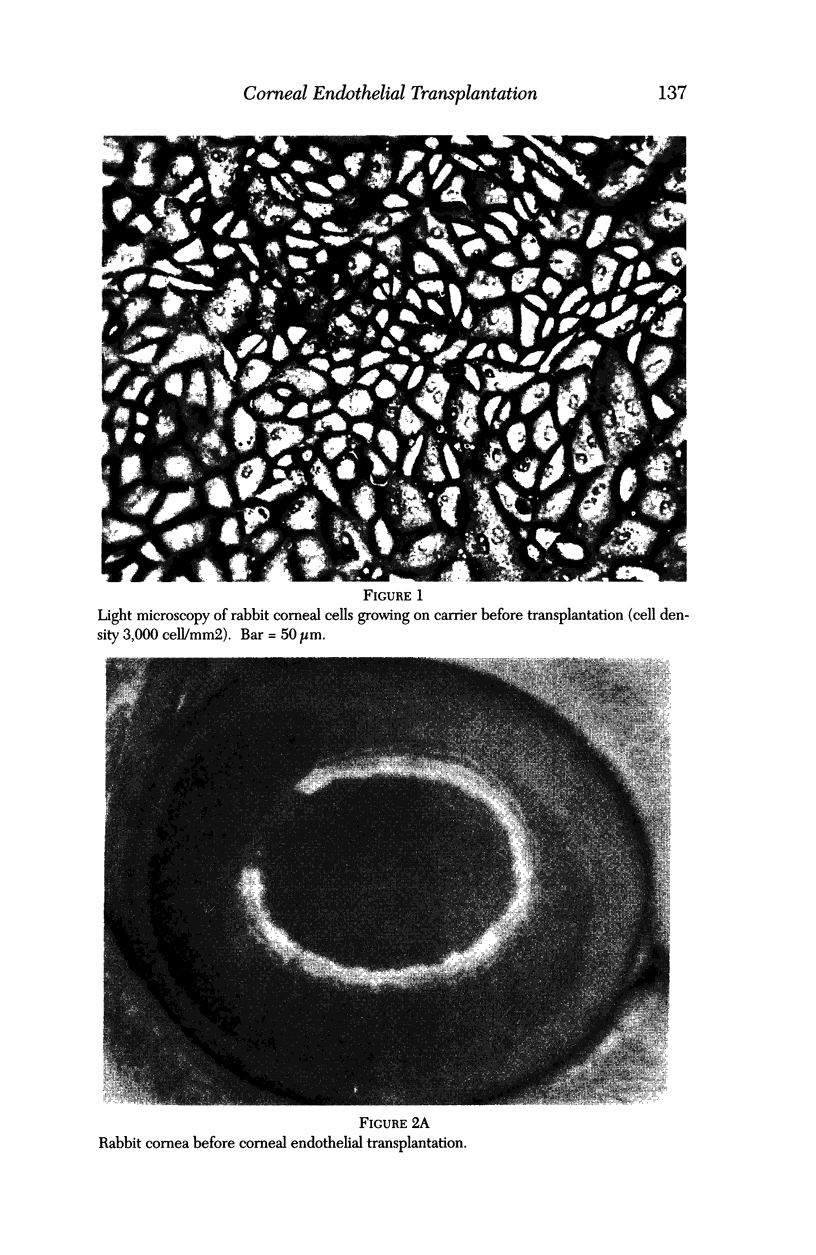
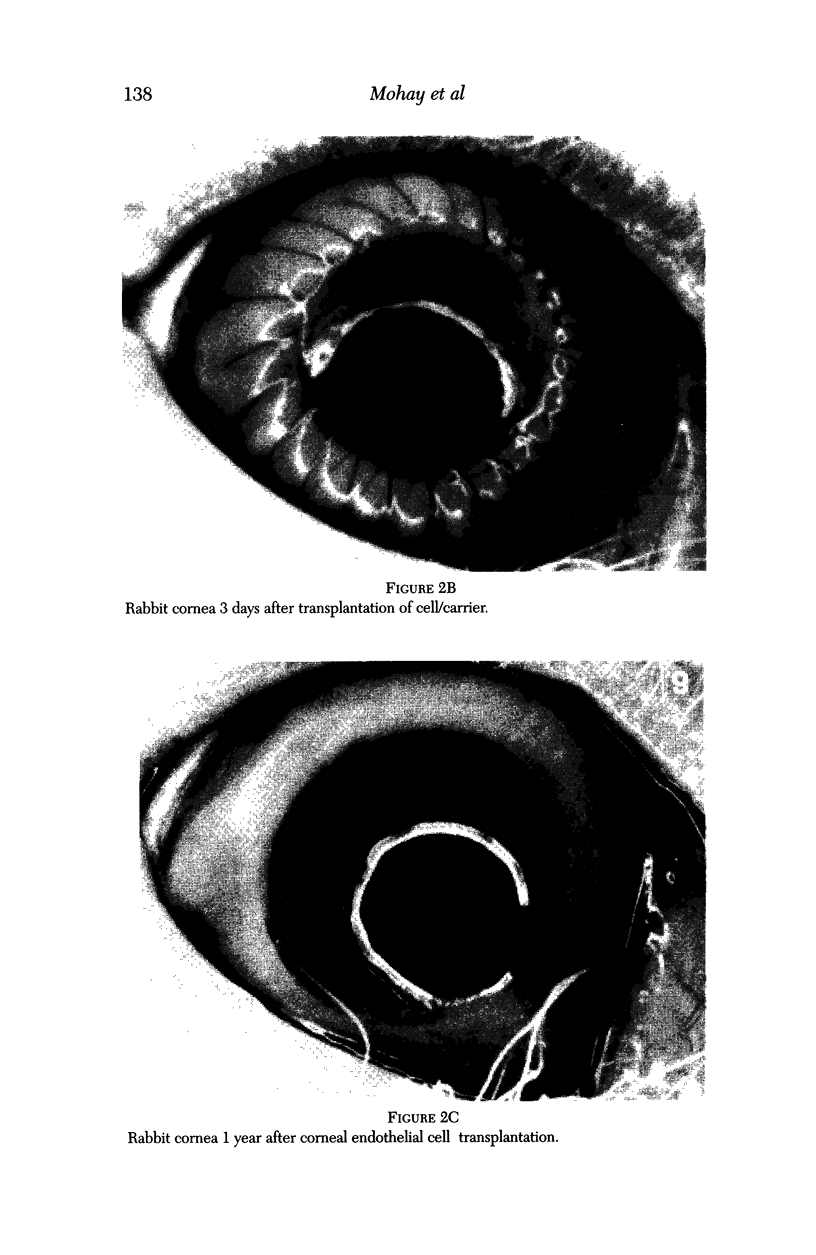
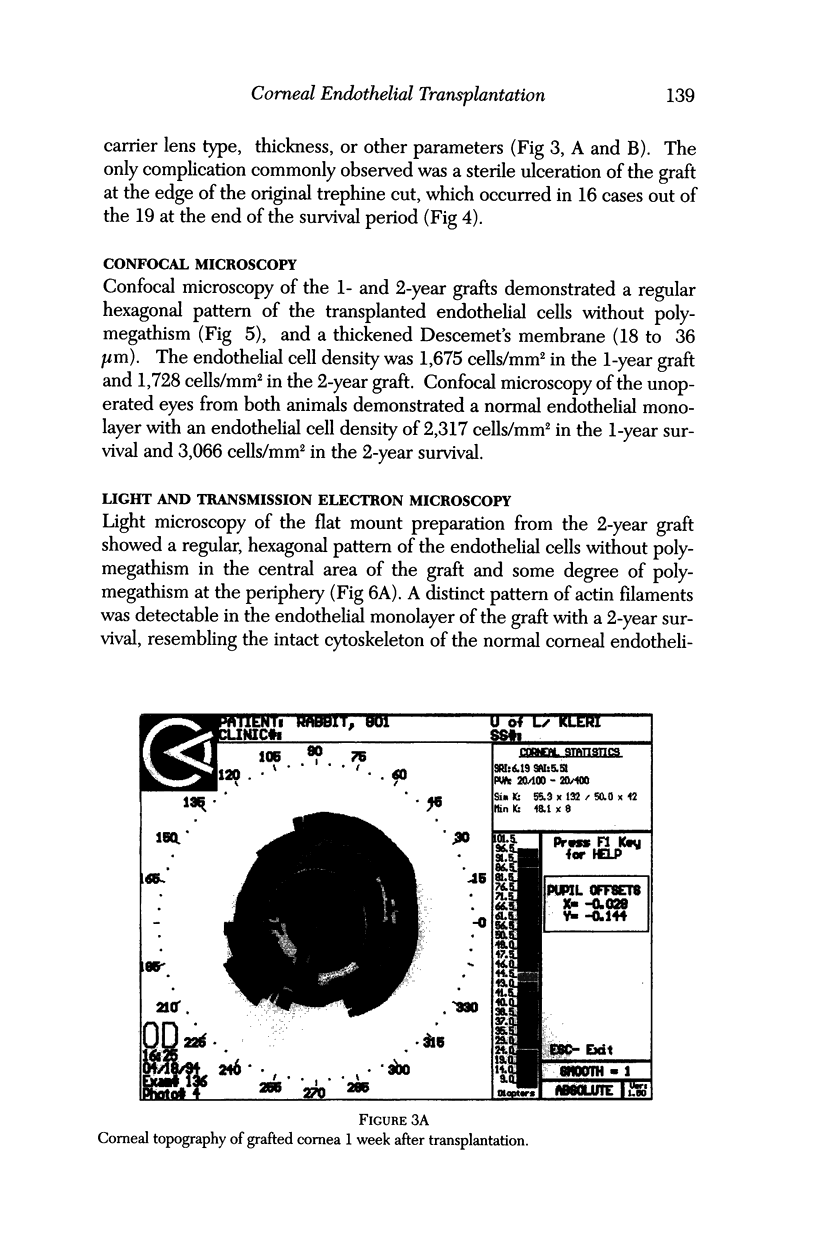
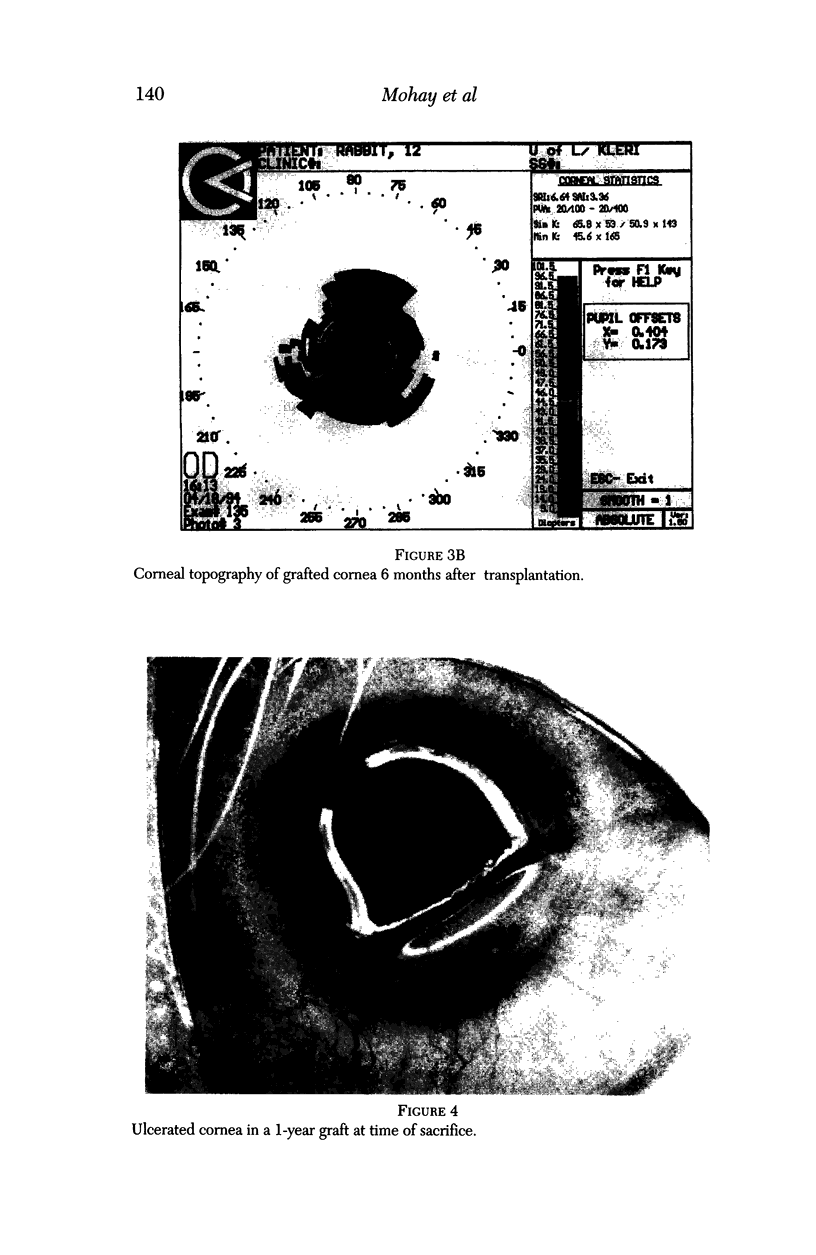
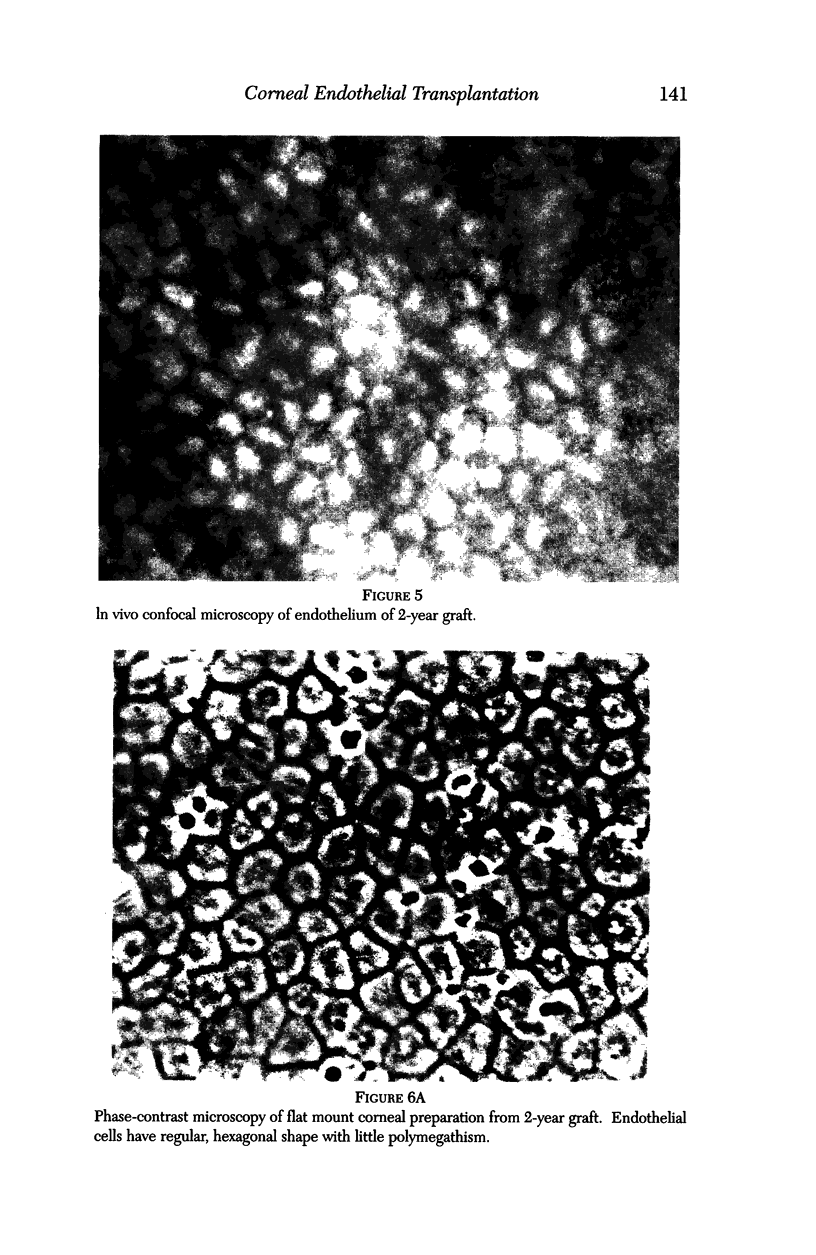
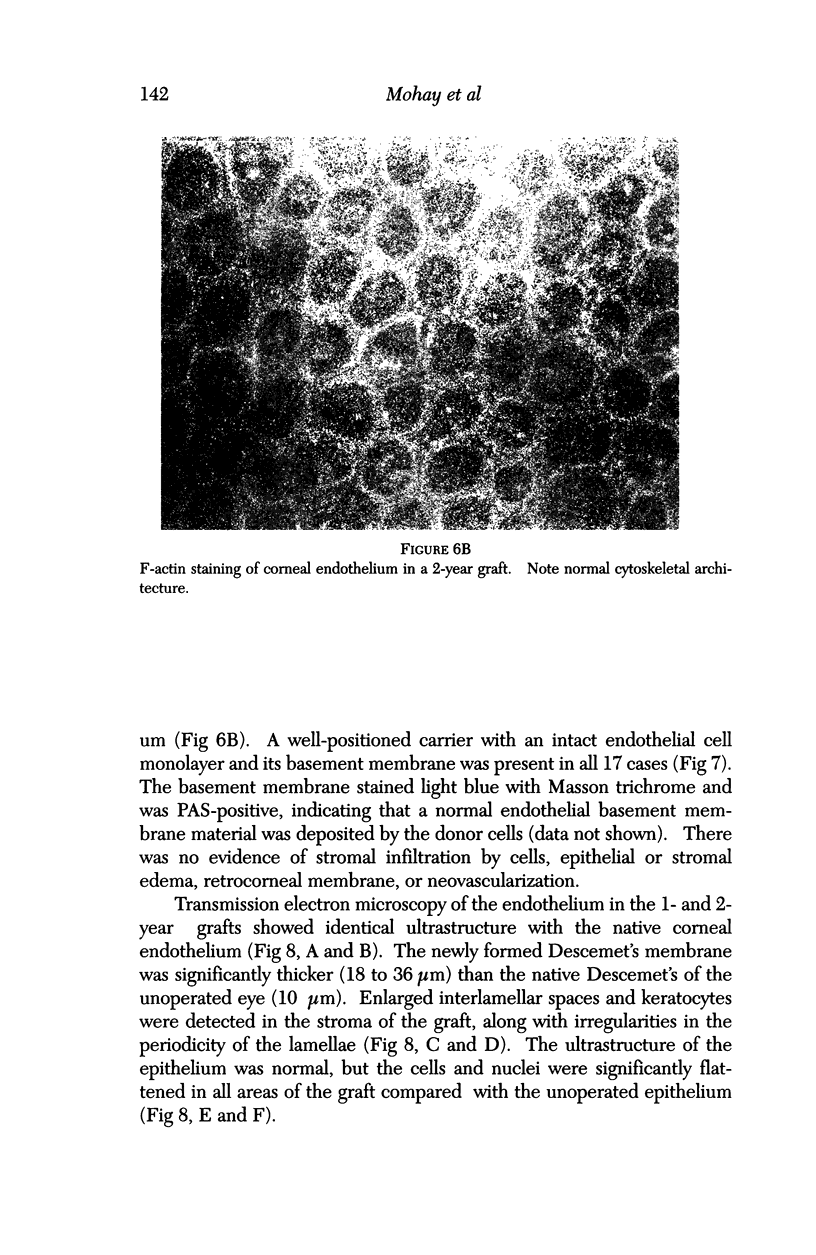
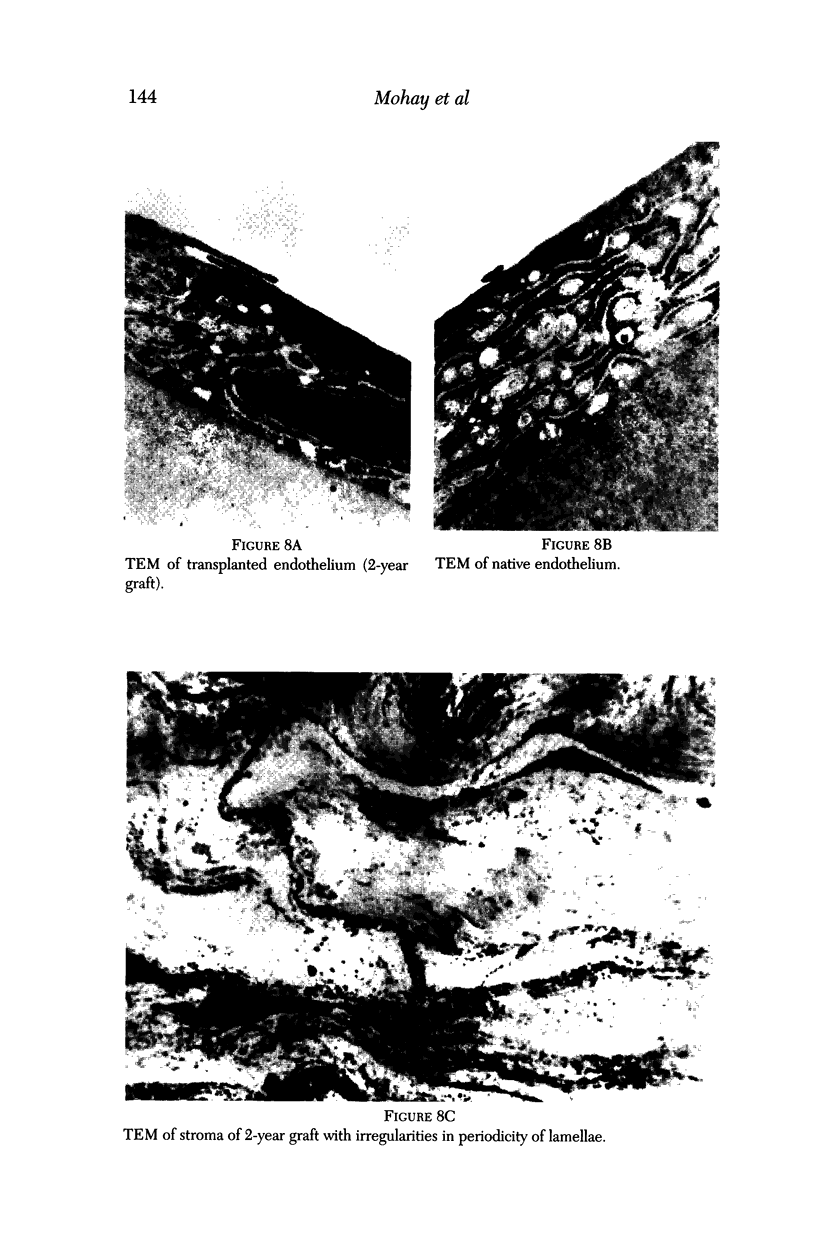
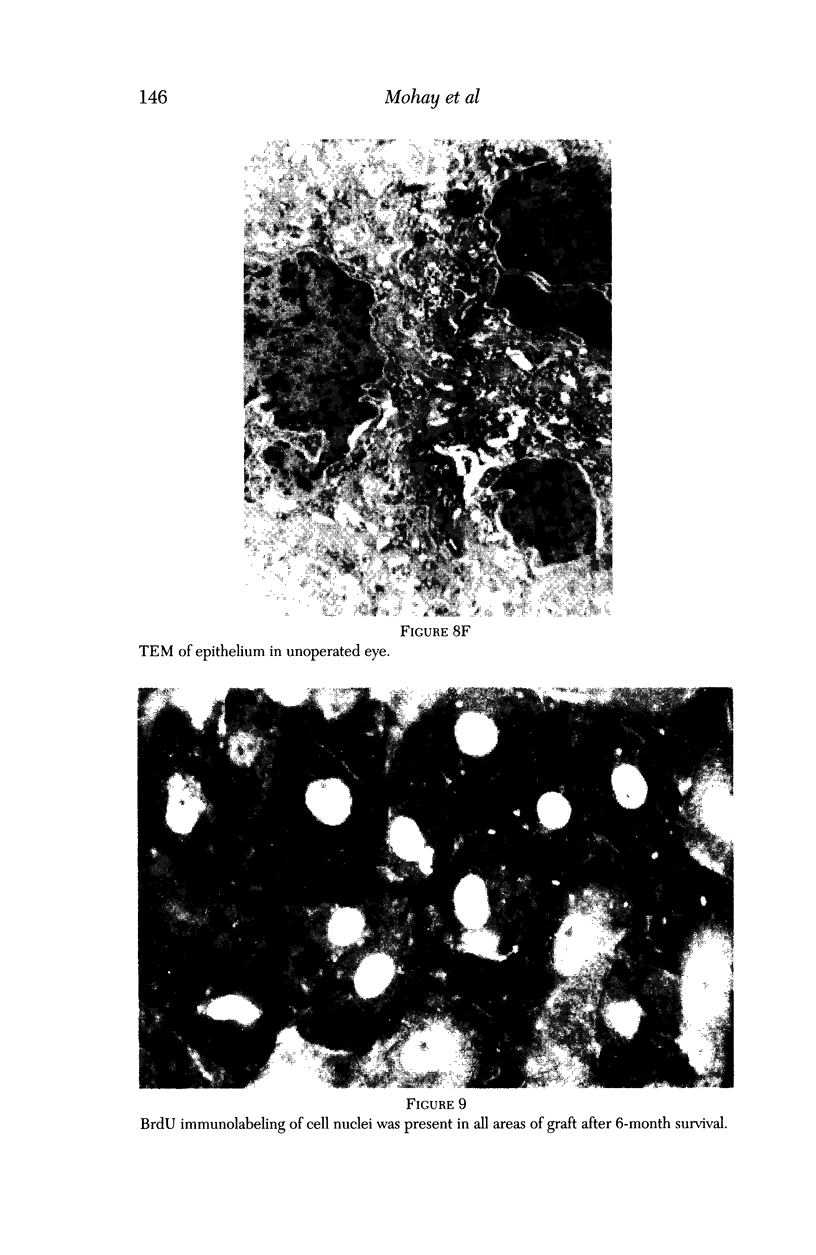
PURPOSE: This report describes the clinical course, refractive changes, confocal microscopic and histological evaluation of corneal endothelial cell transplantation in rabbits with long-term follow-up. METHODS: Transplantation of corneal endothelial cells using a cell/carrier device was performed