Abstract
Protein kinase CK2 (formerly known as casein kinase II) has been viewed traditionally as a stable heterotetrameric complex, but new analytical techniques are bringing a different picture into focus. The transient nature of this complex has been highlighted by the elucidation of its structure. Furthermore, analysis of the spatiotemporal organization of individual CK2 subunits in living cells has shown that they are dynamic and that they integrate into different multimolecular assemblies. These new studies give an additional dimension to the challenge of determining the cellular regulation of this protein kinase.
Keywords: CK2, dynamic subunit interaction, heterotetrameric complex
Introduction
X-ray crystallography studies usually generate static pictures of molecular complexes. However, when combined with information from other approaches that more effectively capture the dynamic behaviour of proteins in living cells, notable insights into the transient nature of molecular complexes can be obtained. Two recent papers on protein kinase CK2 (formerly known as casein kinase II) show how such insights can be gained by the comparison of structural data (Niefind et al, 2001) with cell-culture studies, in which the dynamics of the protein can be visualized directly using green fluorescent protein (GFP)-tagged CK2 (Filhol et al, 2003).
The common view
CK2 is a multifunctional and almost universal protein kinase that has crucial roles in cell differentiation, proliferation and survival (Ahmed et al, 2002; Litchfield, 2003). Mounting evidence indicates that the enzyme is a component of regulatory protein-kinase networks that are involved in several aspects of transformation and cancer (Guerra & Issinger, 1999). In addition, recent studies on the Drosophila clock genes provide strong evidence for the involvement of CK2 in the molecular clock machinery (Akten et al, 2003; Blau, 2003).
Through pioneering work more than 25 years ago, Thornburg and Lindell first described CK2 as a multisubunit protein kinase that is generated by the association of two subunits α and α′ (38–42 kDa) with a dimer of the 27-kDa β-subunit. These observations led the authors to propose that CK2 functions as a stable heterotetrameric complex (Thornburg & Lindell, 1977). Subsequently, the multimeric structure of CK2 purified from different sources was confirmed (Dahmus & Natzle, 1977; Hathaway & Traugh, 1978; Dahmus, 1981). The α/α′- subunits were shown to contain the catalytic domain of the kinase, whereas the βsubunit was identified as the regulatory component (Cochet & Chambaz, 1983). It was later observed that, when added together in vitro, recombinant α- and βsubunits assembled instantaneously into a stable heterotetrameric complex with high affinity (dissociation constant (Kd) = 5.4 nM). This high-affinity complex showed few signs of dissociation in the absence of denaturing agents (Pinna & Meggio, 1997; Battistutta et al, 2000; Martel et al, 2002). Therefore, numerous complementary observations, mostly based on traditional biochemical methods, led to the long-held tenet that CK2 is a strong obligate complex.
Challenging a fixed viewpoint
In the early 1990s, the CK2 field received a jolt when Stigare and colleagues first reported in an epithelial Chironomus cell line that most of the catalytic α-subunit was tightly bound to nuclear structures in the absence of its β-subunit counterpart (Stigare et al, 1993). In addition, studies showed that naturally occurring free monomeric CK2α is relatively common in plants (Yan & Tao, 1982; Dobrowolska et al, 1992) and in Dictyostelium discoideum (Ospina et al, 1992). This knowledge spawned several reports that provided evidence for an unbalanced expression of CK2α and CK2β subunits in different mammalian tissues (Guerra et al, 1999; Stalter et al, 1994; Pinna & Meggio, 1997). Moreover, the identification of several CK2-interacting proteins reinforced the idea that both subunits might have biological functions other than those attributed to the CK2 holoenzyme. For instance, the CK2β subunit was characterized as a regulatory binding partner of several important protein kinases, including A-Raf, c-Mos, p90rsk (for a review, see Guerra & Issinger, 1999), PKCζ (Bren et al, 2000) and, more recently, the checkpoint kinase Chk1 (Guerra et al, 2003). In a two-hybrid screen, more than 40 different proteins were shown to interact with CK2β (see Fly Grid at: http://biodata.mshri.on.ca/fly_grid). In mice, as in the metazoan Caenorhabditis elegans, a functional loss of CK2β is lethal (Fraser et al, 2000; Buchou et al, 2003). This leads to the intriguing possibility that at least some aspects of CK2β function are not based on its interaction with the CK2 catalytic subunit. However, despite these observations, the reported high-affinity interaction of the CK2 subunits has made hugely controversial the existence of independent subpopulations of these molecules in the cell.
