Abstract
DNA mismatch repair (MMR) is essential in the surveillance of accurate transmission of genetic information, and defects in this pathway lead to microsatellite instability and hereditary nonpolyposis colorectal cancer (HNPCC). Our previous study raised the possibility that hMRE11 might be involved in MMR through physical interaction with hMLH1. Here, we show that hMRE11 deficiency leads to significant increase in MSI for both mono- and dinucleotide sequences. Furthermore, RNA-interference-mediated hMRE11-knockdown in HeLa cells results in MMR deficiency. Analysis of seven HNPCC-associated hMLH1 missense mutations located within the hMRE11-interacting domain shows that four mutations (L574P, K618T, R659P and A681T) cause near-complete disruption of the interaction between hMRE11 and hMLH1, and two mutations (Q542L and L582V) cause a 30% reduction of protein interaction. These findings indicate that hMRE11 represents a functional component of the MMR pathway and the disruption of hMLH1–hMRE11 interaction could be an alternative molecular explanation for hMLH1 mutations in a subset of HNPCC tumours.
Keywords: DNA mismatch repair, microsatellite instability, hMLH1, hMRE11, HNPCC
Introduction
DNA mismatch repair (MMR) has a crucial role in ensuring accurate transmission of genetic information, and MMR deficiency in humans is associated with profound genetic instability and a high risk for hereditary nonpolyposis colorectal cancer (HNPCC; Harfe & Jinks-Robertson, 2000). Proteins that are involved in MMR have emerged as central components of the DNA damage-response complex, which includes important MMR proteins and the hMRE11-associated complex (Wang et al, 2000).
Studies performed with various eukaryotic systems indicate that the MMR pathway is evolutionarily conserved, and the function of MMR relies on the concerted action of several MutS- and MutL- homologous proteins (Harfe & Jinks-Robertson, 2000). Although biochemical studies have shown that the 5′–3′ exonuclease I (hExoI) is involved in the process of MMR in human cells (Tishkoff et al, 1998; Dzantiev et al, 2004), the inactivation of ExoI only moderately affects MMR in the mouse (Wei et al, 2003). In addition, mice lacking ExoI are less likely to develop tumours than Msh2- or Mlh1-knockout mice, and this suggests the existence of functionally redundant exonucleases in mammalian MMR (Wei et al, 2003).
It is known that the pathogenesis of HNPCC can be attributed largely to germline mutations in at least five MMR genes; in fact germline mutations in the MMR gene hMLH1 alone are responsible for more than 50% of all HNPCC cases (Database of the International Collaborative Group on HNPCC, http://www.nfdht.nl). Almost 30% of all hMLH1 mutations are scattered missense alterations causing single amino-acid substitutions. Although the functional basis underlying the pathogenic effects for most missense mutations is at present open-ended, these mutations have the potential to alter the properties of hMLH1, which in turn could affect the assembly of functional repair complexes.
Our previous studies have shown a physical link between hMLH1 and hMRE11 (Her et al, 2002). Here, we show that hMRE11 is involved in human MMR, and that HPNCC-associated hMLH1 missense mutations located within the hMRE11-interacting domain disrupt protein interaction between hMLH1 and hMRE11.