in 19 rabbits. Clinical evaluation between 1-25 months included slit-lamp examination, keratometry, retinoscopy and surface topography. Two grafts in rabbits with 12 and 24 month survivals were evaluated in vivo by 3D tandem scanning confocal microscopy. The same grafts were then processed for transmission electron microscopy. BrdU labeling of the grafted cells in one transplant was performed in order to distinguish between host and grafted endothelial cells. RESULTS: All grafts cleared and remained clear for an average of one year without signs of rejection or inflammation. Postoperative refraction data and topography of the transplants showed progressive development of myopia and steep corneas compared to the unoperated eyes in each case. Confocal microscopy in vivo demonstrated a regular hexagonal pattern of the transplanted endothelial cells and a thickened Descemet's membrane, which correlated with the light and electron microscopic findings. BrdU labeling of the grafted endothelial cells showed a homogenous labeling of cell nuclei 6 months after the transplantation. CONCLUSIONS: This study demonstrates that corneal endothelial cells grown on a biomaterial can be replaced and remain functional for a long period of time.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado J. A., Gospodarowicz D., Greenburg G. Corneal endothelial replacement. I. In vitro formation of an endothelial monolayer. Invest Ophthalmol Vis Sci. 1981 Aug;21(2):300–316. [PubMed] [Google Scholar]
- Assil K. K., Barrett A. M., Fouraker B. D., Schanzlin D. J. One-year results of the intrastromal corneal ring in nonfunctional human eyes. Intrastromal Corneal Ring Study Group. Arch Ophthalmol. 1995 Feb;113(2):159–167. doi: 10.1001/archopht.1995.01100020041026. [DOI] [PubMed] [Google Scholar]