To resolve this dispute, the direct visualization of CK2 subunits and an investigation into their interaction behaviour in living cells was required. In a recent paper, Filhol and colleagues observed the individual CK2 subunits on a short timescale in living cells using live-cell fluorescent imaging (Filhol et al, 2003). From this study, it is apparent that the CK2 subunits are highly mobile proteins; more importantly, photobleaching experiments also provided evidence of the independent movement of CK2α and CK2β in cells. Overall, the recovery kinetics showed that the majority of the two subunits are not present in a common holoenzyme. This apparent difference in mobility was also evident at the level of their nuclear translocation: each CK2 subunit enters the nucleus as distinct subunits rather than as a pre-assembled holoenzyme. Qualitative analysis of kinetic parameters indicates that the nuclear accumulation of the two CK2 subunits might proceed in a sequential manner through different mechanisms. Moreover, unlike CK2β, nuclear CK2α can be exported back to the cytosol through an exportin 1/Crm1-dependent pathway. This observation indicates that the residence time of the catalytic subunit in the nucleus might be shorter than that of the regulatory subunit. The dynamic nature of CK2α in growing cells indicates that the continuous exchange of the catalytic subunit is instrumental in the maintenance of cell-cycle progression.
These observations also reveal that the molecular interaction between the CK2 subunits, which was once thought to be stable, is in fact highly dynamic. Therefore, at any one time, α- and β-subunits are not stably and permanently associated with each other, but are also able to interact with specific partners and to participate in the transient formation of distinct multimolecular complexes. The assembly process of such macromolecular complexes is reminiscent of the dynamic and transient assembly of RNA polymerase I (pol I). Combining in vivo microscopy techniques with computational simulations, Misteli and colleagues elegantly showed that the 12–15 subunits of RNA pol I only transiently associate with the ribosomal promoter and that the subunits are recruited to the promoter independently of each other (Dundr et al, 2002; Dundr & Misteli, 2003).
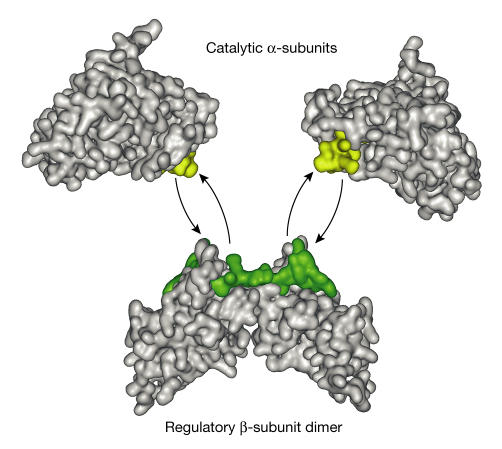
How can we reconcile this dynamic vision of CK2 with the extremely high binding affinity of the α–β interaction that is observed in vitro? A milestone in the CK2 field was the elucidation of the crystal structure of the CK2 holoenzyme, which provided unexpected clues as to the molecular attachment of the CK2β dimer to the CK2α catalytic subunits (Niefind et al, 2001). In the holoenzyme, two CK2α catalytic subunits that are not in contact with each other interact with a central building block that is represented by the CK2β dimer. The C-terminal domain of each of the two CK2β monomers interacts with the central βsheet of the small lobe of each catalytic subunit (Niefind et al, 2001; Ermakova et al, 2003). A key observation from these X-ray crystallography studies is that the surface contacts between the catalytic and regulatory subunits were considerably smaller (832 Å2) than the surface contacts that are usually observed in stable protein complexes (Fig 1). This possible intersubunit flexibility led the authors to propose that the CK2 holoenzyme is a transient heterocomplex (Niefind et al, 2001), which is consistent with the live-cell imaging data.
