Abstract
The formation of new branched actin filament networks at the cell cortex of migrating cells is choreographed by the actin-related protein (Arp) 2/3 complex. Despite the fundamental role of the Arp2/3 complex in actin nucleation and branching, upstream signals that control the functions of p41-Arc, a putative regulatory component of the mammalian Arp2/3 complex, remain unidentified. Here we show that p41-Arc interacts with p21-activated kinase 1 (Pak1) both in vitro and in vivo. Pak1 phosphorylation of p41-Arc regulates its localization with the Arp2/3 complex in the cortical nucleation regions of cells. Pak1 phosphorylates p41-Arc on threonine 21 in the first WD repeat, and its mutation has functional implications in vivo. Threonine 21 phosphorylation by Pak1 is required for both constitutive and growth-factor-induced cell motility. Pak1 regulation of p41-Arc activation status represents a novel mechanism by which signalling pathways may influence the functions of the Arp2/3 complex, leading to motility in mammalian cells.
Introduction
The process of eukaryotic cell motility, or movement past or through a substrate, is important in both normal and disease processes. In many tissues, cell motility functions are normally inactive, but can be activated by appropriate stimuli, receptor tyrosine kinases or oncogenic transformation (Hall, 1998). In mammalian cells, the generation of actin-based dynamic motile structures such as actin stress fibres, filopodia/lamellipodia and membrane ruffles is regulated by the small GTPases Rho, Cdc42 and Rac1, respectively (Bishop & Hall, 2000). Engagement and activation of downstream effectors and/or kinases mediate the cellular effects of the small GTPases. For example, the p21-activated kinases (Paks) are targets for Cdc42 and Rac1. Stimulation of Pak activity produces phenotypic changes similar to those produced by Rac1 and Cdc42 (Kumar & Vadlamudi, 2002). Pak1 also activates LIM kinase 1, which in turn phosphorylates and inactivates cofilin, resulting in reduced actin filament depolymerization (Edwards et al, 1999).
The Arp2/3 complex is required for the formation of branched networks of actin filaments at the cell cortex. A number of proteins that activate the Arp2/3 complex has been identified (Weaver et al, 2003). The Wiskott–Aldrich syndrome (WASP) family comprises well-studied activators of the Arp2/3 complex. Once activated, the Arp2/3 complex nucleates new actin filaments and also crosslinks actin filaments from end to side-branch, thus creating new barbed ends, which drive the extension of the cell's leading edge (Mullins et al, 1998). Although multiple extracellular signals trigger rapid actin polymerization, how signalling pathways ultimately target the Arp2/3 complex is not known.
The Arp2/3 complex consists of seven subunits (Arp2, Arp3, p16, p20, p21, p34 and p41) (Welch, 1999; Mullins & Pollard, 1999). Unlike other components of the Arp2/3 complex, p41-Arc may not be involved in actin polymerization but instead is proposed to have a regulatory role, possibly facilitating assembly and maintenance of the Arp2/3 complex (Gournier et al, 2001). p41-Arc is unique in that it contains seven evenly spaced WD repeats and may undergo post-translational modification producing p40-Arc and p41-Arc isoforms, which may regulate structural aspects of the Arp2/3 complex (Welch et al, 1997). Importantly, p40-Arc is essential for cell viability in budding yeast (Winter et al, 1999). However, little is known about its upstream regulatory signals of p41-Arc. Here we show that p41-Arc is a physiological substrate of Pak1, and that Pak1–p41-Arc interactions have an important role in optimal cell migration in mammalian cells.