- Bahn C. F., MacCallum D. K., Lillie J. H., Meyer R. F., Martonyi C. L. Complications associated with bovine corneal endothelial cell-lined homografts in the cat. Invest Ophthalmol Vis Sci. 1982 Jan;22(1):73–90. [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Alvarado J. Transplantation of cultured bovine corneal endothelial cells to rabbit cornea: clinical implications for human studies. Proc Natl Acad Sci U S A. 1979 Jan;76(1):464–468. doi: 10.1073/pnas.76.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Alvarado J. Transplantation of cultured bovine corneal endothelial cells to species with nonregenerative endothelium. The cat as an experimental model. Arch Ophthalmol. 1979 Nov;97(11):2163–2169. doi: 10.1001/archopht.1979.01020020481016. [DOI] [PubMed] [Google Scholar]
- Insler M. S., Lopez J. G. Heterologous transplantation versus enhancement of human corneal endothelium. Cornea. 1991 Mar;10(2):136–148. doi: 10.1097/00003226-199103000-00009. [DOI] [PubMed] [Google Scholar]
- Insler M. S., Lopez J. G. Transplantation of cultured human neonatal corneal endothelium. Curr Eye Res. 1986 Dec;5(12):967–972. doi: 10.3109/02713688608995178. [DOI] [PubMed] [Google Scholar]
- Jumblatt M. M., Maurice D. M., McCulley J. P. Transplantation of tissue-cultured corneal endothelium. Invest Ophthalmol Vis Sci. 1978 Dec;17(12):1135–1141. [PubMed] [Google Scholar]