Figure 1.
Intersubunit flexibility indicated by the crystal structure of the CK2 holoenzyme. The nature and the surface of the contacts between the catalytic and regulatory subunits (832 A2) revealed by X-ray crystallography studies indicate that the CK2 holoenzyme is a transient heterotetrameric complex that can dissociate into a dimer of the regulatory β-subunit and two molecules of the catalytic α-subunits (Niefind et al, 2001). The α/β contacts on each subunit are coloured yellow (α) and green (β). The interface size between the catalytic subunits and the dimer of regulatory βsubunits is relatively small (832 Å2). The coordinates (Protein Data Bank (PDB) identification number ) from the crystal structure of the CK2 holoenzyme were used with the Web Lab Viewer Pro to generate this figure.
Relevance for CK2 regulation
The subcellular dynamics of the CK2 subunits and the transient nature of their interaction, as revealed by imaging and X-ray crystallography studies, are hallmarks of intracellular signalling molecules. Most non-obligate interactions have a regulatory role, which, in the case of CK2, is illustrated by the notable changes in substrate specificity of its complexed and non-complexed catalytic subunit (Martel et al, 2002; Pinna, 2002). The non-complexed catalytic subunit is spontaneously active on several protein substrates and the high-affinity binding of the CK2β dimer to CK2α does not exert all-or-nothing effects on the activity of the kinase (Meggio et al, 1992). Instead, binding of this regulatory subunit might result in the phosphorylation of a range of substrates that are not, or are only weakly, phosphorylated in its absence. This means that any change in the expression of CK2β might lead to a shift in the balance of phosphorylated CK2α- and holoenzymespecific substrates. As CK2 substrates localize to many different subcellular compartments, a dynamic rather than a static interaction of the CK2 subunits should increase the kinase specificity and ensure that the relevant form of the catalytic subunit is present at each of these locations. Curiously, it is becoming increasingly clear that the tight complexes between CK2 and some of its substrates are often bridged by the CK2β dimer (Guerra & Issinger, 1999; Litchfield, 2003). This raises the possibility that CK2α could be locally and transiently recruited into multimolecular complexes in which the CK2β dimer serves as a scaffold or a docking subunit through high-affinity interactions with substrate or nonsubstrate protein partners (Pinna, 2002). According to this theory, the CK2β dimer represents a building block that exerts a crucial spatiotemporal regulatory role in the assembly of these molecular complexes. Indeed, fluorescence-correlation spectroscopy (FCS) analysis has provided evidence for the existence of fast- and slow-moving populations of both CK2 subunits (Filhol et al, 2003).
These considerations lead to a central question: what are the mechanisms that control the association and dissociation of the CK2 holoenzyme (a process that might be vital to the biological functions of the kinase)? Obligate complexes have Kd values that are usually in the nanomolar range (Nooren & Thornton, 2003), which is similar to the binding affinity that has been determined for recombinant CK2 subunits (Martel et al, 2002). Intuitively, to become non-obligate, a strong transient complex such as the CK2 holoenzyme needs to physicochemically regulate the transient interaction of its subunits through changing their binding affinity by orders of magnitude.