Results and Discussion
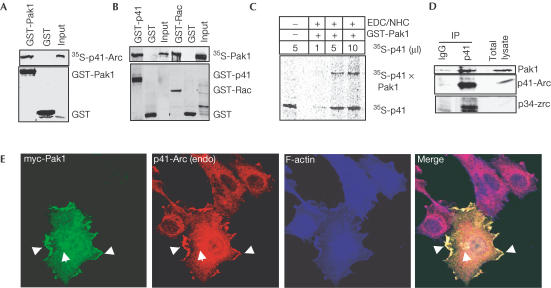
To identify novel Pak1-interacting substrates, we screened a HeLa cDNA expression library using a glutathione-S-transferase (GST)-Pak1 solid-phase-based kinase reaction. One positive clone was identical to human 41 kDa subunit 1A of Arp2/3 (p41-Arc) (GenBank accession number NM006409). In vitro-translated p41-Arc efficiently interacted with GST-Pak1 but not with GST alone, confirming protein interaction (Fig 1A). Conversely, in vitro-translated Pak1 protein also specifically interacted with GST-p41-Arc (Fig 1B). Crosslinking experiments using the zero-length crosslinker EDC/NHS further confirmed that Pak1 interacts with p41-Arc (Fig 1C). To examine endogenous Pak1 and p41-Arc interaction in vivo, cell lysates from exponentially growing MCF-7 cells were immunoprecipitated using an anti-p41-Arc antibody. An anti-p41-Arc antibody but not control IgG coimmunoprecipitated Pak1, confirming in vivo interaction (Fig 1D). Minimum binding regions required for Pak1/p41-Arc binding were mapped to the N-terminal 1–132 amino acids (aa) of Pak1 and the seventh WD domain (aa 283–372) of p41-Arc (see supplementary fig S1 online).
Figure 1.
Pak1 interaction with p41-Arc. (A) p41-Arc was translated in vitro and the 35S-labelled protein was incubated with either GST or GST-Pak1 and interaction was analysed by GST pull-down assays. Input represents 1/20th of the total 35S-labelled p41-Arc used in the assay. (B) 35S-labelled Pak1 was incubated with GST or GST-p41-Arc, and binding was analysed by GST pull-down assays. guanosine 5′-[γ-thio] triphosphate (GTPγS)-loaded GST-v12Rac was used as a positive control. Input represents 1/20th of the total 35S-labelled Pak1 used in the assay. (C) Crosslinking analysis of Pak1 to p41-Arc. Purified GST-Pak1 was incubated with an increasing amount of in vitro-translated 35S-labelled p41-Arc and crosslinked using a zero-length crosslinker EDC/NHC. Crosslinked products were denatured by heating in SDS buffer and separated on an SDS–PAGE gel followed by autoradiography. (D) In vivo interaction of Pak1 and p41-Arc. Total cell lysate from MCF-7 cells growing in 10% serum (2 mg) was immunoprecipitated with either IgG- or a p41-Arc-specific antibody, blotted and probed with antibodies specific for Pak1, p41-Arc and p34. (E) MCF-7 cells were transfected with both myc-tagged Pak1 and immunostained with antibodies against myc tag (green) and endogenous p41-Arc (red), and with phalloidin staining for F-actin (blue). Arrows indicate points of white colour indicating three-colour colocalization.
To explore the spatial relationship between Pak1 and p41-Arc within MCF-7 breast cancer cells, we used confocal scanning microscopy. Because available Pak1- and p41-specific antibodies were of rabbit origin, we transfected myc-tagged Pak1 (Fig 1E) in MCF-7 cells and looked for colocalization with endogenous p41-Arc and with F-actin. Myc-Pak1 was distinctly colocalized with endogenous p41-Arc at the cell periphery within lamellipodia and in distinct intracellular clusters (Fig 1E), which are both areas of cortical actin nucleation.
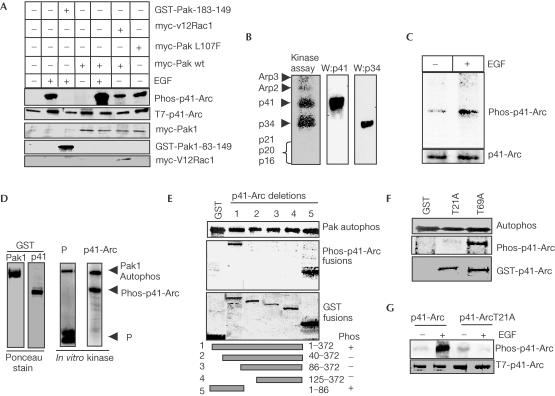
To investigate whether Pak1 can phosphorylate p41-Arc, MCF-7 cells were co-transfected with T7-tagged p41-Arc and various Pak1 regulatory plasmids. Physiological signals, such as epidermal growth factor (EGF), that activate Pak1 substantially stimulated p41-Arc phosphorylation (Fig 2A, lane 2), and were effectively blocked by co-transfection of a Pak1 autoinhibitory peptide, aa 83–149 (Fig 2A; lane 3), but not by the inactivated mutant inhibitory Pak1-83-149-107F (data not shown). Activated Rac1 (V12Rac1) increased both Pak1 activity and p41-Arc phosphorylation (Fig 2A, lane 6). Co-transfection of catalytically activated Pak1 (PakL107F) also significantly increased p41-Arc phosphorylation in the absence of growth factor treatment (Fig 2A, lane 7).