- Mohay J., Lange T. M., Soltau J. B., Wood T. O., McLaughlin B. J. Transplantation of corneal endothelial cells using a cell carrier device. Cornea. 1994 Mar;13(2):173–182. doi: 10.1097/00003226-199403000-00011. [DOI] [PubMed] [Google Scholar]
- Parks R. A., McCarey B. E., Knight P. M., Storie B. R. Intrastromal crystalline deposits following hydrogel keratophakia in monkeys. Cornea. 1993 Jan;12(1):29–34. doi: 10.1097/00003226-199301000-00006. [DOI] [PubMed] [Google Scholar]
- Peppas N. A., Langer R. New challenges in biomaterials. Science. 1994 Mar 25;263(5154):1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- Ratner B. D. New ideas in biomaterials science--a path to engineered biomaterials. J Biomed Mater Res. 1993 Jul;27(7):837–850. doi: 10.1002/jbm.820270702. [DOI] [PubMed] [Google Scholar]
- Samples J. R., Binder P. S., Zavala E. Y., Baumgartner S. D., Deg J. K. Morphology of hydrogel implants used for refractive keratoplasty. Invest Ophthalmol Vis Sci. 1984 Jul;25(7):843–850. [PubMed] [Google Scholar]
- Schwartz B. D., McCulley J. P. Morphology of transplanted corneal endothelium derived from tissue culture. Invest Ophthalmol Vis Sci. 1981 Apr;20(4):467–480. [PubMed] [Google Scholar]