Abstract
Telomere dysfunction induces two types of cellular response: cellular senescence and apoptosis. We analysed the extent to which the cellular level of telomere dysfunction and p53 gene status affect these cellular responses in mouse liver using the experimental system of TRF2 inhibition by a dominant-negative version of the protein (TRF2ΔBΔM). We show that the level of telomere dysfunction correlates with the level of TRF2ΔBΔM protein expression resulting in chromosomal fusions, aberrant mitotic figures and aneuploidy of liver cells. These alterations provoked p53-independent apoptosis, but a strictly p53-dependent senescence response in distinct populations of mouse liver cells depending on the cellular level of TRF2ΔBΔM expression. Apoptosis was associated with higher expression of TRF2ΔBΔM, whereas cellular senescence was associated with low levels of TRF2ΔBΔM expression. Our data provide experimental evidence that induction of senescence or apoptosis in vivo depends on the cellular level of telomere dysfunction and differentially on p53 gene function.
Keywords: telomere, TRF2, Terc, p53, senescence
Introduction
The main function of telomeres is to cap chromosomal ends and thus to prevent chromosomal fusions and activation of DNA damage responses (Blackburn et al, 2000; de Lange, 2002). It has been shown that telomere shortening leads to loss of telomere function and induction of DNA-damage responses (Shay et al, 1991; Chin et al, 1999; Karlseder et al, 1999; d'Adda di Fagagna et al, 2003; Satyanarayana et al, 2004a). In vitro experiments have suggested that a twostage checkpoint response exists in response to different levels of telomere dysfunction (Shay et al, 1991). According to this hypothesis, moderate telomere dysfunction induces senescence at the mortality stage 1 (M1). However, if p53 is lost, cells bypass the senescence checkpoint and continue to proliferate, eventually reaching a second mortality stage (M2) characterized by massive cell death and chromosomal instability (crisis). Whether this model applies to the in vivo situation is under debate.
In telomerase-deficient (Terc−/−) mice, telomere shortening induced two different cellular phenotypes: apoptosis and senescence (Lee et al, 1998; Satyanarayana et al, 2003). In compound mutant mice lacking p53 and Terc, organ phenotypes induced by telomere dysfunction were rescued. However, these reappeared in a late generation of Terc−/− p53−/− double-knockout mice, suggesting that p53-independent signals can mediate responses to telomere dysfunction in vivo (Chin et al, 1999).
To analyse the influence of the level of telomere dysfunction on cellular responses in vivo, we used the experimental system of TRF2 inhibition, which leads to telomere dysfunction and chromosomal fusions (van Steensel et al, 1998). In vitro experiments have established that inhibition of TRF2 provokes the same phenotypes as telomere shortening: senescence and apoptosis (Karlseder et al, 1999, 2002; Smogorzewska & de Lange, 2002).
In the present study, we analysed the consequences of the loss of telomere protection by TRF2 inhibition in the mouse liver. The cellular responses were analysed in correlation with the level of telomere protection and p53 gene status. Our study demonstrates that the cellular level of telomere dysfunction determines the type of damage response that occurs and provides experimental evidence that telomere dysfunction provokes both p53-dependent and p53-independent effects in mouse liver cells.