Figure 2.
Pak1 regulation of p41-Arc phosphorylation. (A) MCF-7 cells were co-transfected with T7–p41-Arc along with Pak1 autoinhibitory domain (GST–Pak1-83-149) Pak1, wild type (wt) activated Pak1 T423E or activated Rac1 (V12Rac1). After 48 h, cells were metabolically labelled with 32P-orthophosphate and treated with or without 100 ng/ml EGF for 5 min. In vivo phosphorylation of T7–p41-Arc was assessed by immunoprecipitation. Western blot with GST antibody was used to analyse the expression of GST–Pak1-83-149, and c-myc tag antibody was used to analyse the expression of Pak1 and Rac1 constructs. (B) Pak1 phosphorylates p41-Arc. Purified Arp2/3 complex from platelet cells was used as a substrate in in vitro Pak1 kinase assays. Reaction components were separated by SDS–PAGE, transferred to a nylon membrane and autoradiographed. Blots were probed using p41-Arc and p34 antibodies. (C) Serum-starved MCF-7 cells were metabolically labelled with 32P-orthophosphate, treated with EGF (100 ng/ml, 2 min), and endogenous p41-Arc was immunoprecipitated using polyclonal antibody raised against it (kindly provided by Dr Mathew Welch). (D) Pak1 phosphorylates p41-Arc in vitro. Pak1 kinase assay was performed using purified Pak1 enzyme and p41-Arc as a substrate. MBP was used as a positive control. (E) Mapping of Pak1 phosphorylation site in p41-Arc. In vitro phosphorylation of GST fusion proteins of p41-Arc deletion mutants by Pak1 enzyme (middle panel). Ponceau stain of the GST p41-Arc fusion proteins used in the assay (bottom panel). Autophosphorylation of Pak1 is shown in the top panel. (F) Phosphorylation of GST-p41-Arc T21A or GST-p41-Arc T69A mutants by Pak1 enzyme. (G) MCF-7 cells were co-transfected with p41-Arc wt or p41-Arc T21A. Cells were metabolically labelled with 32P-orthophosphate, treated with or without EGF, and in vivo phosphorylation of p41-Arc constructs was analysed.
We next examined whether Pak1 phosphorylated p41-Arc in vitro using Arp2/3 complex purified from human platelet cells as a substrate in an in vitro kinase reaction (Fig 2B). The results show that Pak1 phosphorylates p41-Arc in the complex. In addition, we have also noticed that Pak1 can phosphorylate the p34 subunit in in vitro assays. Since we identified p41-Arc as a Pak1 substrate in our initial screen, we have focused this study on Pak1 regulation of p41-Arc. To establish that EGF indeed stimulates phosphorylation of endogenous p41-Arc, serum-starved, 32P-labelled MCF-7 cells were treated with or without EGF. Phosphorylation of endogenous p41-Arc was analysed by immunoprecipitation. EGF treatment substantially increased phosphorylation of endogenous p41-Arc (Fig 2C). Together, these findings establish a role of growth factor signalling through Pak1 in the in vivo regulation of p41-Arc phosphorylation.