Living cells are packed with proteins and other molecules that occupy 20–30% of the total volume; therefore, a test tube cannot be likened to the intracellular environment (Ellis & Minton, 2003). Accordingly, it is important to consider that each CK2 subunit resides in a crowded environment with many potential binding partners with different surface properties. CK2β in particular seems to be multispecific, with many competing binding partners on coinciding or overlapping interfaces (Guerra & Issinger, 1999). A large excess of CK2β is synthesized compared with CK2α and the non-complexed protein is rapidly degraded (Lüscher & Litchfield, 1994). Structural data indicate that the C-terminal tail and the N-terminal acidic loop of free CK2β are in an unfolded conformation, which raises the possibility that the protein might adopt various folded structures on binding to different biological partners. As a consequence of this internal plasticity, C K2β could exist as several rapidly interconverting conformers that are able to recognize many biological targets yet still retain specificity. This gives rise to the speculation that, at any one time in any given cell, CK2 is regulated by several protein–protein interactions. If the binding of the CK2 subunits is assumed to be essentially stochastic in nature, this interaction might be controlled by: contact between their interacting surfaces, which requires co-localization in time and space within a compartment; the local concentration of partners or substrates, which can be changed temporarily; the local physicochemical environment; or post-translational modifications, such as the dynamic phosphorylation of specific surfaces on each CK2 subunit or of their interacting partners. Therefore, conformational changes could shift the balance in favour of, or against, binding between the two subunits. This hypothesis is supported by FCS analysis in mammalian cells, which showed that a fraction of each individual CK2 subunit is engaged in different high-molecular-weight complexes (Filhol et al, 2003). These observations are consistent with the data from a proteome-wide analysis of native protein complexes in yeast, which showed the differential integration of the individual CK2 subunits in many functional multiprotein complexes (Gavin et al, 2002; Ho et al, 2002).
There are several ways to address the biological relevance of this dynamic behaviour of CK2. First, each subunit has many potential phosphorylation sites; therefore, some insight could be gained through a systematic study of the behaviour of mutant CK2 subunits under various phosphorylation conditions. Second, at least in vitro, binding of the regulatory subunit to CK2α results in notable changes in the substrate specificity of the kinase (Martel et al, 2002). Therefore, studies using small interfering RNA (siRNA) to knock down CK2β expression will be crucial for evaluating the involvement of this subunit in the phosphorylation of key protein substrates. This should also encourage the development of specific peptide or non-peptide inhibitors of the interaction between the CK2 subunits. Third, higher order CK2 structures between CK2 tetramers that show differential catalytic activity have been characterized in vitro (Glover, 1986; Valero et al, 1995). The reversible association of different CK2 conformers in vivo might account for regulated changes in their activity. Clearly, this idea would add another level of complexity in interpreting the in vivo behaviour of CK2 (Pinna, 2002).
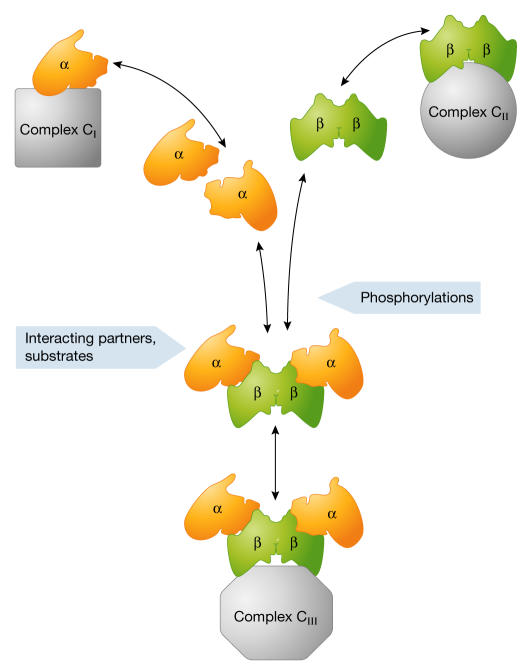
CK2 subunits might not act solely as a single holoenzyme entity; they might also be organized into discrete subcomplexes, each containing a different set of partners or substrates. The presence or absence of CK2β in CK2α-containing multimolecular assemblies would prevent or trigger the phosphorylation of specific CK2 substrates that are present in close proximity, and thereby contribute to a subtle but powerful combinatorial control. Additionally, specific phosphorylation events could control the integration of the appropriate combinations of each individual CK2 subunit into these assemblies, so that they interact correctly with substrates (Fig 2). It is likely that during cell-extract preparation, the subtle equilibrium between these transient multiprotein complexes and/or the state of phosphorylation of their components could be notably altered by the dilution of the cellular environment. This would artificially favour the high-affinity interaction of the CK2 subunits (Fig 3; see supplementary information online for calculations).