Results And Discussion
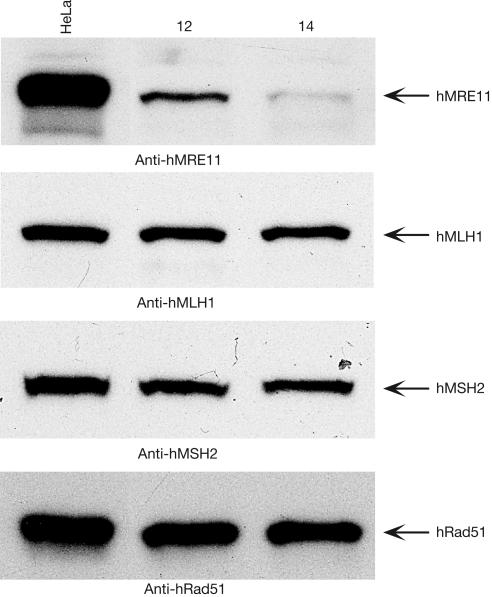
To examine the role of hMRE11 in MMR, we first investigated whether hMRE11 deficiency causes microsatellite instability (MSI)—the hallmark of defective MMR. We created two MSI reporters in which the open reading frame (ORF) of the green fluorescence protein (GFP) was interrupted by the insertion of either mononucleotide (A)17 or dinucleotide (CA)25 microsatellite repetitive sequences. These two out-of-frame (OF) GFP ORFs were then cloned and placed under the control of the human cytomegalovirus (CMV) immediate-early promoter. Then, a series of hMRE11-deficient cell lines were generated from MMR-proficient HeLa cells using RNA interference (RNAi) technology. The levels of hMRE11 silencing were estimated to be in the range of 45–97% in this series of individual clones, of which HeLa clone #14 (HeLa 14) showed the most significant knockdown (Fig 1). Despite being an essential gene, cells derived from HeLa 14 grew at rates similar to those of HeLa cells, and showed no detectable morphological changes (supplementary Fig S1 online). Immunoblotting analysis showed that hMRE11 RNAi had no effects on the expression of hMLH1, hMSH2 and hRad51 proteins, indicating that hMRE11 RNAi was highly specific (Fig 1).
Figure 1.
Generation of stable hMRE11-knockdown HeLa cells. Gene silencing of hMRE11 in HeLa cells was achieved by the use of small interfering RNAs. To generate HeLa cells stably displaying hMRE11-knockdown, two pmH1P-neo-based RNAi constructs encoding short hairpin RNAs were used to transfect HeLa cells. Neomycin-resistant clones were selected with 400 μg/ml G418, and 150 μg of cell extracts was analysed by immunoblotting. HeLa, parental control; 12 and 14, two representative stable cell lines.
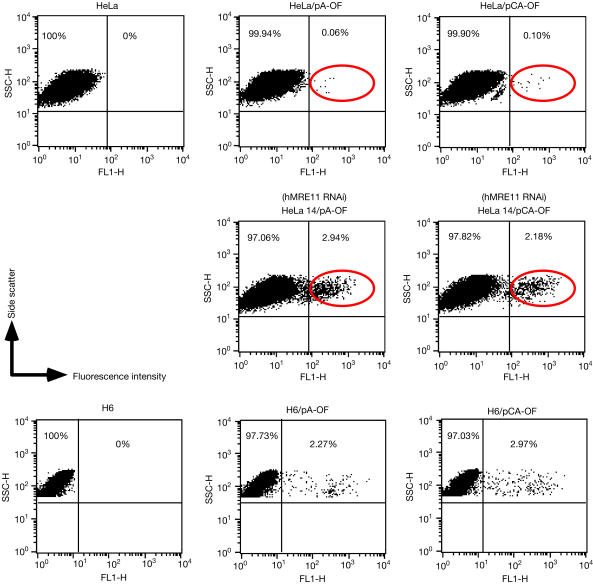
Cells of HeLa 14 were then examined for MSI. The results of the MSI functional assay, using pA-OF and pCA-OF, indicated that hMRE11-knockdown in HeLa cells increased mononucleotide (A)17 instability about 50-fold and dinucleotide (CA)25 instability 22-fold (Fig 2). Analysis of hMLH1-deficient H6 cells using the same reporters showed that hMLH1-deficient H6 cells showed similar levels of MSI (Fig 2). Therefore, cells defective in hMRE11 seem to show a mutator phenotype similar to that observed in hMLH1-deficient cells. In addition, human cells harbouring an integrated copy of an in-frame reporter also showed an increase in MSI when treated with constructs encoding hMRE11 short hairpin RNAs (supplementary Fig S2 online). These observations suggest a crucial role for hMRE11 in MMR, presumably through the interplay with hMLH1.
Figure 2.