We next performed in vitro kinase assays with purified Pak1 enzyme and GST-p41-Arc as a substrate. Pak1 efficiently phosphorylated GST-p41-Arc (Fig 2D). Deletion of the N-terminal 40 aa of p41-Arc completely prevented Pak1 phosphorylation, while Pak1 phosphorylated a GST fusion protein containing the N-terminal 86 aa of p41-Arc (Fig 2E), indicating that this region alone is sufficient for Pak1 binding and phosphorylation. However, the lack of Pak1 binding to this region in GST pull-down binding assays suggests that the affinity of Pak1 interaction is weak. However, the presence of a strong binding site at the C-terminal region of p41-Arc suggests that this high-affinity site may be responsible for strong binding of the substrate. It is also possible that the C-terminal region of p41-Arc may facilitate Pak1 recruitment to the Arp2/3 complex for some other purpose.
There are two possible Pak1 consensus phosphorylation sites (K/R-K/R)-X-S/T) in the N-terminal 86 aa of p41-Arc. Mutation of threonine 21 to alanine, but not threonine 69 to alanine, completely abolished the ability of Pak1 to phosphorylate p41-Arc (Fig 2F). EGF treatment readily phosphorylated wild-type (wt) p41-Arc but not mutant p41-Arc T21A in transfected MCF-7 cells metabolically labelled with 32P-orthophosphate (Fig 2G). Examination of the three-dimensional structure of p41-Arc (Robinson et al, 2001) revealed that the minimal Pak1-binding region of p41-Arc (aa 323–372) is immediately adjacent to T21 of p41-Arc, suggesting that Pak1 binding and phosphorylation sites are localized in close proximity (see supplementary fig S3 online). Together, these findings identify T21 in p41-Arc as a putative Pak1 phosphorylation site.
To understand the functional significance of Pak1 and p41-Arc interactions, we show the partial colocalization of tagged p41-Arc and Pak1 in serum-stimulated cells (supplementary fig S2 online). As some coactivators of the Arp2/3 complex are shown to promote actin nucleation in vitro, we performed pyrene–actin assays with or without Pak1. The addition of Pak1 minimally accelerated the actin nucleation activity of the Arp2/3 complex (data not shown).
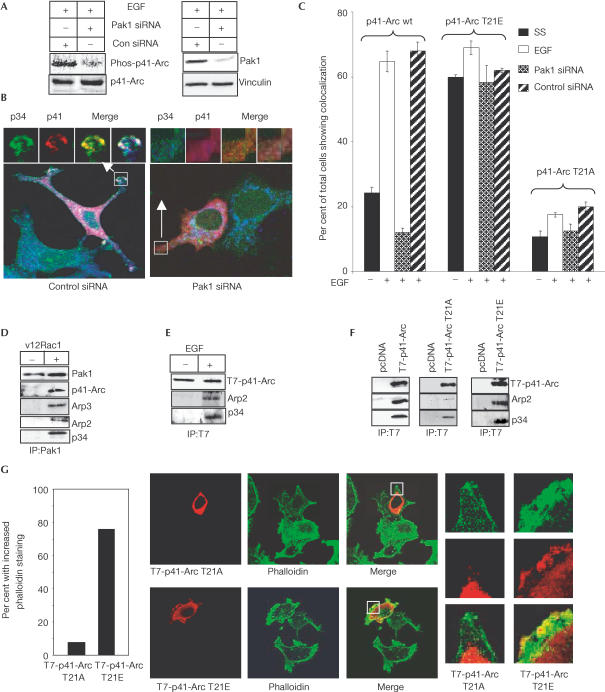
To demonstrate the physiological significance of T21 phosphorylation by Pak1, we transiently transfected T7-tagged wt p41-Arc, a T7-p41-Arc T21A mutant, which lacks the Pak1 phosphorylation site, or a T7-p41-Arc T21E mutant, which introduces a negatively charged side chain and mimics Pak1 phosphorylation, into MCF-7 cells. The localization of T7-p41-Arc constructs, endogenous p34 as a marker of the Arp2/3 complex, and actin were determined by confocal microscopy. Some cells were also co-transfected with Pak1-specific short interfering RNA (siRNA) (Wang et al, 2002) or nonspecific siRNA. The efficacy of Pak1 knockdown by siRNA was confirmed by control transfections (see supplementary fig S4 online). Pak1-specific siRNA but not nonspecific siRNA reduced Pak1 protein levels and EGF-induced p41-Arc phosphorylation (Fig 3A). EGF treatment stimulated the colocalization of T7 wt p41, p34 and actin at the cell periphery and in focal areas of the cytoplasm in transfected cells (Fig 3B, left panel). Pak1-specific siRNA effectively decreased p41-Arc, p34 and actin colocalization (Fig 3B, right panel). These findings suggest a specific regulatory role for Pak1 in EGF-stimulated colocalization of the Arp2/3 complex subunits. T7-p41-Arc T21E, which mimicked Pak1 phosphorylation, showed increased constitutive colocalization with p34 and actin, which could not be blocked by Pak1-specific siRNA (Fig 3C). Furthermore, colocalization of mutated p41-Arc T21A with p34 and actin was markedly reduced as compared with wt T7-p41-Arc (Fig 3C). These data strengthen the rationale for Pak1-mediated regulation of complex formation and an essential role for Pak1 phosphorylation of p41-Arc T21 in this process.