Figure 2.
A schematic view of several dynamic pools of CK2 subunits. In a living cell, high local concentrations of interacting partners, substrates or accessory proteins that are present in specific cellular locations might stabilize weakly associating interactions of each CK2 subunit, leading to the transient formation of macromolecular complexes (CI and CII). The segregation of individual CK2 subunits might therefore contribute to signal specificity by sequestering the CK2 subunits in different pathways. Alternatively, CK2 subunits can bind to each other with high affinity. The resulting holoenzyme might be reversibly integrated/recruited into other complexes (CIII). The presence of interacting partners or substrates and dynamic post-translational modifications, such as phosphorylation, might shift the balance between the different forms of CK2 subunits.
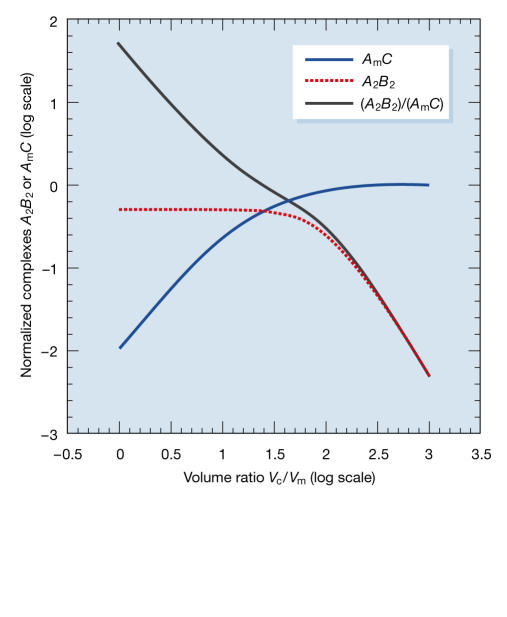
Figure 3.
Mathematical model of the distribution of CK2 subunits in macromolecular complexes. We assume that CK2α subunits, as well as associating with their high-affinity CK2β2 partner, can also bind with low affinity to other molecular complexes. The latter reaction is restricted to a cell subcompartment of volume Vm, whereas the former takes place everywhere in the cell (volume Vc). High Vc/Vm favours CK2α binding to the low-affinity target (curve AmC in blue), which predominates over the binding to CK2β2 (curve A2B2 in red). Conversely, if both compartments have the same volume (low Vc/Vm), only CK2α2β2 complexes are observed and the ratio of A2B2/AmC (black curve) is high (see supplementary information online for details and simulations).
In summary, the picture that emerges from the sum of these recent structural and in vivo imaging studies is that the molecular interaction between CK2 subunits is highly dynamic. The transient association of the CK2 subunits argues against a model in which the two proteins exclusively exert their functions through the formation of a static oligomeric structure. These new data provide further evidence for the simultaneous existence of several independently regulated populations of non-complexed and complexed CK2 subunits in a living cell (Litchfield, 2003). The likelihood is that cells have developed specific mechanisms to actively segregate the CK2 subunits or to trigger their interaction to create a functional holoenzyme. It can be predicted that such a balance is crucial in the control of the many cellular processes that are governed by this pleiotropic kinase. How the relative abundance of these different pools is regulated in response to the activation of specific signalling pathways remains a challenging question.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank T. Misteli for encouragement and critical comment on the manuscript, and J.P. Andrieu for help in the preparation of Fig 1. The research of C.C. was supported by grants from the Institut National de la Santé et de la Recherche Medicale (INSERM), the Centre Nationale de la Recherche Scientifique (CNRS; contract 8BC06G), the Commissariat à l'Energie Atomique and the Ligue Nationale contre le Cancer (Équipe Labellisée).
References
- Ahmed K, Gerber DA, Cochet C (2002) Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol 12: 226–230 [DOI] [PubMed] [Google Scholar]
- Akten B, Jauch E, Genova GK, Kim EY, Edery I, Raabe T, Jackson FR (2003) A role for CK2 in the Drosophila circadian oscillator. Nat Neurosci 6, 251–257 [DOI] [PubMed] [Google Scholar]
- Battistutta R, Sarno S, De Moliner E, Marin O, Issinger OG, Zanotti G, Pinna LA (2000) The crystal structure of the complex of Zea mays α subunit with a fragment of human β subunit provides the clue for the architecture of protein kinase CK2 holoenzyme. Eur J Biochem 267: 5184–5190 [DOI] [PubMed] [Google Scholar]
- Blau J (2003) A new role for an old kinase: CK2 and the circadian clock. Nat Neurosci 6: 208–210 [DOI] [PubMed] [Google Scholar]
- Bren GD, Pennington KN, Paya CV (2000) PKC-ζ associated CK2 participates in the turnover of free IκBα. J Mol Biol 297: 1245–1258 [DOI] [PubMed] [Google Scholar]
- Buchou T, Vernet M, Blond O, Jensen HH, Pointu H, Olsen BB, Cochet C, Issinger OG, Boldyreff B (2003) Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol 23: 908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet C, Chambaz EM (1983) Oligomeric structure and catalytic activity of G type casein kinase. J Biol Chem 258: 1403–1406 [PubMed] [Google Scholar]
- Dahmus ME (1981) Purification and properties of calf thymus casein kinases I and II. J Biol Chem 256: 3319–3325 [PubMed] [Google Scholar]
- Dahmus ME, Natzle J (1977) Purification and characterization of Novikoff ascites tumor protein kinase. Biochemistry 16: 1901–1908 [DOI] [PubMed] [Google Scholar]
- Dobrowolska G, Meggio F, Szczegielniak J, Muszynska G, Pinna LA (1992) Purification and characterization of maize seedling casein kinase IIB, a monomeric enzyme immunologically related to the α subunit of animal casein kinase-2. Eur J Biochem 204: 299–303 [DOI] [PubMed] [Google Scholar]
- Dundr M, Misteli T (2003) Gene expression dynamics. Biomed Pharmacother 57: 179–181 [Google Scholar]
- Dundr M, Hoffmann-Rohrer U, Hu Q, Grummt I, Rothblum LI, Phair RD, Misteli T (2002) A kinetic framework for a mammalian RNA polymerase in vivo. Science 298: 1623–1626 [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Minton AP (2003) Cell biology: join the crowd. Nature 245, 27–28 [DOI] [PubMed] [Google Scholar]
- Ermakova I, Boldyreff B, Issinger OG, Niefind K (2003) Crystal structure of a C-terminal deletion mutant of human protein kinase CK2 catalytic subunit. J Mol Biol 330: 925–934 [DOI] [PubMed] [Google Scholar]
- Filhol O, Nueda A, Martel V, Gerberscokaert D, Benitez MJ, Souchier C, Saoudi Y, Cochet C (2003) Live cell fluorescence imaging reveals the dynamics of protein kinase CK2 individual subunits. Mol Cell Biol 23: 975–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J (2000) Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408: 325–330 [DOI] [PubMed] [Google Scholar]
- Gavin AC et al. (2002) Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415: 141–147 [DOI] [PubMed] [Google Scholar]
- Glover CV (1986) A filamentous form of Drosophila casein kinase II. J Biol Chem 261: 13349–13354 [PubMed] [Google Scholar]
- Guerra B, Issinger OG (1999) Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis 20: 391–408 [DOI] [PubMed] [Google Scholar]