Results
Dose-dependent induction of telomere dysfunction
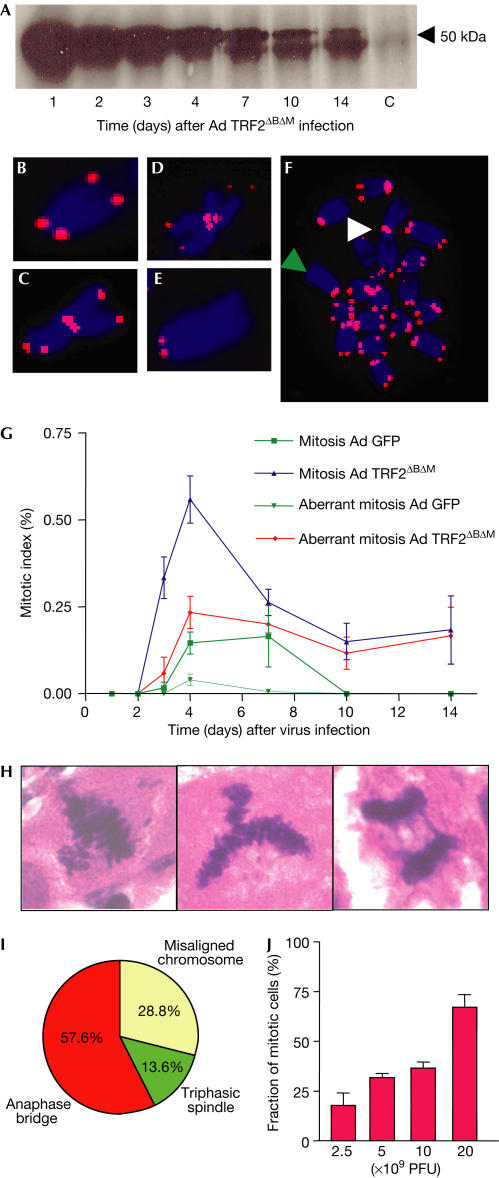
We analysed chromosomal fusions, anaphase bridges and ploidy of liver cells in response to adenoviral-mediated TRF2ΔBΔM expression in mouse liver. The level of TRF2ΔBΔM protein expression peaked 24 h after virus application (Fig 1A). Chromosomal fusions were detected at a rate of 1.03 fusions per metaphase 72 h after adenovirus TRF2ΔBΔM (Ad TRF2ΔBΔM) infection. Chromosomal fusions often contained telomere sequence, and there were high rates of telomere free ends (Fig 1B–F). We did not detect a significant degradation of the telomeric Gstrand in mouse liver infected with Ad TRF2ΔBΔM (supplementary Fig 1 online), which is possibly due to the relatively low percentage of TRF2ΔBΔM-infected liver cells in our in vivo system (supplementary Fig 2 online; also see below and Fig 3F) compared with previous in vitro experiments. In our in vivo system, the majority of infected liver cells were hepatocytes, as determined on the basis of cell morphology (data not shown).
Figure 1.
TRF2ΔBΔM induces telomere dysfunction in mouse liver. Mice were injected intravenously with Ad TRF2ΔBΔM (1 × 1010 PFU/ml) or with control Ad GFP (1 × 1010 PFU/ml, n=21 mice per group, three mice per time point). (A) Protein expression level of TRF2ΔBΔM in protein extracts (100 μg) from whole-cell lysates of mouse liver. (B–F) Representative photographs of metaphase chromosomes from mouse liver cells, 72 h after Ad TRF2ΔBΔM (1 × 1010 PFU/ml) infection. A telomerespecific probe was used for fluorescence in situ hybridization (FISH; counter staining: DAPI). (B) Normal chromosome with telomere signal on either side of chromosome arms. (C) Fusion involving both p-arms of the fused chromosomes with telomeric sequence at the fusion point. (D) Single short-arm chromatid fusion involving only one p-arm with telomeric sequence at the fusion point. (E) Telomere free end at the q-arm of the chromosome. (F) Metaphase: the white arrowhead shows chromosomal fusion, and the green arrowhead points to telomere-free end. (G) Graph of the rate of aberrant mitosis at the indicated time points after virus infection. The rate of aberrant mitosis was significantly higher in the liver of Ad TRF2ΔBΔM-infected mice compared with Ad GFP-infected mice (e.g. rate of aberrant mitosis at day 4 after virus infection: 0.233±0.033 in Ad TRF2ΔBΔM-infected mice versus 0.040±0.033 in Ad GFP-infected mice, P=0.006). (H) Representative photographs and (I) piechart of the types of aberrant mitotic figure observed in Ad TRF2ΔBΔM-infected mouse liver. (H) From left to right: misaligned chromosome, triphasic spindle and anaphase bridge (magnification: × 1,000). (J) Percentage of aberrant mitotic figures 120 h after Ad TRF2ΔBΔM infection at different viral concentrations.