Figure 3.
Pak1 regulation of the p41-Arc interaction with the Arp2/3 complex in vivo. (A) MCF-7 cells were transfected with control or Pak1-specific siRNA, metabolically labelled with 32P-orthophosphate, and treated with EGF (2 min), and phosphorylation of p41-Arc was measured by immunoprecipitation. An aliquot of total lysate was analysed by western blotting for expression of Pak1. Vinculin was used as a loading control. (B,C) MCF-7 cells were transfected with T7-tagged wt p41-Arc, T7-p41-Arc T21A or T7-p41-Arc T21E, serum starved for 48 h, and then treated with 1 nM EGF for 30 min before processing for immunostaining of T7 (red), endogenous p34 (green) and F-actin (blue). Additional experimental groups were co-transfected with either Pak1-specific siRNA or with control noninhibitory siRNA, serum starved for 48 h, and then stimulated with EGF. (B) Examples of p34 and p41-Arc colocalization in control and Pak1 siRNA-treated cells. (C) The mean (±s.d.) percentage of cells with focal cytoplasmic or membranous colocalization points was assessed for each group (n=250 cells per group). Data are mean±s.d. of three experiments. (D) MCF-7 cells were transfected with or without activated Rac1 (V12Rac1) to activate Pak1. Pak1 was immunoprecipitated from cell lysates and analysed for Arp2/3 complex proteins. (E) MCF-7 cells were transfected with T7-p41-Arc, serum starved for 48 h, treated with EGF for 2 min, and cell lysates were immunoprecipitated with anti-T7 antibody and analysed for the presence of Arp complex proteins by western blotting. (F) MCF-7 cells were transfected with wt, T21E or T21A mutants of T7-p41-Arc. After 72 h, cell lysates were immunoprecipitated with anti-T7 antibody and analysed for the presence of Arp complex proteins by western blotting. (G) MCF-7 cells were transfected with either T21A or T21E mutants of T7-p41-Arc. F-actin levels in these cells were analysed by confocal microscopy. The percentage of transfected cells (n=100) showing greater overall pixel intensity of phalloidin taining than neighbouring untransfected cells as quantified using Zeiss image analysis software.