- Guerra B, Siemer S, Boldyreff B, Issinger OG (1999) Protein kinase CK2: evidence for a protein kinase CK2β subunit fraction, devoid of the catalytic CK2α subunit, in mouse brain and testicles. FEBS Lett 462: 353–357 [DOI] [PubMed] [Google Scholar]
- Guerra B, Issinger OG, Wang JYJ (2003) Modulation of human checkpoint kinase Chk1 by the regulatory βsubunit of protein kinase CK2. Oncogene 22: 4933–4942 [DOI] [PubMed] [Google Scholar]
- Hathaway GM, Traugh JA (1978) Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. J Biol Chem 254: 762–768 [PubMed] [Google Scholar]
- Ho Y et al. (2002) Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectroscopy. Nature 415: 180–183 [DOI] [PubMed] [Google Scholar]
- Litchfield DW (2003) Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J 369: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B, Litchfield DW (1994) Biosynthesis and degradation of casein kinase II in lymphoid cell lines. Eur J Biochem 220: 521–524 [DOI] [PubMed] [Google Scholar]
- Martel V, Filhol O, Nueda A, Cochet C (2002) Dynamic localization/association of protein kinase CK2 subunits in living cells: a role in its cellular regulation? Ann NY Acad Sci 973: 272–277 [DOI] [PubMed] [Google Scholar]
- Meggio F, Boldyreff B, Marin O, Pinna LA, Issinger OG (1992) Role of the β subunit of casein kinase-2 on the stability and specificity of the recombinant reconstituted holoenzyme. Eur J Biochem 204: 293–297 [DOI] [PubMed] [Google Scholar]
- Niefind K, Guerra B, Ermakowa I, Issinger OG (2001) Crystal structure of human protein kinase CK2: insights into basic properties of the CK2 holoenzyme. EMBO J 20: 5320–5331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooren IM, Thornton JM (2003) Diversity of protein–protein interactions. EMBO J 22: 3486–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina B, Nunez A, Fernàndez-Renart M (1992) Purification of a soluble casein kinase II from Dictyostelium discoideum lacking the β subunit: regulation during proliferation and differentiation. Mol Cell Biochem 118: 49–60 [DOI] [PubMed] [Google Scholar]
- Pinna LA (2002) Protein kinase CK2: a challenge to canons. J Cell Sci 115: 3873–3878 [DOI] [PubMed] [Google Scholar]
- Pinna LA, Meggio F (1997) Protein kinase CK2 (“casein kinase-2”) and its implication in cell division and proliferation. Prog Cell Cycle Res 3: 77–97 [DOI] [PubMed] [Google Scholar]
- Stalter G, Siemer S, Becht E, Ziegler M, Remberger K, Issinger OG (1994) Asymmetric expression of protein kinase CK2 subunits in human kidney tumors. Biochem Biophys Res Commun 202: 141–147 [DOI] [PubMed] [Google Scholar]
- Stigare J, Buddelmeijer N, Pigon A, Egyhazi E (1993) A majority of casein kinase II α subunit is tightly bound to intranuclear components but not to the β subunit. Mol Cell Biochem 129: 77–85 [DOI] [PubMed] [Google Scholar]
- Thornburg W, Lindell TJ (1977) Purification of rat liver nuclear protein kinase NII. J Biol Chem 252: 6660–6665 [PubMed] [Google Scholar]
- Valero E, De Bonis S, Filhol O, Wade RH, Langowski J, Chambaz EM, Cochet C (1995) Quaternary structure of casein kinase II. J Biol Chem 270: 8345–8352 [DOI] [PubMed] [Google Scholar]
- Yan TF, Tao M (1982) Purification and characterization of a wheat germ protein kinase. J Biol Chem 257: 7037–7043 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information