Ad TRF2ΔBΔM-infected mouse liver showed high rates of aberrant mitosis (Fig 1G), including misaligned metaphase chromosomes, triphasic spindles, and most importantly, frequent anaphase bridges (Fig 1H,I). The prevalence of higher rates of mitotic figures in Ad TRF2ΔBΔM-infected mice compared with control mice was in line with previous observations showing that telomere dysfunction induces a delay in mitosis progression, thus increasing the rate of mitotic figures in regenerating liver (Rudolph et al, 2000). Telomere dysfunction and aberrant mitosis resulted in severe polyploidy of liver cells in TRF2ΔBΔM-infected mice (supplementary Fig 3 online). Adenovirus (Ad) green fluorescent protein (GFP)-infected control mice did not show telomere dysfunction, anaphase bridges or polyploidy (Fig 1G; supplementary Fig 3 online). The analysis of aberrant mitosis and anaphase bridges in mouse liver after application of different doses of Ad TRF2ΔBΔM showed a strong correlation between the level of Ad TRF2ΔBΔM infection and induction of aberrant mitosis (Fig 1J).
Induction of apoptosis and senescence
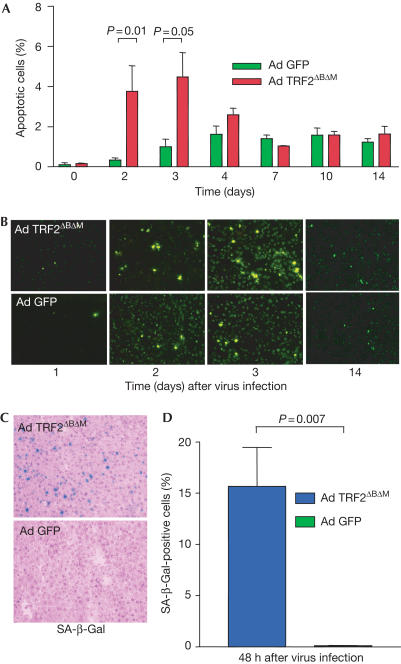
TRF2ΔBΔM adenovirus produced an apoptotic response peaking at 48–72 h after virus application, which was not seen in Ad GFP-infected control mice (Fig 2A,B). At later time points (day 7 after virus infection), Ad TRF2ΔBΔM-infected mouse liver no longer showed elevated rates of apoptosis compared with Ad GFP-infected control mouse liver. Senescence-associated β-galactosidase (SA-β-Gal)-positive liver cells were detected first at 48 h after adenoviral delivery of TRF2ΔBΔM but not in Ad GFP-infected control mice (Fig 2C,D).
Figure 2.
TRF2ΔBΔM induces apoptosis and senescence in the mouse liver. Apoptosis was determined by TUNEL assay on liver sections from mice infected with Ad TRF2ΔBΔM or Ad GFP (1 × 1010 PFU/ml, n=21 mice per group, n=3 mice per time point). (A) Percentage of apoptotic liver cells at the indicated time points. Note that Ad TRF2ΔBΔM induced significantly higher rates of apoptosis compared with Ad GFP-infected mice at early time points when maximal levels of TRF2ΔBΔM were expressed (P=0.01 at day 2 after virus infection; P=0.05 at day 3 after virus infection). (B) Representative photographs of apoptotic liver cells as detected by TUNEL assay at the indicated time points after virus infection (magnification: × 200). (C) Representative photographs of SA-β-Gal-positive liver cells 48 h after Ad TRF2ΔBΔM (top) and Ad GFP infection (bottom) (magnification: × 200). (D) Percentage of liver cells positive for SA-β-Gal staining in Ad TRF2ΔBΔM-infected mice compared with Ad GFP-infected mice.