To further establish Pak1 interaction with the Arp2/3 complex in vivo, we activated Pak1 by transfecting activated V12Rac1 (Fig 3D). Cell lysates were immunoprecipitated with an anti-Pak1 antibody and analysed for the presence of Arp2/3 complex proteins by western blotting using specific antibodies. The results show that Pak1 associates with the Arp2/3 complex in activated cells (Fig 3D). To verify that EGF stimulates association of p41-Arc with the Arp2/3 complex, MCF-7 cells transfected with T7-p41-Arc were stimulated with EGF, and its association with the Arp2/3 complex was analysed by immunoprecipitation. The results showed EGF-stimulated association of Arp2/3 complex proteins Arp2 and p34 with T7-p41-Arc (Fig 3E). To determine whether the p41-Arc T21A mutant associates with the endogenous Arp2/3 complex, wt, T21A and T21E mutants of T7-p41-Arc were transfected into MCF-7 cells. The expressed proteins were immunoprecipitated with a T7 monoclonal antibody and then analysed for the presence of Arp2/3 complex proteins. wt p41-Arc and T21E p41-Arc immunoprecipitated p34 and Arp2, two known interactors of p41-Arc in stoichiometric proportions, while p41-Arc T21A immunoprecipitated substantially less p34 and Arp2 (Fig 3F). To examine further the role of p41-Arc T21 in actin polymerization in vivo, MCF-7 cells were transfected with either p41-Arc T21A or T21E mutants and cells were stained for F-actin. p41-Arc T21E- but not p41-Arc T21A-expressing cells showed consistently increased F-actin compared with neighbouring untransfected cells (Fig 3G). Thus, Pak1-mediated modification of p41-Arc may be important for both the localization of p41-Arc to the regions of actin polymerization and, presumably, p41-Arc regulation of the Arp2/3 complex.
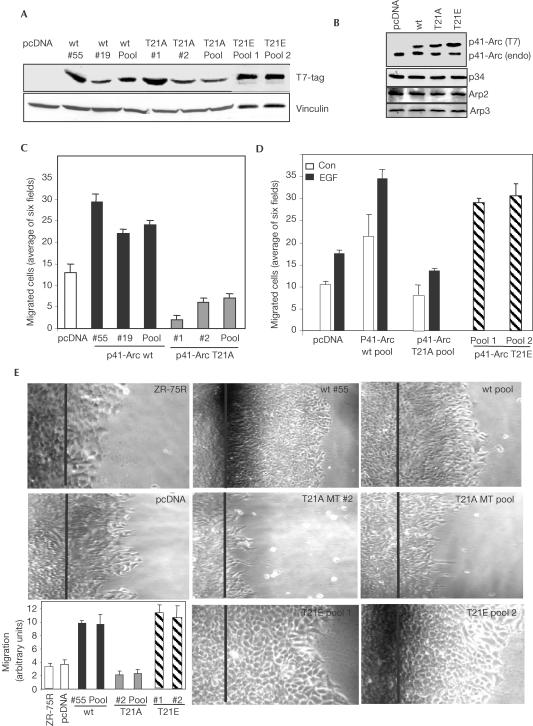
To explore the importance of p41-Arc in the biology of breast cancer cells, we next established stable ZR-75R breast cancer cell clones expressing T7-tagged p41-Arc, p41-Arc T21A, p41-Arc T21E or control vector (Fig 4A). Expressed T7-tagged p41-Arc protein is larger than the endogenous p41-Arc due to the addition of epitope tag at the N-terminus, and thus migrates slower on a 12% SDS–PAGE gel (Fig 4B). We have generated several individual as well as pooled clones, which express the T7-p41 transgene 1–2-fold higher than endogenous p41-Arc levels. The relative levels of other Arp2/3 components were not altered (Fig 4B). The physiological significance of Pak1 phosphorylation of p41-Arc on cell migratory potential was examined using wt and p41-Arc T21A mutant clones in Boyden chamber assays (Albini et al, 1987). wt p41-Arc clones exhibited increased migratory potential, while p41-Arc T21A clones exhibited decreased migratory potential (Fig 4C). p41-Arc T21E clones exhibited increased migratory potential even in the absence of EGF stimulation (Fig 4D). We also measured cell motility using a wound-healing assay (Fig 4E) (Sells et al, 2002). wt p41-Arc and p41-Arc T21E clones consistently exhibited a 3–4-fold increase in the distance migrated compared to pcDNA-expressing clones, while p41-Arc T21A mutant-expressing clones showed reduced motility. Together, these findings validate a mechanistic role of p41-Arc and its phosphorylation by Pak1 in cell motility.
Figure 4.