Effects of hMRE11-knockdown on microsatellite instability (MSI). Two out-of-frame MSI reporters, pA-OF and pCA-OF, were used to transfect a roughly equivalent number of parental and hMRE11-knockdown HeLa cells (HeLa 14), as well as the hMLH1-deficient cell line H6 as a control. The number of cells that expressed GFP was determined by FACS analysis performed with a total of 20,000 cells for HeLa and HeLa 14, and a total of 10,000 cells for parental and transfected H6 cells.
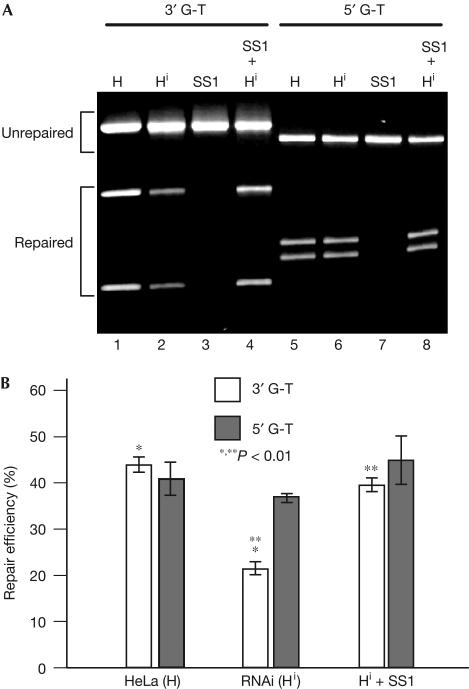
We then assessed the role of hMRE11 in MMR by a functional in vitro MMR assay. Nuclear extracts derived from HeLa 14 and its parental HeLa cells were tested for their abilities to repair heteroduplexes containing a single G-T mismatch and a strand break either 3′ (3′ G-T) or 5′ (5′ G-T) to the mismatch (Guo et al, 2004). Consistent with earlier reports (Holmes et al, 1990; Thomas et al, 1991), HeLa extracts carried out repair reactions for both substrates at comparable levels (Fig 3). Remarkably, the repair activities for the 3′ and 5′ heteroduplexes in extracts of HeLa 14 were markedly different; the 3′ activity was more than 50% lower than that of the control, and addition of partially purified hMRE11-enriched HeLa nuclear fraction SS1 (supplementary Fig S3 online) efficiently restored the 3′ activity to the control level (Fig 3B). As the SS1 fraction alone cannot carry out both 5′ and 3′ nick-directed MMR (Fig 3A), the results of the MMR assay show that hMRE11 is involved in the process of MMR. Although the repair of 5′ G-T was about 90% competent in extracts of HeLa 14 cells as compared with that of parental HeLa cells, the observed difference was not statistically significant (Fig 3B). The preferential reduction in 3′ G-T repair suggests that hMRE11 is involved in 3′ nick-directed MMR, which is well reconciled with the fact that hMRE11 possesses DNA 3′–5′ exonuclease activity (Paull & Gellert, 1998; Trujillo et al, 1998; Moreau et al, 1999). The remaining 3′ G-T repair activity observed in HeLa 14 could be attributed to the residual hMRE11 protein and/or the involvement of other functional redundant proteins in MMR, such as hExoI. It is noteworthy that the inactivation of ExoI caused only partial reduction of MMR activities in Saccharomyces cerevisiae and mouse (Tishkoff et al, 1997; Tran et al, 1999; Wei et al, 2003), which suggests the existence of redundant nucleases in MMR.
Figure 3.
Effects of hMRE11-knockdown on DNA mismatch repair (MMR). A 50 μg portion of nuclear extracts derived from parental HeLa (H) and HeLa 14 (Hi) cells and 17.6 μg of a partially purified hMRE11-enriched HeLa nuclear fraction SS1 were used to perform MMR reactions with 5′ G-T and 3′ G-T mismatch substrates. Repaired products were distinguished from substrates by restriction digestion, and repair efficiency was scored by dividing repaired products with the total substrate DNA. (A) MMR reactions were performed as indicated. The hMRE11-enriched SS1 fraction is defective for both 3′ and 5′ repairs. (B) Average repair efficiencies and standard deviations (error bars) were determined from three independent data points. *P=0.003, **P=0.007, t-test.