Outcome – dependent on level of mutant TRF2
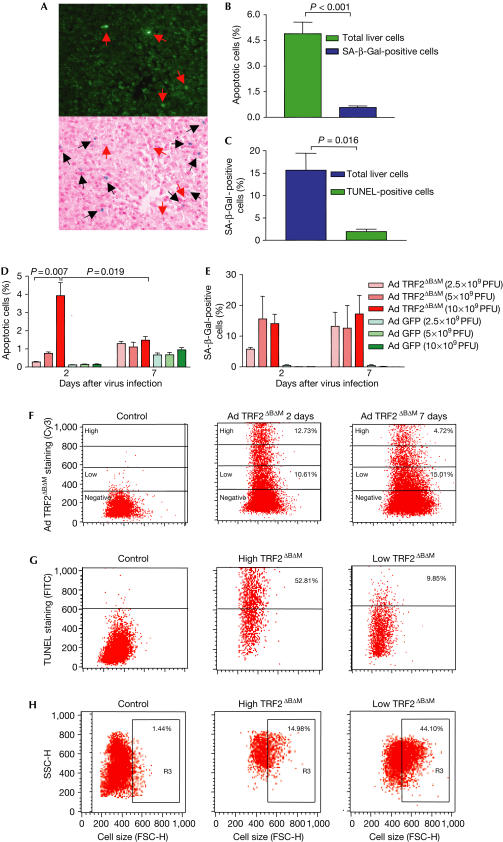
Co-staining of SA-β-Gal and TUNEL showed that senescence and apoptosis were induced in different populations of mouse liver cells in response to TRF2ΔBΔM infection (Fig 3A–C). Costaining of TUNEL, SA-β-Gal and TRF2ΔBΔM showed that apoptotic cells showed stronger staining for TRF2ΔBΔM, whereas SA-β-Gal-positive liver showed weaker staining for TRF2ΔBΔM (supplementary Fig 4A–C online). After infection of mice with different titres of Ad TRF2ΔBΔM, a strong dose–response correlation between the titre of TRF2ΔBΔM infection and the rate of apoptotic liver cells was observed at day 2 after viral infection (Fig 3D). In contrast to the apoptotic response, the induction of SA-β-Gal activity was sustained and showed a slight increase at day 7 after Ad TRF2ΔBΔM infection (Fig 3E). These data correlated with the observation that the percentage of liver cells expressing high levels of TRF2ΔBΔM decreased at day 7 compared with day 2 after virus infection, whereas the percentage of liver cells expressing weak levels of TRF2ΔBΔM increased at the same time interval (Fig 3F). Fluorescence-activated cell sorting (FACS) analysis on liver cell suspension costained for TUNEL and TRF2ΔBΔM confirmed a positive correlation between the cellular level of TRF2ΔBΔM expression and apoptosis of liver cells (Fig 3G). To analyse the correlation between senescence and the level of TRF2ΔBΔM, cell size was monitored according to previous studies, which have shown a significant increase in cell size of senescent cells, detectable by FACS analysis (Gorbunova et al, 2003; Martin-Ruiz et al, 2004). This analysis showed an inverse relationship between the cellular level of TRF2ΔBΔM expression and the rate of senescent liver cells (Fig 3H).
Figure 3.
Induction of apoptosis or senescence depends on the level of TRF2ΔBΔM expression in mouse liver. The localization of apoptotic and senescent cells was analysed in liver sections of mice infected with Ad TRF2ΔBΔM (1 × 1010 PFU/ml; n=4). (A) Representative photographs of TUNEL and SA-β-Gal costaining. Upper panel: TUNEL staining; lower panel: SA-β-Gal staining with haemotoxylin and eosin counterstaining. Note that the SA-β-Gal-positive cells (black arrows) can be discriminated from the green fluorescent apoptotic liver cells (red arrows) (magnification: × 200). (B) Histogram of the prevalence of SA-β-Gal-positive liver cells 48 h after Ad TRF2ΔBΔM infection (1 × 1010 PFU/ml): 15.7% of total liver cells were SA-β-Gal positive, whereas only 1.9% of TUNEL-positive liver cells were SA-β-Gal positive (P=0.016). (C) Histogram of the prevalence of apoptotic liver cells 48 h after Ad TRF2ΔBΔM infection (1 × 1010 PFU/ml): 4.9% of total liver cells were TUNEL positive, whereas only 0.6% of SA-β-Gal-positive liver cells were TUNEL positive (P<0.001). (D) Dose–response analysis of the percentage of TUNEL-positive cells in correlation with the titre of virus infection. The dose of Ad TRF2ΔBΔM infection correlates with the level of apoptosis at day 2 after virus infection. (E) Dose–response analysis of the percentage of SA-β-Gal-positive cells in correlation with the titre of virus infection. There was no significant dose–response correlation. Note that in contrast to the apoptosis response, the rate of SA-β-Gal-positive liver cells increased at day 7 after virus infection. (F) Percentage of TRF2ΔBΔM-positive liver cells as determined by FACS analysis of liver-cell suspensions at the indicated time points after virus infection (1 × 1010 PFU/ml). Left: Ad GFP-infected control mouse liver; middle: Ad TRF2ΔBΔM-infected mouse liver at day 2 after viral infection; right: Ad TRF2ΔBΔM-infected mouse liver at day 7 after viral infection. Note the decrease in liver cells expressing a high level of TRF2ΔBΔM but the increase in liver cells expressing a low level of TRF2ΔBΔM at day 7 compared with day 2 after viral infection. (G) Representative graphs of the percentage of TUNEL-positive cells in liver-cell suspensions analysed by FACS at day 2 after virus infection (1 × 1010 PFU/ml). Left: control mouse liver; middle: fraction of liver cells expressing high levels of TRF2ΔBΔM (52.8% of the cells stain positive for TUNEL); right: fraction of liver cells expressing low levels of TRF2ΔBΔM (9.85% of the cells stain positive for TUNEL). The gates for the expression level of TRF2ΔBΔM were set according to the labelling in (F). (H) Representative graphs of the percentage of senescent liver cells as analysed on the basis of cell size by FACS of liver-cell suspension at day 7 after virus infection. The gate R3 contains cells that have an increase in cell size (FSC, increase in forward scatter). Left: control mouse liver; middle: fraction of liver cells expressing high levels of TRF2ΔBΔM (29.1% of the cells show a senescence-associated increase in cell size); right: fraction of liver cells expressing low levels of TRF2ΔBΔM (44.1% of the cells show a senescence-associated increase in cell size). The gates for the expression level of TRF2ΔBΔM were set according to the labelling in (F).
Role of p53 in apoptosis and senescence
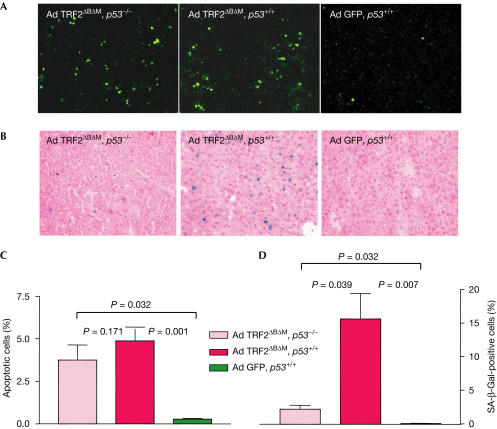
To identify the role of p53 in response to different levels of telomere dysfunction induced by TRF2ΔBΔM in mouse liver cells, we studied induction of apoptosis and senescence in p53−/− and p53+/+ mice 48 h after infection with Ad TRF2ΔBΔM (Fig 4). The rate of apoptosis induced by Ad TRF2ΔBΔM infection was almost identical in p53+/+ and p53−/− mice (Fig 4A,C). In contrast, the induction of senescence by Ad TRF2ΔBΔM infection was markedly reduced in p53−/− mice compared with p53+/+ mice (Fig 4B,D).
Figure 4.
TRF2ΔBΔM expression induces p53-independent apoptosis but p53-dependent senescence. The impact of p53 status on the effects of the loss of telomere protection by Ad TRF2ΔBΔM (1 × 1010 PFU/ml) infection was analysed by comparing p53 wild-type (p53+/+) and p53-knockout (p53−/−) mice (n=5). (A) Representative photographs of TUNEL-positive liver cells 48 h after TRF2ΔBΔM infection in p53−/− mice (left), p53+/+ mice (middle) and GFP control-infected p53+/+ mice (right) (magnification: × 200). (B) Representative photographs of SA-β-Galstained liver sections 48 h after Ad TRF2ΔBΔM infection of p53−/− mice (left), Ad TRF2ΔBΔM infection of p53+/+ mice (middle) and Ad GFP infection of p53+/+ mice (right) (magnification: × 200). (C) Percentage of apoptotic liver cells 48 h after Ad TRF2ΔBΔM or Ad GFP infection. Note that the increase in apoptotic cells in Ad TRF2ΔBΔM-infected mice is p53 independent. (D) Percentage of SA-β-Gal-positive liver cells 48 h after Ad TRF2ΔBΔM infection. Note that the increase in SA-β-Gal-positive cells in Ad TRF2ΔBΔM-infected mice is p53 dependent.
Discussion
Our current study shows that TRF2ΔBΔM expression provokes acute telomere dysfunction, chromosomal fusions, aberrant mitosis and polyploidy in mouse liver cells in vivo. One limitation of this experimental system is that it does not mimic the gradual telomere attrition that is observed in physiological processes in vivo. Conversely, TRF2 inhibition seems to be a functional surrogate for telomere dysfunction arising from telomere attrition, as shown by its ability to promote anaphase bridging in a dose-dependent manner. This system allowed us to pinpoint specific downstream signalling responses to different levels of telomere dysfunction in the mouse liver.
Our study shows that the cellular level of the loss of telomere protection dictates the type of damage response induced in liver cells in vivo. The data show that senescence induced by low levels of TRF2ΔBΔM expression is p53 dependent, whereas apoptosis induced by high levels of TRF2ΔBΔM expression is p53 independent. Our results differ from previous reports, which showed a strict p53 dependency of apoptosis induced by TRF2ΔBΔM in mouse fibroblasts and human cancer cell lines in vitro (Karlseder et al, 1999). A possible explanation is that the accumulation of genetic alterations in mouse embryo fibroblasts (Parrinello et al, 2003) and human cancer cells alters the cellular responses to telomere dysfunction. Our data suggest that in vivo a twostage checkpoint exists in response to different levels of telomere dysfunction. These data are in agreement with the classical model of two mortality stages of primary human fibroblasts in response to moderate or severe telomere dysfunction (Shay et al, 1991). Given that telomere shortening is a property of ageing cells and an early feature of various cancers (Djojosubroto et al, 2003; Satyanarayana et al, 2004b), it is possible that this twostage checkpoint response to telomere dysfunction is necessary for tumour suppression in vivo. An increase in senescent cells has been demonstrated in various mammalian tissues during ageing (Dimri et al, 1995; Krishnamurthy et al, 2004). It remains to be analysed how frequently cells bypass the M1 checkpoint of senescence in vivo and how this contributes to cancer initiation in aged human tissue.
Methods
Mice. For this study, we used 12- to 14-week-old female mice in a C57Bl/6J background and, for the p53-related experiments, C57Bl/6J-Trp53tm1Tyj.
Western blotting. Proteins subjected to SDS–polyacrylamide gel electrophoresis were detected using antibody against human TRF2 (1:10,000; anti-TRF2 05-521, Upstate, Charlottesville, VA, USA), which recognizes TRF2ΔBΔM but not endogenous mouse TRF2.
G-strand overhang assay. The assay was carried out as described previously (Cimino-Reale et al, 2001; Keys et al, 2004).
Adenovirus amplification and purification. All viruses were produced in HEK-293 cells and titred at the same time point in the same manner according to standard protocols. To assure a similar rate of adenovirus infection in different areas of mouse liver, the expression level of GFP was monitored 48 h after infection in all five liver lobes of an individual mouse infected with Ad GFP (supplementary Fig 5 online).
Apoptosis staining. The rate of apoptosis was determined by TUNEL assay (In situ cell detection kit, Roche, Mannheim, Germany). The number of apoptotic cells was determined in 20 low-power fields (× 200) and expressed as a percentage of all cells counted.
Senescence-associated β-galactosidase staining. SA-β-Gal staining was carried out as described previously (Dimri et al, 1995). The number of SA-β-Gal-positive cells was determined in 20 randomly chosen low-power fields (× 100) and expressed as a percentage of all cells counted.
Apoptosis–SA-β-galactosidase co-staining. The SA-β-Gal-stained sections were permeabilized with 1% Triton X-100 and 0.1% sodium citrate followed by TUNEL staining and counterstaining with DAPI. The number of apoptotic (n=422) and SA-β-Gal-positive cells (n=1,200) was analysed randomly in low-power fields (× 200) chosen at random (n=4 mice).
SA-β-galactosidase–TRF2 co-staining. The SA-β-Gal-stained sections were permeabilized in 10 mM citric acid–sodium phosphate buffer at 95°C for 7 min followed by incubation with anti-TRF2 antibody (1:300) for 1 h. The detection was performed by Cy3-labelled rabbit anti-mouse antibody (1:500; Zymed, San Francisco, CA, USA). The number of SA-β-Gal-positive cells (n=600) in correlation with the level of TRF2ΔBΔM expression was counted in randomly chosen low-power fields (× 200; n=4 mice).
Apoptosis–TRF2 co-staining. TUNEL-stained slides were incubated with TRF2 antibody (1:300) for 1 h followed by incubation with a secondary Cy3-labelled rabbit anti-mouse antibody (1:500) for 30 min. The number of apoptotic cells (n=400) in correlation with the level of TRF2ΔBΔM expression was counted in randomly chosen low-power fields (× 200; n=4 mice).
Feulgen staining. Feulgen staining was performed according to standard methods. The intensity of Feulgen staining was analysed with ACAS software (Ahrens Cytometry Analysis System for flow and static cytometry, Bargteheide/Hamburg, Germany). For each sample, 300 hepatocyte nuclei were analysed.
Metaphase spreads. After 48 h of adenovirus infection (Ad TRF2ΔBΔM, n=3; Ad GFP, n=4), the mice were treated with colcemid (a total of 20 μg/mouse) by intraperitoneal injection. At 24 h after colcemid injection, the liver cells were collected by liver perfusion as described previously (Satyanarayana et al, 2003).
Telomere fluorescence in situ hybridization. Quantitative fluorescence in situ hybridization (Q-FISH) was carried out as described previously (Plentz et al, 2004). Signals were detected using an epifluorescence microscope (Carl Zeiss Jena GmbH, Jena, Germany) equipped with a CCD camera and FISH View software (Applied Spectral Imaging Ltd, Migdal HaEmek, Israel).
FACS analysis. Cells were collected by liver perfusion at different time points and immediately used for TRF2 and TUNEL staining. They were then analysed by FACS Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). The cells were double stained for TRF2 with Cy3-labelled goat anti-mouse antibody (1:100; Zymed) and TUNEL to analyse the apoptosis. For measuring senescence, cells were stained for TRF2 with FITC-labelled goat anti-mouse (1:100; Caltag, Burlingame, CA, USA) and cell size was monitored by forward scatter (FSC). The gating for selection of large-senescent and small non-senescent cells was used as described previously (Gorbunova et al, 2003; Martin-Ruiz et al, 2004) using Ad GFP-infected and non-infected mice as controls.
Statistical programs. Student's t-test, Graphpad Instat, Graphpad Prism and Microsoft Excel software was used to calculate the statistical significance and standard deviations.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent /extref/7400352s1.pdf).
Supplementary Material
Supplemental Figures
Acknowledgments
We thank T. de Lange for providing the Ad TRF2ΔBΔM virus and Dr Hecker for statistical analysis. K.L.R. is supported by the Deutsche Forschungsgemeinschaft (Emmy-Noether-Programm: Ru 745/2-1, Ru745 4-1 and KFO119) and a grant from the Deutsche Krebshilfe e.V. (10-2236-Ru 2).
References
- Blackburn EH, Chan S, Chang J, Fulton TB, Krauskopf A, McEachern M, Prescott J, Roy J, Smith C, Wang H (2000) Molecular manifestations and molecular determinants of telomere capping. Cold Spring Harb Symp Quant Biol 65: 253–263 [DOI] [PubMed] [Google Scholar]
- Chin L, Artandi SE, Shen Q, Tam A, Lee SL, Gottlieb GJ, Greider CW, DePinho RA (1999) p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97: 527–538 [DOI] [PubMed] [Google Scholar]
- Cimino-Reale G, Pascale E, Starace G, Verna R, D'Ambrosio E (2001) The length of telomeric G-rich strand 3′-overhang measured by oligonucleotide ligation assay. Nucleic Acids Res 29: E35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198 [DOI] [PubMed] [Google Scholar]
- de Lange T (2002) Protection of mammalian telomeres. Oncogene 21: 532–540 [DOI] [PubMed] [Google Scholar]
- Dimri GP et al. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92: 9363–9367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djojosubroto MW, Choi YS, Lee HW, Rudolph KL (2003) Telomeres and telomerase in aging, regeneration and cancer. Mol Cells 15: 164–175 [PubMed] [Google Scholar]
- Gorbunova V, Seluanov A, Pereirasmith OM (2003) Evidence that high telomerase activity may induce a senescent-like growth arrest in human fibroblasts. J Biol Chem 278: 7692–7698 [DOI] [PubMed] [Google Scholar]
- Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T (1999) p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283: 1321–1325 [DOI] [PubMed] [Google Scholar]
- Karlseder J, Smogorzewska A, de Lange T (2002) Senescence induced by altered telomere state, not telomere loss. Science 295: 2446–2449 [DOI] [PubMed] [Google Scholar]
- Keys B, Serra V, Saretzki G, Von Zglinicki T (2004) Telomere shortening in human fibroblasts is not dependent on the size of the telomeric-3′-overhang. Aging Cell 3: 103–109 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE (2004) Arf expression is a biomarker of aging. J Clin Invest 114: 1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Blasco MA, Gottlieb GJ, Horner JW II, Greider CW, DePinho RA (1998) Essential role of mouse telomerase in highly proliferative organs. Nature 392: 569–574 [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C, Saretzki G, Petrie J, Ladhoff J, Jeyapalan J, Wei W, Sedivy J, von Zglinicki T (2004) Variation in telomere shortening rate causes heterogeneity of human fibroblast replicative life span. J Biol Chem 279: 17826–17833 [DOI] [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J (2003) Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol 5: 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plentz RR, Caselitz M, Bleck JS, Gebel M, Flemming P, Kubicka S, Manns MP, Rudolph KL (2004) Hepatocellular telomere shortening correlates with chromosomal instability and the development of human hepatoma. Hepatology 40: 80–86 [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA (2000) Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science 287: 1253–1258 [DOI] [PubMed] [Google Scholar]
- Satyanarayana A, Wiemann SU, Buer J, Lauber J, Dittmar KEJ, Wüstefeld T, Blasco M, Manns MP, Rudolph KL (2003) Telomere shortening impairs organ regeneration by inhibiting cell cycle re-entry of a subpopulation of cells. EMBO J 22: 4003–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana A, Greenberg RA, Schaetzlein S, Buer J, Masutomi K, Hahn WC, Zimmermann S, Martens U, Manns MP, Rudolph KL (2004a) Mitogen stimulation cooperates with telomere shortening to activate DNA damage responses and senescence signaling. Mol Cell Biol 24: 5459–5474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana A, Manns MP, Rudolph KL (2004b) Telomeres and telomerase: a dual role in hepatocarcinogenesis. Hepatology 40: 276–283 [DOI] [PubMed] [Google Scholar]
- Shay JW, Pereirasmith OM, Wright WE (1991) A role for both RB and p53 in the regulation of human cellular senescence. Exp Cell Res 96: 1–6 [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T (2002) Different telomere damage signaling pathways in human and mouse cells. EMBO J 21: 4338–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B, Smogorzewska A, de Lange T (1998) TRF2 protects human telomeres from end-to-end fusions. Cell 92: 401–413 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figures