Requirement of p41-Arc T21 phosphorylation in cell migration. (A) Immunoblot of T7-p41-Arc wt, T7-p41-Arc T21A and T7-p41-Arc T21E in stable cell lines. Vinculin was used as a loading control. (B) Analysis of Arp2/3 complex proteins in pooled ZR-75R breast cancer cells stably expressing various p41-Arc constructs. (C) Effect of T7-p41-Arc expression on cell motility. Migratory potential of p41-Arc wt or p41-Arc T21A-expressing clones was measured by Boyden chamber assay. Migrated cells were recorded by microscopy. Data are the mean±s.e.m. of three experiments. (D) Clones stably expressing p41-Arc wt and p41-Arc T21A were treated with or without EGF, and p41-Arc T21E cells were not treated with EGF. Motility of the clones was measured by Boyden chamber assay. (E) Effect of expression of p41-Arc, p41-Arc T21A and p41-Arc T21E on cell migration in wound-healing assay. Data are the mean±s.e.m. of three experiments.
Overall, our results present work demonstrating that Pak1 phosphorylation of p41-Arc regulates its localization and interaction with the Arp2/3 complex in the cortical nucleation regions of cells. Mechanistic results suggest an essential role for Pak1 phosphorylation of p41-Arc T21 in optimal cell motility. Pak1 may be a critical regulator of the localization of p41-Arc to the Arp2/3 complex upon growth factor stimulation. Pak1-specific siRNA results and studies with the p41-Arc T21A mutant further support the idea that Pak1 signalling is required for efficient colocalization of p41-Arc with the Arp2/3 complex. Further expression of upstream Pak1 activators, constitutively activated Pak1 or a p41-Arc T21E mutant mimicked the effects of growth factor signalling in localization of p34 with p41-Arc. In accordance with these conclusions, breast cancer cells expressing wt p41-Arc or p41-Arc T21E exhibited increased migratory potential in Boyden chamber assays, while p41-Arc T21A clones showed decreased migratory potential even in the presence of growth factor (Fig 4D). In summary, we have identified a novel pathway by which Pak1 can interact directly with and phosphorylate p41-Arc, the regulatory subunit of the Arp2/3 complex. This functional interaction may regulate cell motility and invasiveness.
Speculation
The formation of motile lamellipodia is a functional outcome of dynamic polymerization and depolymerization of actin. Our results suggest that in addition to its regulation of actin depolymerization through the activity of LIM kinase, Pak1 may also use p41-Arc to influence the assembly and reorganization of actin. Moreover, p41-Arc is the first component of the Arp2/3 complex to be phosphorylated directly by a signalling kinase. Our results suggest that Pak1 mediates p41-Arc phosphorylation and thus may provide an explanation for the existence of p40 and p41 forms. Earlier studies also suggested that p41-Arc is the only subunit of the Arp2/3 complex that can exist as a monomer and also as a part of the Arp2/3 complex (Mullins & Pollard, 1999). Pak1 regulation of p41-Arc demonstrates new functions for both Pak1 and p41-Arc, and represents a new mechanism by which extracellular growth factors and cellular signalling cascades may control the functions of the Arp2/3 complex and subsequently the motility of mammalian cells.
Methods
Cell culture and reagents Breast cancer MCF-7 and ZR-75R cells were obtained from ATCC. Antibodies against Pak1 were purchased from Cell signaling and Zymed Inc. Antibodies for myc tag, the T7 tag and phalloidin were from MBL Laboratories, Novagen and Molecular Probes, respectively. Polyclonal antibody against p41-Arc used for immunoprecipitations was obtained from Dr Mathew Welch. p41-Arc antibody used for western analysis was obtained from ABCAM (#ab2837). Zero-length crosslinker EDC and NHC were obtained from Pierce. The Pak1 antibody was obtained from Cell Signaling. Arp2 and p34 antibodies were obtained from Upstate Biotechnology.
Screening of HeLa expression library Construction and screening of the expression library by solid-phase phosphorylation was carried out using the procedure described previously (Li et al, 2002, see supplementary online).
Plasmid construction T7- and GST-tagged p41-Arc was constructed by PCR amplification and subcloning into pcDNA3.1 and PGEX5X1 vectors. p41-Arc T21A and p41-Arc T21E mutations were created using a site-directed mutagenesis kit (Li et al, 2002).
In vitro kinase and GST pull-down assays In vitro kinase assays were performed as described previously (Li et al, 2002). Crosslinking experiments were performed according to the protocol described (Weaver et al, 2002).