Our data support the notion that the functional interplay between hMLH1 and hMRE11 has a crucial role in human MMR. Despite the well-established roles of the hMRE11-associated complex in DNA damage response and the repair of DNA double-strand breaks (DSBs), the role of hMRE11-associated nuclease activities in DSB repair has not been fully established (Bressan et al, 1999). Although the effect of hMRE11-knockdown on 5′ G-T repair is not statistically significant, we could not eliminate the possibility that the residual hMRE11 protein in cells from HeLa 14 may be sufficient for 5′ repair. This view is consistent with the observation that human hExoI may be involved in both 5′ and 3′ nick-initiated and mismatch-induced MMR reactions (Dzantiev et al, 2004), despite the fact that hExoI is known to possess only a 5′–3′ hydrolytic activity (Tishkoff et al, 1997; Lee & Wilson, 1999). Recent studies have shown the presence of hMRE11 somatic mutations in chromosomal instability (CIN) cancers, MMR-deficient colorectal tumours and cancer cell lines (Giannini et al, 2002; Wang et al, 2004). Although the close correlation between an intronic (T)11 mutation in the hMRE11 gene and MMR deficiency has led to the speculation that hMRE11 might represent a target in tumorigenesis driven by MMR deficiency (Giannini et al, 2002), our data indicate that hMRE11 deficiency directly results in elevated MSI and defective MMR.
So far, studies performed with HNPCC families have shown that hMLH1 gene mutations account for about 52.9% of all HNPCC cases (http://www.nfdht.nl), among which about 30% of hMLH1 mutations are single amino-acid substitutions. Despite this prevalence, the molecular basis underlying the pathogenic effects of these single amino-acid substitutions has not been fully explained. As the interaction between hMRE11 and hMLH1 might be involved in recruiting hMRE11 during the MMR process (Her et al, 2002), it is plausible that some of the functional effects of hMLH1 mutations could result from the disruption of hMRE11–hMLH1 interaction.
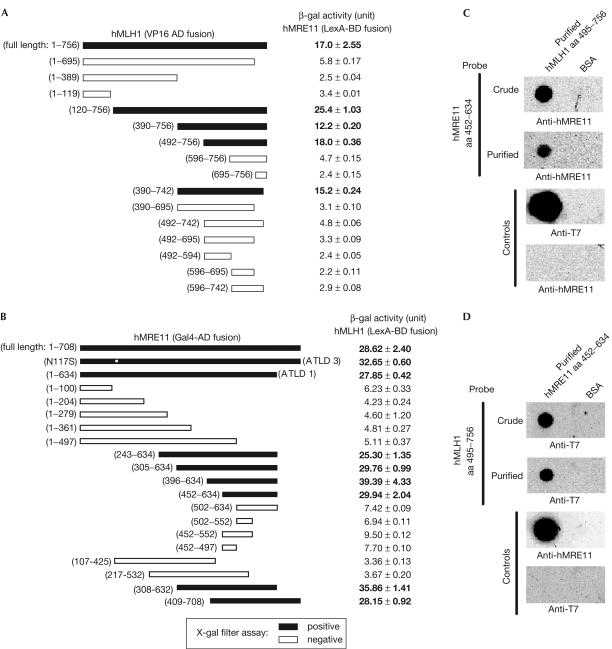
Hence, to test whether the disruption of hMRE11–hMLH1 interaction is an immediate consequence of hMLH1 mutations in HNPCC, we determined the effects of seven hMLH1 mutations on protein interaction. The minimal interacting domains of hMLH1 and hMRE11 were first determined by yeast two-hybrid analysis performed with a series of hMLH1 and hMRE11 deletion mutants. As shown in Fig 4A, experiments performed with LexA-BD–hMRE11 (full-length) and a series of hMLH1 VP16-AD fusion mutants showed that the minimal hMRE11-interacting region was located within the carboxy-terminal portion of hMLH1 corresponding to amino-acid residues 492–756, which overlapped with hMLH1 domains implicated in mediating protein interactions with hPMS2, hMLH3, hPMS1, BLM or hExo1 (Kondo et al, 2001; Pedrazzi et al, 2001; Schmutte et al, 2001). Similarly, the minimal region of hMRE11 that mediated interaction with hMLH1 was resolved to comprise amino-acid residues 452–634 (Fig 4B). The interaction between these two domains was then validated by far-western analysis (Fig 4C,D). Immobilized hMLH1 amino acid (aa) 495–756 fragment specifically interacted with hMRE11 aa 452–634 fragment either in purified form or crude lysate (Fig 4C). Conversely, immobilized hMRE11 aa 452–634 protein could specifically capture hMLH1 aa 495–756 fragment (Fig 4D). Thus, the C-terminal portion of hMLH1 interacts with a region of hMRE11 protein that resides in between two DNA-binding motifs (de Jager et al, 2001).
Figure 4.
Interaction domain mapping for hMLH1 and hMRE11. (A) The 756-amino-acid full-length hMLH1 and a series of deletion mutants were encoded in pVPd as VP16-AD fusion proteins. The average β-galactosidase (β-gal) activity units and standard errors (s.e.) from at least three independent data points are listed. L40 double transformant harbouring full-length hMRE11-BD plasmid and an empty pVPd vector showed a background β-gal activity reading of 2.60±0.12. (B) cDNA fragments encoding the 708-amino-acid full-length hMRE11 and a series of hMRE11 truncation mutants in the form of Gal4-AD fusions were used to examine protein interactions with the full-length hMLH1 in LexA-BD fusion form. The protein interaction was ascertained as described in (A). The L40 double transformant harbouring full-length hMLH1-BD plasmid and an empty pGADT7 vector showed a background β-gal activity reading of 4.59±0.42. The two naturally occurring hMRE11 mutations R633stop and N117S are indicated by ATLD1 and ATLD3, respectively. In (A,B), the black bars indicate positive protein interactions monitored by X-gal filter assay. (C,D) Characterization of interacting domains by far-western analysis. The T7 tag was removed from the construct encoding hMRE11 aa 452–634 fragment. (C) Purified T7 tagged hMLH1 aa 495–756 protein (3 μg) and BSA were immobilized directly on the same nitrocellulose membranes, which were then probed with either purified MRE11 aa 452–634 proteins or the corresponding crude preparation. The anti-hMRE11 and anti-T7 antibodies were used to detect the presence of hMRE11 aa 452–634 and T7-hMLH1 aa 495–756 proteins, respectively. (D) A reciprocal far-western analysis of purified, immobilized hMRE11 aa 452–634 protein and BSA. The membranes were incubated with T7-hMLH1 aa 495–756 crude lysate or purified protein. The same antibodies as in (C) were used for detection.
We next investigated whether the formation of hMLH1–hMRE11 complex could be disrupted by the common HNPCC-associated hMLH1 missense mutations located within the interacting domain. Of the seven mutations studied, two (L574P and R659P) completely disrupted the interactions of hMLH1 with both hMRE11 and hPMS2 (Table 1). Two mutations (K618T and A681T) showed differential effects on their interactions with hMRE11 and hPMS2; both point mutations abolished the interaction with hMRE11 but remained at 50% of their capacity to associate with hPMS2 (Table 1). The remaining hMLH1 mutations (Q542L, E578G and L582V) had relatively small (less than 30%) but differential effects on hMLH1 interaction with hMRE11 and hPMS2 (Table 1). The observed defects of various hMLH1 missense mutations in protein interaction with hPMS2 are consistent with those reported previously (Kondo et al, 2003). The different effects of these hMLH1 mutations on protein interactions are probably due to protein conformational changes caused by amino-acid sequence variations, which is supported by previous observations that most of the missense mutations did not affect the expression levels of hMLH1 variant proteins (Kondo et al, 2003).
Table 1.
Effects of various hMLH1 HNPCC missense mutations on protein interaction
|
β-gal activity (units±s.e.) (Gal4-AD fusion) |
||
|---|---|---|
| hMLH1 (495–756 LexA-BD) | hMRE11 | hPMS2 |
| Wild type | 128.89±10.27 | 192.77±14.00 |
| Q542L | 93.21±7.84 | 164.55±12.72 |
| L574P | 4.25±0.29 | 3.91±0.29 |
| E578G | 124.92±7.29 | 145.01±14.16 |
| L582V | 99.72±8.57 | 191.83±10.49 |
| K618T | 4.22±0.44 | 80.45±1.62 |
| R659P | 2.64±0.27 | 3.62±0.24 |
| A681T | 2.47±0.32 | 90.23±7.65 |
| R659P/A681T | 2.69±0.36 | 3.53±0.09 |
β-Galactosidase activity was determined with the β-gal liquid assay using ONPG as a substrate. The coding sequences for wild-type hMLH1 aa 495–756 and various missense mutants were fused to LexA-BD in plasmid pBTM116. R659P/A681T is a double mutant containing two independent mutations. Full-length hMRE11 and hPMS2 proteins were fused with Gal4-AD in plasmid pGADT7. Analysis of protein interaction was carried out in reporter strain L40. The average β-gal activity units and standard errors (s.e.) were determined with at least three independent data points.
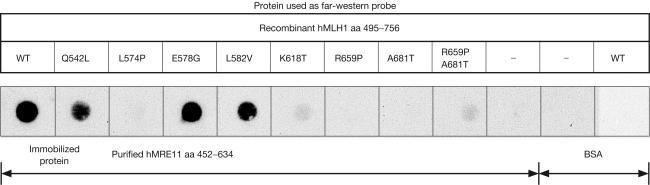
To validate the results obtained from yeast two-hybrid analysis, we performed a far-western assay to examine the effects of the same set of hMLH1 missense mutations on the interaction between hMLH1 and hMRE11. As shown in Fig 5, the result of far-western analysis was consistent with that of the two-hybrid assay, which suggests that the disruption of hMLH1–hMRE11 interaction might represent an alternative molecular basis underlying the functional effects of hMLH1 mutations in human cells.
Figure 5.
Far-western analysis of the effects of hMLH1 HNPCC missense mutations on the hMLH1 and hMRE11 interaction. Membranes with purified, immobilized hMRE11 aa 452–634 protein and BSA were probed independently with crude lysate containing wild-type and various hMLH1 mutant proteins. Conventional western blot analysis was carried out using anti-T7 antibody to detect the captured wild-type and mutant hMLH1 aa 495–756 proteins.
Our results show, for the first time, that the human hMRE11 protein is involved in 3′ nick-directed MMR, presumably through interaction with hMLH1. Our data indicate that hMRE11 deficiency leads to MSI and defective MMR in human cells. Furthermore, our study suggests that the biological consequence of hMLH1 HNPCC mutations might be attributed to the uncoupling of the hMRE11–hMLH1 protein complex. Obviously, for a better appreciation of the hMRE11–hMLH1 interplay in human cells, further deciphering of the precise enzymatic and/or structural roles of hMRE11 in the process of MMR will be required. The participation of hMRE11 in MMR should predict that the dynamic interplay between MMR components and DSB repair proteins is essential for the surveillance of genomic integrity.
Methods
Western blot analysis and antibodies. Proteins were separated on 10% SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, NH, USA). Antibodies used in this study included anti-hMRE11 polyclonal (Novus Biologicals Inc., Littleton, CO, USA), anti-hMRE11 12D7 (GeneTex Inc., San Antonio, TX, USA), anti-hMLH1 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), anti-hMSH2 (Calbiochem, San Diego, CA, USA), anti-RAD51 (Oncogene Research Products, Cambridge, MA, USA) and anti-T7 tag (Novagen, Madison, WI, USA). For far-western analysis, immobilization of affinity-purified proteins on nitrocellulose membranes was carried out with Bio-Dot Microfiltration Apparatus (Bio-Rad, Hercules, CA, USA). The procedure for far-western analysis was as described previously (Wu et al, 2000).
HeLa hMRE11–RNAi stable transfectants. HeLa cells were transfected with a mixture of pmH1P-neo-based constructs (Carmell et al, 2003) targeting two regions of the hMRE11 transcript at positions 400–418 (5′-GGGGCAGATGCACTTTGTG) and 1242–1260 (5′-TGGGAAACTTATCACAAAG). Transfected HeLa cells were selected with 400 μg/ml G418 (Clontech, Palo Alto, CA, USA). Individual G418-resistant clones were expanded and analysed by immunoblotting techniques.
Yeast two-hybrid analysis. Complementary DNA fragments encoding full-length and relevant regions of hMRE11, hMLH1 and hPMS2 proteins were cloned into vectors pBTM116, pVPd, pGADT7 and pACT2. Protein interactions were ascertained with the transcription activation of lacZ gene in the reporter strain L40. β-Galactosidase (β-gal) activities were either qualitatively monitored with X-gal filter assays or quantitatively measured by the β-gal liquid assay using o-nitrophenyl β-D-galactopyranoside (ONPG) as a substrate.
Microsatellite instability reporter assay. Microsatellite repetitive sequences ATGGC(A)17 or ATGGC(CA)25 were cloned upstream of a GFP ORF devoid of initial ATG start codon in pcDNA6 (Invitrogen, Carlsbad, CA, USA) to create the MSI reporter constructs pA-OF and pCA-OF. Cells that were transfected with pA-OF and pCA-OF were counted with a fluorescence-activated cell sorter (FACSort, Becton Dickinson, Franklin Lakes, NJ, USA), and the percentage of GFP-expressing cells was analysed with BD CellQuest Pro.
Nuclear extracts and repair assay. Nuclear extracts were prepared from parental HeLa and HeLa 14 cells as described previously (Holmes et al, 1990). DNA heteroduplexes used in this study contained a G-T mismatch and a strand-break either 5′ (5′ G-T) or 3′ (3′ G-T) to the mismatch (Guo et al, 2004). MMR assays were performed as described previously (Holmes et al, 1990).
Purification of recombinant proteins. hMRE11 and hMLH1 cDNA fragments encoding protein interaction domains were subcloned into pET-28a (Novagen, San Diego, CA, USA), and the resulting constructs were transformed into BL21(DE3)-RIL (Stratagene, La Jolla, CA, USA) to produce recombinant T7-hMLH1 aa 495–756 and hMRE11 aa 452–634 as His6 fusion proteins. Recombinant proteins were purified with the TALON Metal Affinity Resins (Clontech).
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400392s1.pdf).
Supplementary Material
Supplementary Information
Acknowledgments
We thank Y. Chen for technical assistance, Dr S. Fukushige for VP16 based yeast two-hybrid constructs, Dr P. Concannon for hMRE11 deletion mutants and Dr T. Rosenquist for pmH1P-neo vector. This work was supported in part by NIH grant CA101796 (C.H.).
References
- Bressan DA, Baxter BK, Petrini JH (1999) The Mre11–Rad50–Xrs2 protein complex facilitates homologous recombination-based doublestrand break repair in Saccharomyces cerevisiae. Mol Cell Biol 19: 7681–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmell MA, Zhang L, Conklin DS, Hannon GJ, Rosenquist TA (2003) Germline transmission of RNAi in mice. Nat Struct Biol 10: 91–92 [DOI] [PubMed] [Google Scholar]
- de Jager M, Dronkert ML, Modesti M, Beerens CE, Kanaar R, van Gent DC (2001) DNA-binding and strand-annealing activities of human Mre11: implications for its roles in DNA doublestrand break repair pathways. Nucleic Acids Res 29: 1317–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzantiev L, Constantin N, Genschel J, Iyer RR, Burgers PM, Modrich P (2004) A defined human system that supports bidirectional mismatch-provoked excision. Mol Cell 15: 31–41 [DOI] [PubMed] [Google Scholar]
- Giannini G et al. (2002) Human MRE11 is inactivated in mismatch repair-deficient cancers. EMBO Rep 3: 248–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Presnell SR, Yuan F, Zhang Y, Gu L, Li GM (2004) Differential requirement for proliferating cell nuclear antigen in 5′ and 3′ nick-directed excision in human mismatch repair. J Biol Chem 279: 16912–16917 [DOI] [PubMed] [Google Scholar]
- Harfe BD, Jinks-Robertson S (2000) DNA mismatch repair and genetic instability. Annu Rev Genet 34: 359–399 [DOI] [PubMed] [Google Scholar]
- Her C, Vo AT, Wu X (2002) Evidence for a direct association of hMRE11 with the human mismatch repair protein hMLH1. DNA Repair (Amst) 1: 719–729 [DOI] [PubMed] [Google Scholar]
- Holmes J Jr, Clark S, Modrich P (1990) Strandspecific mismatch correction in nuclear extracts of human and Drosophila melanogaster cell lines. Proc Natl Acad Sci USA 87: 5837–5841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo E, Horii A, Fukushige S (2001) The interacting domains of three MutL heterodimers in man: hMLH1 interacts with 36 homologous amino acid residues within hMLH3, hPMS1 and hPMS2. Nucleic Acids Res 29: 1695–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo E, Suzuki H, Horii A, Fukushige S (2003) A yeast two-hybrid assay provides a simple way to evaluate the vast majority of hMLH1 germ-line mutations. Cancer Res 63: 3302–3308 [PubMed] [Google Scholar]
- Lee BI, Wilson DM III (1999) The RAD2 domain of human exonuclease 1 exhibits 5′–3′ exonuclease and flap structurespecific endonuclease activities. J Biol Chem 274: 37763–37769 [DOI] [PubMed] [Google Scholar]
- Moreau S, Ferguson JR, Symington LS (1999) The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol Cell Biol 19: 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Gellert M (1998) The 3′–5′ exonuclease activity of Mre 11 facilitates repair of DNA doublestrand breaks. Mol Cell 1: 969–979 [DOI] [PubMed] [Google Scholar]
- Pedrazzi G et al. (2001) Direct association of Bloom's syndrome gene product with the human mismatch repair protein MLH1. Nucleic Acids Res 29: 4378–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutte C, Sadoff MM, Shim KS, Acharya S, Fishel R (2001) The interaction of DNA mismatch repair proteins with human exonuclease I. J Biol Chem 276: 33011–33018 [DOI] [PubMed] [Google Scholar]
- Thomas DC, Roberts JD, Kunkel TA (1991) Heteroduplex repair in extracts of human HeLa cells. J Biol Chem 266: 3744–3751 [PubMed] [Google Scholar]
- Tishkoff DX, Boerger AL, Bertrand P, Filosi N, Gaida GM, Kane MF, Kolodner RD (1997) Identification and characterization of Saccharomyces cerevisiae EXO1, a gene encoding an exonuclease that interacts with MSH2. Proc Natl Acad Sci USA 94: 7487–7492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff DX, Amin NS, Viars CS, Arden KC, Kolodner RD (1998) Identification of a human gene encoding a homologue of Saccharomyces cerevisiae EXO1, an exonuclease implicated in mismatch repair and recombination. Cancer Res 58: 5027–5031 [PubMed] [Google Scholar]
- Tran HT, Gordenin DA, Resnick MA (1999) The 3′ → 5′ exonucleases of DNA polymerases δ and ɛ and the 5′ → 3′ exonuclease Exo1 have major roles in postreplication mutation avoidance in Saccharomyces cerevisiae. Mol Cell Biol 19: 2000–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KM, Yuan SS, Lee EY, Sung P (1998) Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J Biol Chem 273: 21447–21450 [DOI] [PubMed] [Google Scholar]
- Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, Qin J (2000) BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev 14: 927–939 [PMC free article] [PubMed] [Google Scholar]
- Wang Z et al. (2004) Three classes of genes mutated in colorectal cancers with chromosomal instability. Cancer Res 64: 2998–3001 [DOI] [PubMed] [Google Scholar]
- Wei K et al. (2003) Inactivation of exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev 17: 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Davies SL, North PS, Goulaouic H, Riou JF, Turley H, Gatter KC, Hickson ID (2000) The Bloom's syndrome gene product interacts with topoisomerase III. J Biol Chem 275: 9636–9644 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information