Cell migration and wound-healing assays Cell migration assays were performed as described (Albini et al, 1987). Wound-healing assays were performed as described previously (Sells et al, 2002).
Immunofluorescence and confocal imaging Confocal imaging was performed as described (Li et al, 2002). Scoring in Fig 3C was based on the presence of colocalization mainly at the cell periphery in motile structures, since that is where the majority of colocalization occurred. The cytoplasmic foci occurred less frequently, and were not seen independently of colocalization at the cell periphery.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figures
Acknowledgments
We thank Matthew D. Welch and Erin D. Goley for generously providing the purified native Arp2/3 complex, antibodies to p34-Arc and p41-Arc, and performing actin–pyrene assays. We also thank Laura Machesky for providing antibodies to Arp2 and Arp3, and Melanie Cobb for providing Pak1-L107F plasmid. This work was supported by the National Institute of Health grant CA90970, CA80066 and CA65746 (R.K.).
References
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson AS, Kozlowski JM, Mcewan RN (1987) A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 47: 3239–3245 [PubMed] [Google Scholar]
- Bishop AL, Hall A (2000) Rho GTPases and their effector proteins. Biochem J 348: 241–255 [PMC free article] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN (1999) Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signaling to actin cytoskeletal dynamics. Nat Cell Biol 1: 253–259 [DOI] [PubMed] [Google Scholar]
- Gournier H, Goley ED, Niederstrasser H, Trinh T, Welch MD (2001) Reconstitution of human Arp2/3 complex reveals critical roles of individual subunits in complex structure and activity. Mol Cell 8: 1041–1052 [DOI] [PubMed] [Google Scholar]
- Hall A (1998) Rho GTPases and the actin cytoskeleton. Science 279: 509–514 [DOI] [PubMed] [Google Scholar]
- Kumar R, Vadlamudi R (2002) Emerging functions of p21-activated kinases in human cancer cells. J Cell Physiol 193: 133–144 [DOI] [PubMed] [Google Scholar]
- Li F, Adam L, Vadlamudi RK, Zhou H, Sen S, Chernoff J, Kumar R (2002) p21 activating kinase 1, a new chromosomal passenger, phosphorylates histone H3 in eukaryotic cells. EMBO Rep 3: 767–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RD, Heuser JA, Pollard TD (1998) The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci USA 95: 6181–6186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RD, Pollard TD (1999) Structure and function of the Arp2/3 complex. Curr Opin Struct Biol 9: 244–249 [DOI] [PubMed] [Google Scholar]
- Robinson RC, Turbedsky K, Kaiser DA, Marchand J, Higgs HN, Choe S, Pollard TD (2001) Crystal structure of Arp2/3 complex. Science 294: 1679–1684 [DOI] [PubMed] [Google Scholar]
- Sells MA, Pfaff A, Chernoff J (2002) Temporal and spatial distribution of activated Pak1 in fibroblasts. J Cell Biol 151: 1449–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Mazumdar A, Vadlamudi RK, Kumar R (2002) P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-α and promotes hyperplasia in mammary epithelium. EMBO J 21: 5437–5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver AM, Heuser JE, Karginov AV, Lee WL, Parsons JT, Cooper JA (2002) Interaction of cortactin and N-WASp with Arp2/3 complex. Curr Biol 12: 1270–1278 [DOI] [PubMed] [Google Scholar]
- Weaver AM, Young ME, Lee W, Cooper JA (2003) Integration of signals to the ARP2/3 complex. Curr Opin Cell Biol 15: 23–30 [DOI] [PubMed] [Google Scholar]
- Welch MD (1999) The world according to Arp: regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol 9: 423–427 [DOI] [PubMed] [Google Scholar]
- Welch MD, DePace AH, Verma S, Iwamatsu A, Mitchison TJ (1997) The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J Cell Biol 138: 375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DC, Elizabeth YC, Li R (1999) Genetic dissection of the budding yeast Arp2/3 complex: a comparison of the in vivo and structural roles of individual subunits. Proc Natl Acad Sci USA 96: 7288–7293 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures