Abstract
A provisional matrix consisting of fibrin and fibronectin (FN) is deposited at sites of tissue damage and repair. This matrix serves as a scaffold for fibroblast migration into the wound where these cells deposit new matrix to replace lost or damaged tissue and eventually contract the matrix to bring the margins of the wound together. Tenascin-C is expressed transiently during wound repair in tissue adjacent to areas of injury and contacts the provisional matrix in vivo. Using a synthetic model of the provisional matrix, we have found that tenascin-C regulates cell responses to a fibrin-FN matrix through modulation of focal adhesion kinase (FAK) and RhoA activation. Cells on fibrin-FN+tenascin-C redistribute their actin to the cell cortex, downregulate focal adhesion formation, and do not assemble a FN matrix. Cells surrounded by a fibrin-FN+tenascin-C matrix are unable to induce matrix contraction. The inhibitory effect of tenascin-C is circumvented by downstream activation of RhoA. FAK is also required for matrix contraction and the absence of FAK cannot be overcome by activation of RhoA. These observations show dual requirements for both FAK and RhoA activities during contraction of a fibrin-FN matrix. The effects of tenascin-C combined with its location around the wound bed suggest that this protein regulates fundamental processes of tissue repair by limiting the extent of matrix deposition and contraction to fibrin-FN-rich matrix in the primary wound area.
INTRODUCTION
Wound repair is a dynamic chain of events involving both soluble factors, blood proteins, and cells in the synthesis of a provisional matrix that is deposited at sites of tissue injury. The provisional matrix is a covalently cross-linked network consisting predominantly of fibrin and plasma fibronectin (pFN) that is formed during the terminal steps of blood coagulation (Clark et al., 1982). At areas of tissue damage, fibrin-FN deposits fill the wound to prevent further blood loss. This matrix also supports cell adhesion and migration into the site of injury and is subsequently remodeled to regain normal tissue structure and function. The synthesis of the fibrin-FN provisional matrix can be recapitulated in vitro using purified components (Wilson and Schwarzbauer, 1992; Corbett et al., 1996). The resulting matrix allows examination of the effects of individual extracellular matrix proteins and dissection of molecular signaling pathways in an environment with physiological relevance. Using a synthetic fibrin-FN provisional matrix, we have previously shown that FN is required for fibroblast attachment and spreading on this matrix and have characterized the molecular requirements for maximal fibrin-FN cross-linking (Corbett et al., 1996, 1997).
Fibroblasts carry out several functions once they have migrated into the provisional matrix. The first major task is the deposition of granulation tissue, which is composed of newly synthesized FN and other matrix proteins (Grinnell et al., 1981; Welch et al., 1990). Fibroblasts then differentiate into myofibroblasts, which express increased amounts of contractile proteins such as alpha smooth muscle actin (Masur et al., 1996). These cells exert mechanical force on the matrix. This contraction is essential for bringing the margins of the wound together to minimize the wound area and the extent of scarring (Clark, 1996).
The fibrin-FN matrix also contacts uninjured tissue adjacent to the wound bed, thus allowing resident extracellular matrix proteins to affect provisional matrix functions. Tenascin-C is an extracellular matrix protein that exhibits a restricted pattern of expression in vivo. It is limited to developing tissues and sites of active remodeling, such as tumors and wounds (Mackie et al., 1988; Erickson and Bourdon, 1989; Crossin, 1996). Tenascin-C is significantly but transiently upregulated in tissues adjacent to injury sites, suggesting that it may act to regulate cellular response to the provisional matrix. In tenascin-C knockout mice, wound healing is defective and FN matrix deposition at wound sites is decreased (Forsberg et al., 1996; Mackie and Tucker, 1999). A major role for tenascin-C in adhesion modulation is suggested by its adhesive and antiadhesive properties. This protein can antagonize the adhesive effects of matrix proteins such as FN and can cause changes in actin cytoskeleton organization and focal adhesion formation (Chiquet-Ehrismann et al., 1988; Spring et al., 1989; Murphy-Ullrich et al., 1991; Sage and Bornstein, 1991; Orend and Chiquet-Ehrismann, 2000; Huang et al., 2001).
We have shown that tenascin-C dramatically alters fibroblast spreading on a three-dimensional fibrin-FN matrix. In our fibrin-FN matrix model, both fibroblast morphology and actin organization are modulated by tenascin-C via regulation of RhoA GTPase activity (Wenk et al., 2000). The Rho family of GTPases controls actin cytoskeletal organization. In particular, RhoA mediates the formation of actin stress fibers and regulates cellular functions including adhesion, contractility, cell cycle progression, and gene transcription (Hall, 1998).
Based on the RhoA-mediated effects of tenascin-C on fibroblast interactions with a fibrin-FN matrix, we hypothesized that the presence of this protein may regulate wound contraction. A role for FN in fibrin-FN matrix contraction has been previously demonstrated (Corbett and Schwarzbauer, 1999). Here we show that tenascin-C inhibits matrix contraction and that this inhibition can be reversed by treatments that activate RhoA. We also show that tenascin-C downregulates FAK phosphorylation and that expression and phosphorylation of FAK is required for matrix contraction. Deposition of a FN-rich matrix is also blocked by tenascin-C. Our results suggest a model whereby tenascin-C serves a major role in determining the boundaries of the wound. The presence of this protein limits both the deposition of new extracellular matrix and the extent of wound contraction to within the boundaries of the provisional matrix.
MATERIALS AND METHODS
Protein Production
Rat pFN was purified by gelatin-Sepharose (Pharmacia Biotech, Piscataway, NJ) affinity chromatography from freshly drawn plasma (Wilson and Schwarzbauer, 1992). The production of recombinant proteins 70-kDa, 70Ten, and 70TenS has previously been described (Schwarzbauer, 1991; Luczak et al., 1998; Wenk et al., 2000). Native human tenascin-C from U251 glioma cells, consisting of >90% large splice variant, was purchased from Life Technologies (Rockville, MD).
Cell Culture
NIH 3T3 fibroblasts were maintained in DMEM and 10% calf serum (Hyclone Laboratories, Logan, UT). Rat-1 fibroblasts stably transfected with activated RhoA-V14 or control vector cDNA (Qiu et al., 1995; gifts from Dr. Marc Symons, Picower Institute, New York, NY) were maintained in DMEM containing 10% fetal calf serum and 400 μg/ml G418 (GIBCO, Rockville, MD). Because RhoA-V14 is driven by a tetracycline-repressible promoter, medium also contained 2.5 μg/ml puromycin and 2 μg/ml tetracycline. Tetracycline was withdrawn from the medium 48 h before the start of each experiment. Fibroblasts derived from wild-type or FAK-deficient mouse embryos (Ilic et al., 1995; gifts from Dr. Dusko Ilic, UCSF) were cultured in DMEM plus 10% fetal bovine serum (Hyclone Laboratories).
Immunofluorescence
Matrices were prepared as described previously (Corbett and Schwarzbauer, 1999; Wenk et al., 2000). A mass ratio of 20:1 fibrinogen/FN was used, which gives identical results to matrices prepared at a physiological ratio of 10:1 (Wenk et al., 2000). The ratio of FN/tenascin-C was 1:3, a 1:1 M ratio of dimeric FN to hexameric tenascin-C. 600 μg/ml fibrinogen (America Diagnostica Inc., Greenwich, CT), 30 μg/ml FN and 120 μg/ml tenascin-C or 70Ten, and 15 μg/ml coagulation factor XIIIa (Calbiochem, La Jolla, CA) were mixed with 1 mg/ml aprotinin in 150 mM NaCl, 50 mM CaCl2, 10 mM Tris-HCl, pH 7.4. Immediately after the addition of thrombin at 2 U/ml, the mixture was pipetted onto a glass coverslip (Fisher Scientific, Pittsburgh, PA). After overnight incubation at 4°C, the clots were carefully aspirated from the coverslip leaving a matrix attached to the surface (Corbett et al., 1996), and the substrate was blocked with 1% BSA in PBS. Fibrinogen and thrombin were reconstituted and contaminating FN removed from fibrinogen as previously described (Wilson and Schwarzbauer, 1992; Corbett et al., 1996). Covalent cross-linking of matrices was monitored by SDS-PAGE (Wilson and Schwarzbauer, 1992).
Cells were released from tissue culture dishes using 0.2 mg/ml EDTA in PBS, washed with PBS, resuspended in serum-free DMEM at 4 × 104/ml, and added to coverslips. Cells were allowed to spread on substrate-coated glass coverslips for 1 h, after which time cells were washed with PBS, fixed for 15 min with 3.7% formaldehyde in PBS, and permeabilized for 15 min with 0.5% NP-40 (Calbiochem) in PBS. Cells were incubated with primary or secondary antibody or phalloidin in 2% ovalbumin (Sigma Chemical Co., St. Louis, MO) in PBS at 37°C for 30 min. Antibodies were used at the following dilutions: anticortactin monoclonal (Upstate Biotechnology, Lake Placid, NY) at 1:100, PT66 antiphosphotyrosine monoclonal (Sigma Chemical Co.) at 1:300, antivinculin monoclonal (Sigma Chemical Co.) at 1:300 and fluorescein-conjugated goat anti-mouse secondary antibody (Molecular Probes Inc., Eugene, OR) at 1:500. For staining actin filaments, rhodamine-conjugated phalloidin (Molecular Probes Inc.) was used at 1:1000 as described (Corbett et al., 1996). Coverslips were mounted with SlowFade Light Antifade Kit (Molecular Probes Inc.). Cells were visualized with a Nikon Optiphot-2 microscope (Garden City, NY), and images were captured using a Photometrics Coolsnap camera (Tucson, AZ) and analyzed using Coolsnap and IP labs software.
FN matrix assembly was assessed by immunofluorescence staining (Sechler et al., 2001). Cells were plated on fibrin-FN matrix on glass coverslips in 24-well dish (Nunc Inc., Napierville, IL) and allowed to spread. Cells were then incubated with 25 μg/ml human pFN for 2 h, after which time cells were washed with PBS + 0.5 mM MgCl2 and then fixed with 3.7% formaldehyde in PBS for 15 min at room temperature. FN matrix was detected with culture supernatant from hybridoma cells producing anti-human FN antibody 7.1 at 1:200 (Brenner et al., 2000) and fluorescein-conjugated goat anti-mouse secondary antibody (Molecular Probes Inc.) at 1:500. Coverslips were mounted with SlowFade Light Antifade Kit (Molecular Probes Inc.).
Immunoprecipitation
Matrices and cells were prepared as for immunofluorescence studies. Cells were plated in serum-free DMEM at a density of 1.5 × 106 per 35-mm dish and allowed to spread on matrices for 15, 30, or 60 min. At the end of the incubation period cells were washed with PBS and then lysed in 200 μl RIPA lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.25% Na-deoxycholate, 1 mM PMSF, 1 mM NaVO4, 1 mM EDTA, 50 mg/ml leupeptin, 0.5% aprotinin) on ice for 15 min (Kanner et al., 1989). The cells were scraped with a rubber policeman, and lysate was collected and centrifuged for 10 min at 4°C. The pellet was discarded, and the protein concentration of the supernatant was determined using the BCA Protein Assay (Pierce, Rockford, IL). Four micrograms of anti-FAK mAb (Upstate Biotechnology) was added to 250 μg of total cell lysate and incubated at 4°C overnight while rotating. Fifty microliters of washed and packed protein-G agarose beads (Calbiochem) was added to the lysates. After an additional 2-h incubation the beads were washed three times with cold RIPA buffer. Protein was eluted from the beads by boiling in electrophoresis sample buffer (2% SDS, 80 mM Tris-HCl, pH 6.8, 10% glycerol, 0.01% bromophenol blue, 100 mM DTT) for 5 min.
Samples were run on a 6% polyacrylamide-SDS gel and transferred to nitrocellulose. Proteins were detected using anti-FAK mAb (Transduction Laboratories, Lexington, KY) at 1:1000, or PT66 antiphosphotyrosine mAb (Sigma Chemical Co.) at 1:3333. Primary antibodies were detected using horseradish peroxidase–conjugated goat anti-mouse secondary antibody (Pierce) diluted 1:50,000 and Supersignal chemiluminescent detection reagent (Pierce).
Contraction Assays
Matrices were prepared as for immunofluorescence except 1 × 106 cells/ml were added to the matrix components. Immediately after the addition of thrombin, the mixture was pipetted into 48-well plates that had been coated with 1% BSA overnight at 4°C. The cell-matrix mixture was allowed to polymerize for 30 min at 37°C. The clots were then carefully detached from the dishes, and matrix contraction was visualized. The area of the matrix was measured over time using a ruler, subtracted from the starting area, and expressed as a percentage of the starting area.
Before the treatment with activators or inhibitors, cells were serum starved for 24 h. After release with EDTA cells were incubated with 5 μM LPA (Sigma Chemical Co.) or 10 μM Y27632 (BIOMOL, Plymouth Meeting, PA) for 30 min at 37°C in suspension before addition to matrix proteins. Cells were serum starved for 24 h with 25 μg/ml C3 transferase (Cytoskeleton, Denver, CO) or 10 μM simvastatin (Calbiochem) added to the medium before being released from tissue culture dishes as described above and added to matrix proteins. Simvastatin was activated before use as described previously (Laufs et al., 1998). In some experiments, serum-starved cells were treated with 5 μM phenylarsine oxide (Sigma Chemical Co.) or 0.1 mM pervanadate as described (Miao et al., 2000). The pretreatments had no effect on the cross-linking of the matrices as determined by SDS-PAGE.
Membrane Fractionation
Cells plated in serum-free DMEM at a density of 1.5 × 106 per 35-mm dish were allowed to spread on matrices for 1 h. At the end of the incubation period cells were washed with PBS and then lysed in 200 μl hypotonic lysis buffer (20 mM Tris-HCl, pH 8.0, 140 mM NaCl, 1 mM EDTA, 10 mM PMSF, 20 mM β-mercaptoethanol) on ice for 15 min (Gohla et al., 1998). The cells were scraped with a rubber policeman, and lysate was collected and centrifuged for 5 min at 4°C. The supernatant was centrifuged at 50,000 × g for 30 min at 4°C. The pellet was resuspended in lysis buffer without NaCl containing 20 μg/ml leupeptin, and the protein concentration of both the pellet and supernatant was determined using the BCA Protein Assay (Pierce). Equal amounts of total protein were run under reducing conditions on a 13% polyacrylamide-SDS gel and transferred to nitrocellulose. Proteins were detected using anti-Rho mAb (Transduction Laboratories) at 1:250 dilution. Primary antibodies were detected using horseradish peroxidase–conjugated goat anti-mouse secondary antibody (Pierce) diluted 1:50,000 and Supersignal chemiluminescent detection reagent (Pierce).
Analysis of DOC-insoluble Material
Cells were plated at 1.5 × 105/ml in fibrin-FN–coated 24-well dishes and incubated with 25 μg/ml human pFN for 2 h. Cells were then washed with cold PBS and lysed with 200 μl of deoxycholate (DOC) lysis buffer (2% DOC, 0.02 M Tris-HCl, pH 8.8, 2 mM PMSF, 2 mM EDTA, 2 mM iodoacetic acid, and 2 mM N-ethylmaleimide; Sechler et al., 2001). DOC-insoluble material was isolated by centrifugation at 14,000 rpm for 15 min at 4°C, and then solubilized in 25 μl of 1% SDS, 25 mM Tris-HCl, pH 8.0, plus protease inhibitors. Total protein concentration was determined using BCA Protein Assay (Pierce). Equal amounts of DOC-insoluble material were electrophoresed under reducing conditions on a 5% polyacrylamide SDS gel. Separated proteins were transferred to nitrocellulose (Sartorius Corp., Long Island, NY), and FN was detected using culture supernatant from hybridoma cells producing anti-human FN antibody 7.1 at 1:2000. Primary antibodies were detected using horseradish peroxidase–conjugated goat anti-mouse secondary antibody (Pierce) diluted 1:50,000 and Supersignal chemiluminescent detection reagent (Pierce).
RESULTS
Tenascin-C Prevents Focal Contact Formation and Induces Reorganization of Cytoskeleton-associated Proteins
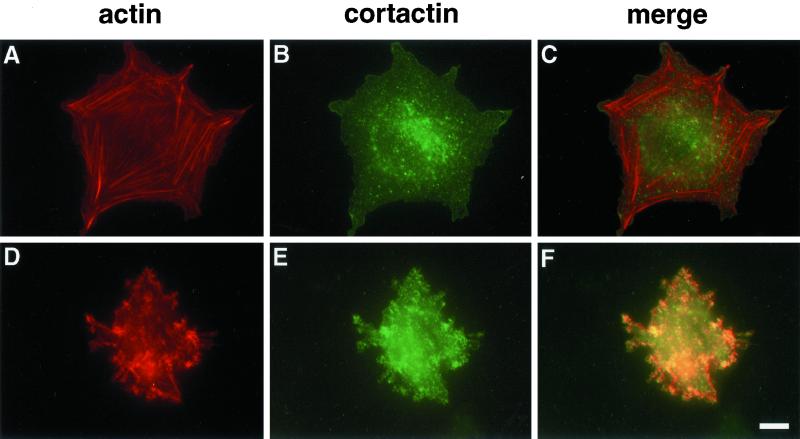
Tenascin-C induces changes in actin organization by suppressing RhoA activation in cells adherent to fibrin-FN matrices (Wenk et al., 2000). To examine the effect of tenascin-C on the distribution of actin filaments, we followed the association of actin with cortactin. Cortactin is a cytoskeletal protein that binds specifically to cortical actin (Wu and Parsons, 1993). It is involved in the signaling pathways of adhesion molecules mediating cytoskeletal reorganization and has been shown to influence cell migration and invasion (Patel et al., 1998). NIH 3T3 fibroblasts plated on fibrin-FN matrices displayed extensive actin stress fibers. Very little actin was associated with cortactin (Figure 1, A–C). In contrast, the addition of tenascin-C to the matrix resulted in a redistribution of actin; no stress fibers were seen and much of the actin became cortical, as indicated by its colocalization with cortactin (Figure 1, D–F).
Figure 1.
Tenascin-C induces a distinct cytoskeletal organization. NIH 3T3 fibroblasts were allowed to interact with fibrin-FN (A–C) or fibrin-FN+tenascin-C (D–F) matrices for 1 h before fluorescent staining for actin with rhodamine-labeled phalloidin (A and D) and for cortactin with an anticortactin mAb (B and E). Sites of colocalization are shown in yellow (C and F). Bar, 10 μm.
We have previously described the production of a highly purified recombinant form of tenascin-C, 70Ten. 70Ten contains the amino-terminal 70-kDa region of FN, including the fibrin cross-linking site, connected to all the type III repeats and the terminal knob of tenascin-C, containing adhesive and antiadhesive domains. 70Ten behaves identically to full-length tenascin-C when included in a fibrin-FN matrix (Wenk et al., 2000). The addition of 70Ten to a fibrin-FN matrix resulted in the same reorganization of the cytoskeleton to cortical actin as tenascin-C. This effect, therefore, is not due to other proteins present in preparations of native tenascin-C.
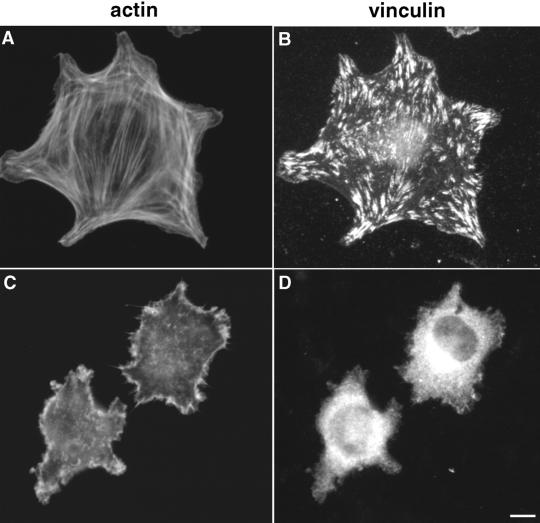
Focal adhesion assembly, cytoskeleton-associated protein distribution, and intracellular signaling were also altered upon cell interaction with a fibrin-FN + 70Ten matrix. Immunofluorescence staining of cells on fibrin-FN matrices revealed typical focal adhesions, which are rich in vinculin, a protein involved in connections to the actin cytoskeleton (Figure 2, A and B). These focal adhesions were not formed by fibroblasts on fibrin-FN + 70Ten matrices (Figure 2, C and D). Staining patterns similar to vinculin were observed with antiphosphotyrosine antibodies that detect signaling proteins such as FAK.
Figure 2.
Tenascin-C inhibits the formation of focal adhesions. NIH 3T3 fibroblasts were allowed to interact with fibrin-FN (A and B) or fibrin-FN + 70Ten (C and D) matrices for 1 h before staining for actin (A and C) and for vinculin with an antivinculin mAb (B and D). Bar, 10 μm.
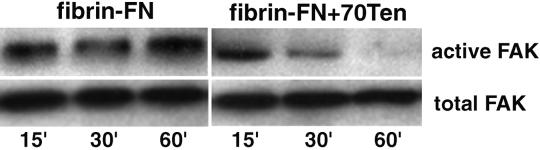
Biochemical data show that although total FAK remains the same in all cell lysates, FAK phosphorylation was sustained on fibrin-FN substrates but was transient in fibrin-FN matrices including 70Ten (Figure 3). Together these results show that focal adhesion assembly is impaired by the addition of tenascin-C, which has a dramatic effect on cytoskeletal organization and subsequent signaling events.
Figure 3.
Transient FAK phosphorylation in the presence of tenascin-C. NIH 3T3 fibroblasts were allowed to adhere to fibrin-FN+/−70Ten matrices for 15, 30, and 60 min before lysis and immunoprecipitation with anti-FAK antibodies. Proteins were separated by SDS-PAGE, and immunoblots were probed with an anti-FAK mAb to detect total cellular FAK and an antiphosphotyrosine mAb to detect active FAK.
Tenascin-C Inhibits Matrix Contraction
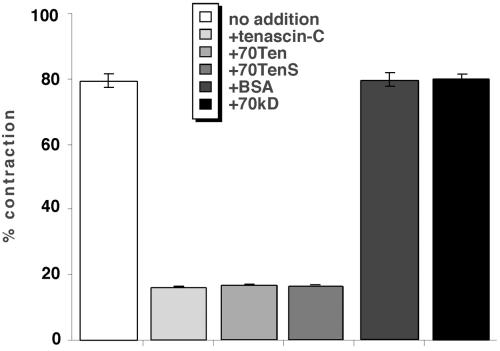
Fibroblast interactions with fibrin-FN–based matrices are important in the generation of force required to contract the provisional matrix found at sites of tissue injury in vivo (Clark, 1996). To determine the effects of matrix composition on cell contractility and to examine cell phenotype in a more quantitative manner, we used a well-characterized matrix contraction assay (Corbett et al., 1997; Corbett and Schwarz-bauer, 1999). NIH 3T3 fibroblasts were incorporated into three-dimensional matrices consisting of fibrin-FN or fibrin-FN+tenascin-C. This cell-matrix mixture was allowed to polymerize in 48-well dishes and then released from the sides of the dish. The ability of cells to contract the matrix was assessed by measuring the area of the matrix over time. This value was subtracted from the starting area, and contraction was expressed as a percentage of the starting area. Fibroblasts rapidly contracted matrices consisting of fibrin-FN. In contrast, fibrin-FN matrix contraction was completely inhibited by the inclusion of tenascin-C or 70Ten (Figure 4). 70TenS is a small splice variant of tenascin-C, which lacks five alternatively spliced type III repeats and has been shown to have the same effects on cell spreading as 70Ten (Wenk et al., 2000). The addition of 70TenS to a fibrin-FN matrix also inhibited the ability of fibroblasts to contract the matrix to the same extent as full-length tenascin-C and 70Ten (Figure 4). The addition of equal molar ratios of BSA or the recombinant protein 70-kDa, which consists of the 70-kDa amino terminal of FN, instead of tenascin-C had no effect on the ability of cells to contract a fibrin-FN matrix (Figure 4). These results show that inhibition of contraction is due to the specific inclusion of tenascin-C in the matrix and demonstrate an important modulatory role for tenascin-C in cell contractility.
Figure 4.
Tenascin-C inhibits cell contractility. NIH 3T3 fibroblasts were mixed together with fibrin-FN and fibrin-FN+tenascin-C, 70Ten, 70TenS, BSA, or 70 kDa, and the matrices were allowed to polymerize in a 48-well dish. The matrix was detached from the dish, and the area of the matrix was measured after 4 h, subtracted from the starting area, and expressed as a percentage of the starting area. The data are expressed as the mean ± SEM of triplicate experiments.
Rho Activation Relieves Inhibitory Effects of Tenascin-C
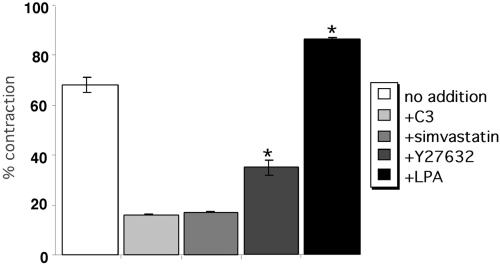
Tenascin-C modulates cell morphology and actin organization on fibrin-FN matrices by regulating RhoA GTPase activity (Wenk et al., 2000). RhoA activation is also essential for contraction of a fibrin-FN matrix. Inhibition of RhoA activity by treatment of cells with C3 transferase or inhibition of RhoA translocation to the membrane by treating cells with simvastatin inhibited fibroblast contraction of a fibrin-FN matrix (Figure 5). Stimulation of the activity of RhoA by preincubation of cells with LPA (Figure 5) or by expression of constitutively active RhoA-stimulated contraction of a fibrin-FN matrix over untreated or vector control cells. The LPA effect was dose dependent. Treatment of cells with Y27632, an inhibitor of ROCK, a downstream target of RhoA involved in promoting stress fiber and focal adhesion formation (Amano et al., 1997), also inhibited matrix contraction (Figure 5), suggesting that RhoA mediation of this process is via a ROCK-dependent pathway. These results show an important role for RhoA in cell contraction of fibrin-FN matrices.
Figure 5.
The role of RhoA in the contraction of fibrin-FN matrices. NIH 3T3 fibroblasts treated with LPA, simvastatin, C3 transferase (C3), or Y27632 were mixed together with fibrin-FN and allowed to polymerize in a 48-well dish. Matrix contraction was measured as described in the legend to Figure 4. The data are expressed as the mean ± SEM of duplicate experiments. Contraction of matrices by cells treated with LPA and Y27632 was significantly different from that of untreated cells (★p < 0.05, Student's paired t test).
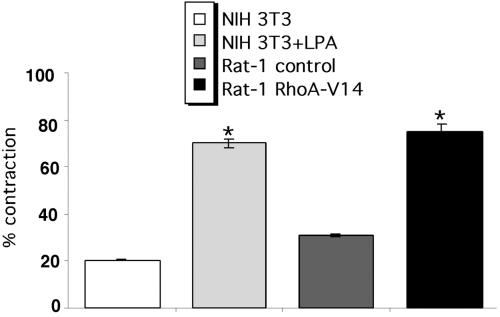
To determine whether RhoA acts downstream of tenascin-C during contraction, the effect of stimulating RhoA activity on the contraction of fibrin-FN + 70Ten matrices was examined. Treatment of NIH 3T3 cells with LPA or expression of constitutively active RhoA in Rat-1 fibroblasts restored normal levels of contraction of a fibrin-FN + 70Ten matrix (Figure 6). Therefore tenascin-C inhibition of cell contractility can be circumvented by downstream activation of RhoA.
Figure 6.
RhoA activation reverses inhibition by 70Ten. NIH 3T3 fibroblasts treated with LPA and Rat-1 fibroblasts expressing constitutively active RhoA or control DNA were incorporated into a fibrin-FN + 70Ten matrix. Contraction data are expressed as the mean ± SEM of duplicate experiments. Compared with untreated cells, the addition of LPA or expression of active RhoA significantly increased contraction of matrices (★p < 0.05; Student's paired t test).
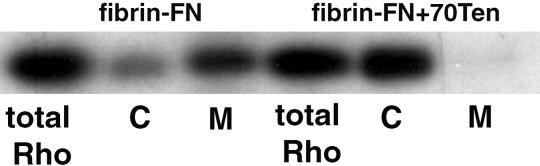
The fact that prevention of RhoA movement to the membrane by simvastatin inhibits matrix contraction suggests that RhoA must be both in its active GTP-bound form and localized properly to mediate contraction. To determine whether tenascin-C regulates RhoA function by affecting its subcellular distribution, we examined the effect of tenascin-C on the localization of RhoA. In cells plated on a fibrin-FN matrix, RhoA was predominantly localized in the membrane fraction of cell lysates (Figure 7). On the addition of 70Ten to the matrix, the majority of RhoA was localized in the cytoplasmic fraction. These data indicate that tenascin-C inhibits the recruitment of RhoA to the cell membrane.
Figure 7.
Tenascin-C inhibits RhoA localization to the cell membrane. NIH 3T3 fibroblasts were allowed to adhere to fibrin-FN+/−70Ten matrices for 1 h before lysis and ultracentrifugation to separate cytoplasmic (C) and membrane (M) fractions. Proteins were separated by SDS-PAGE, and immunoblots were probed with an anti-Rho mAb.
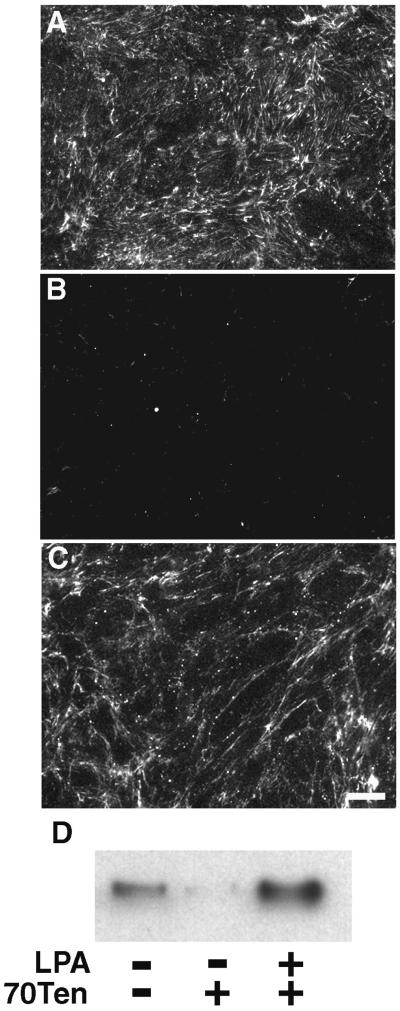
A major role for fibroblasts within the provisional matrix is to deposit a FN-rich matrix as a framework for tissue repair (Grinnell et al., 1981). In addition to regulating matrix contraction, tenascin-C in the surrounding tissue may act to prevent inappropriate FN matrix deposition. To test this idea, NIH 3T3 fibroblasts on a fibrin-FN matrix were examined for the ability to assemble FN into a matrix. In the absence of 70Ten, cells on a fibrin-FN matrix assembled extensive FN fibrils (Figure 8A). In contrast, cells treated with 70Ten showed a marked decrease in fibril formation (Figure 8B). However, LPA stimulation was able to reverse the effects of 70Ten (Figure 8C). The assembly of FN was also monitored by conversion into DOC-insoluble material, and these results confirmed the immunofluorescence data (Figure 8D). FN matrix assembly has previously been shown to be dependent on Rho GTPase signaling (Zhang et al., 1997). Our data indicate that tenascin-C modulates cell-mediated formation of FN fibrils by suppressing RhoA activity.
Figure 8.
Tenascin-C inhibits matrix assembly. Untreated NIH 3T3 fibroblasts (A) or fibroblasts treated with 70Ten (B) or both 70Ten and LPA (C) were allowed to adhere to fibrin-FN matrices in the absence of serum and in the presence of exogenous human pFN for 2 h, before immunofluorescence staining with an anti-human FN antibody 7.1 (Bar, 50 μm). In parallel experiments, matrix assembly was assessed by isolation of DOC-insoluble material. Proteins were separated by SDS-PAGE, and immunoblots were probed with antihuman FN antibody 7.1 (D).
FAK Is Required for Matrix Contraction
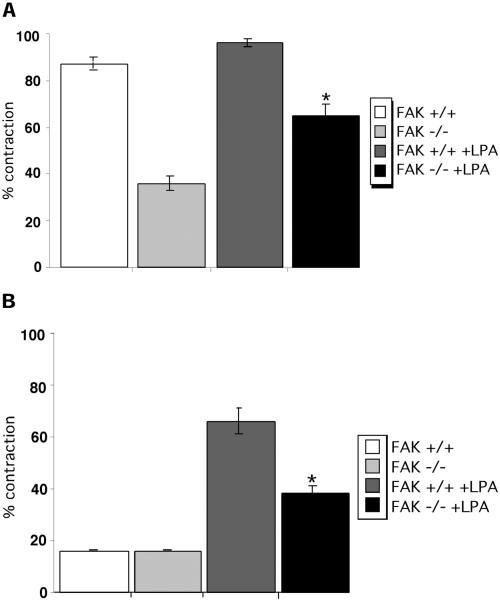
In addition to effects on Rho signaling, tenascin-C inhibits sustained activation of FAK in fibroblasts adherent to fibrin-FN matrices. To determine whether there is a connection between the suppression of FAK activity by tenascin-C and cell contractility, we examined matrix contraction by cells that do not express FAK. Our results show that expression of FAK is required for maximal contraction of a fibrin-FN matrix. FAK-null fibroblasts showed a 52% reduction in matrix contraction compared with wild-type fibroblasts (Figure 9A). Tenascin-C caused a further decrease in contraction by FAK-null cells (Figure 9B). Stimulation of RhoA activity can only partially compensate for the absence of FAK. Treatment of FAK-null cells with LPA stimulated contraction of a fibrin-FN and of a fibrin-FN+tenascin-C matrix over untreated cells (Figure 9, A and B). However, levels of contraction did not reach those of LPA-treated wild-type cells in either matrix, indicating that activation of RhoA cannot replace the loss of FAK expression.
Figure 9.
FAK is required for matrix contraction. Untreated fibroblasts derived from wild-type (+/+) or FAK-deficient (−/−) mouse embryos or fibroblasts treated with LPA (+LPA) were mixed together with fibrin-FN (A) or fibrin-FN + 70Ten (B) and allowed to polymerize in a 48-well dish. Matrix contraction data are expressed as the mean ± SEM of duplicate experiments. The addition of LPA significantly stimulated contraction of FAK-deficient cells, compared with untreated cells (★p < 0.05; Student's paired t test).
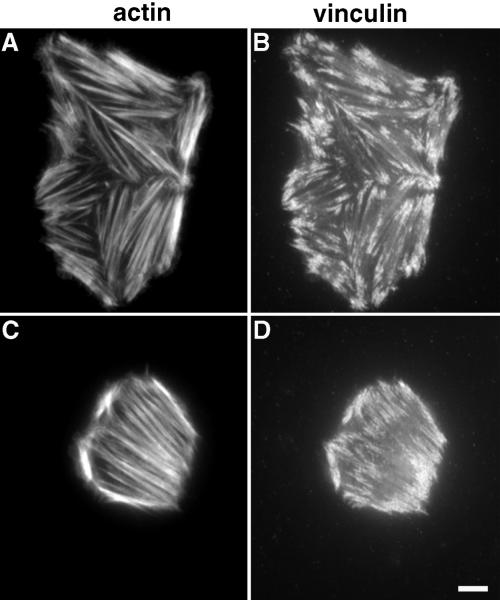
Reduced matrix contraction by FAK-null cells was not due to an inability to organize the actin cytoskeleton. FAK-null fibroblasts formed robust stress fibers and focal adhesions on fibrin-FN matrices (Figure 10, A and B) and, unlike NIH 3T3 cells, stress fibers also formed in the presence of tenascin-C (Figure 10, C and D). In contrast, wild-type fibroblasts derived from mouse embryos expressing FAK behaved identically to NIH 3T3 fibroblasts. They were well spread with actin stress fibers and focal adhesions on a fibrin-FN matrix and were rounded with no stress fibers or focal adhesions on a fibrin-FN matrix containing tenascin-C.
Figure 10.
FAK-deficient fibroblasts form stress fibers and focal adhesions. Fibroblasts derived from FAK-deficient mouse embryos were allowed to interact with fibrin-FN (A and B) or fibrin-FN + 70Ten (C and D) matrices for 1 h before staining for actin (A and C) and for vinculin (B and D). Bar, 10 μm.
Inclusion of tenascin-C did impact spread cell areas, which were significantly less than without tenascin-C. Areas of cells plated on fibrin-FN and fibrin-FN + 70Ten matrices both in the presence and absence of LPA were measured. Both wild-type and FAK-null cells treated with LPA were smaller than untreated cells and yet when included within matrices showed increased levels of contraction of both types of matrix (Table 1). Taken together, these results indicate that activation of both FAK and RhoA is required for maximal matrix contraction.
Table 1.
Comparison of cell size and matrix contraction
| Fibrin-FN
|
Fibrin-FN+70Ten
|
|||
|---|---|---|---|---|
| Cell area (μm2) | Matrix contraction (%) | Cell area (μm2) | Matrix contraction (%) | |
| FAK+/+ | 812 ± 31 | 88 ± 2 | 314 ± 15 | 16 ± 0 |
| FAK+/++LPA | 346 ± 17 | 96 ± 3 | 174 ± 6 | 66 ± 0 |
| FAK −/− | 226 ± 12 | 36 ± 2 | 158 ± 10 | 16 ± 5 |
| FAK −/− +LPA | 126 ± 10 | 65 ± 5 | 162 ± 11 | 38 ± 3 |
Untreated fibroblasts derived from wild type (+/+) or FAK-deficient (−/−) mouse embryos or fibroblasts treated with LPA (+LPA) were mixed together with fibrin-FN or fibrin-FN+70Ten and allowed to polymerize in a 48-well dish. Matrix contraction data are expressed as the mean ± SEM of duplicate experiments. In parallel experiments, cells were plated on fibrin-FN+/− 70Ten matrices for 1 h, after which time areas for 50–75 cells from random fields were measured using IPlab Software and expressed in μm2 ± SD.
Sustained FAK Phosphorylation Rescues Contraction of Fibrin-FN + 70Ten Matrices
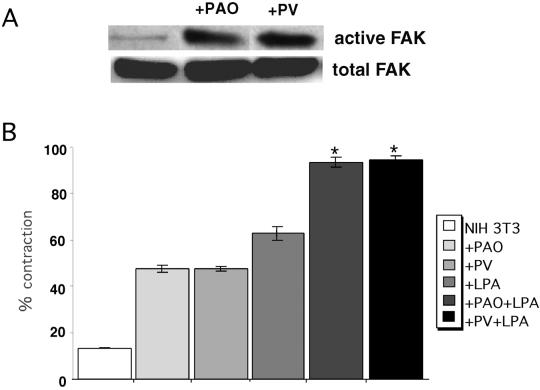
If FAK is required for maximal matrix contraction, then stimulation of FAK activity may modulate contraction of fibrin-FN + 70Ten matrices. NIH 3T3 cells were treated with phosphatase inhibitors pervanadate or phenylarsine oxide. Both inhibitors have been shown to inhibit FAK dephosphorylation and phenylarsine oxide predominantly prevents the dephosphorylation of FAK and paxillin (Miao et al., 2000; Retta et al., 1996). Immunodetection of phosphorylated and total FAK demonstrated that both phosphatase inhibitors prevent FAK dephosphorylation in response to tenascin-C in a fibrin-FN matrix without affecting total FAK levels (Figure 11A). Neither inhibitor affected FAK phosphorylation in cells adherent to a fibrin-FN matrix, suggesting that FAK is already maximally activated by this substrate.
Figure 11.
Sustained FAK phosphorylation rescues contraction of fibrin-FN + 70Ten matrices. (A) NIH 3T3 fibroblasts were treated with phenylarsine oxide or pervanadate and then allowed to adhere to fibrin-FN + 70Ten matrices for 60 min before lysis and immunoprecipitation with anti-FAK antibodies. Proteins were separated by SDS-PAGE, and immunoblots were probed with an anti-FAK mAb to detect total cellular FAK and an antiphosphotyrosine mAb to detect active FAK. (B) NIH 3T3 fibroblasts treated with phenylarsine oxide (PAO), pervanadate (PV), LPA, PAO+LPA or PV+LPA were mixed together with fibrin-FN + 70Ten and allowed to polymerize in a 48-well dish. Matrix contraction was measured as described in the legend to Figure 4. The data are expressed as the mean ± SEM of duplicate experiments. The addition of both LPA and PAO or PV significantly stimulated contraction of matrices, compared with cells treated with LPA alone (★p < 0.05; Student's paired t test).
Cells treated with either phosphatase inhibitor contracted a fibrin-FN + 70Ten matrix to an extent equivalent to LPA-treated cells (Figure 11B). Therefore, tenascin-C inhibition of matrix contraction can be partially overcome by downstream activation of FAK. To achieve complete contraction of fibrin-FN + 70Ten matrix required treatment with both a phosphatase inhibitor and LPA (Figure 11B). These results further support the idea that both FAK and RhoA activities are required for maximal matrix contraction.
DISCUSSION
Fibroblasts participate in multiple stages of tissue repair including wound contraction. In this article, we have demonstrated that tenascin-C downregulates matrix contraction. This reduction correlates with transient FAK activation, the absence of focal adhesions, and cortical actin filament distribution. Partial reversion of tenascin's suppressive effects can be obtained by activation of either FAK or RhoA. Maximal matrix contraction, however, requires both FAK and RhoA activities. Thus, the combined effects of these two signaling molecules appear to be critical components of the intracellular machinery that regulates matrix contraction.
The disruption of focal adhesions and modulation of downstream signaling pathways by tenascin-C has many implications for the cell. Of particular interest at sites of tissue repair is the effect on cell contractility. The ability of cells to contract matrices is essential in bringing the margins of a wound together (Clark, 1996). Changes in matrix content have previously been shown to control cell contractility. For example, polymerized FN included in collagen gels stimulates cell spreading and contractility via a RhoA-dependent mechanism, but nonpolymerized FN does not (Hocking et al., 2000). Strong interactions between integrins and FN are also required for maximal fibrin-FN matrix contraction by fibroblasts (Corbett et al., 1997). Focal adhesions serve to connect the actin cytoskeleton to the matrix, and this physical link enables cells to exert force on the surrounding matrix (Burridge and Chrzanowska-Wodnicka, 1996). It follows that cells unable to assemble focal adhesions and stress fibers would be unable to contract surrounding matrices as we have observed with cells in fibrin-FN+tenascin-C matrix.
Activation of the GTPase RhoA induces stress fiber formation via a ROCK-dependent pathway (Amano et al., 1997). We observed that RhoA signaling through ROCK is required for contraction of fibrin-FN matrices. The inhibitory effects of tenascin-C can be reversed by stimulation of RhoA activity with LPA or overexpression of activated RhoA. Tenascin-C also disrupts intracellular localization of RhoA, which is not recruited to the cell membrane under these matrix conditions. The capacity of Rho to cycle on and off membranes is thought to be integral to its biological activity. Vascular smooth muscle proliferation is inhibited by the prevention of the membrane localization of Rho (Laufs et al., 1999), and LPA-induced cytoskeletal contraction of neuronal cells requires translocation of Rho from the cytosol to the plasma membrane (Kranenburg et al., 1997). It appears that RhoA function and targeting to the plasma membrane are also necessary for matrix contraction. Tenascin-C has no apparent effect on the activation of other members of the Rho family GTPases in this assay. Rac and Cdc42 showed similar levels of activity in cells on fibrin-FN matrices both in the presence and absence of tenascin-C (unpublished observations).
Our data show that contraction of fibrin-FN matrices involves the activation of both FAK and RhoA. FAK plays an important role in the remodeling of focal adhesions and control of cytoskeletal tension (Crowley and Horwitz, 1995; Parsons et al., 2000). FAK expression is also required for smooth muscle cell contractility (Tang and Gunst, 2001). FAK-deficient fibroblasts showed decreased motility and enhanced focal adhesion formation on planar FN substrates, implicating FAK in the regulation of focal adhesion turnover (Ilic et al., 1995). We have shown that, in contrast with wild-type cells, FAK-null cells form robust stress fibers and focal adhesions on fibrin-FN+tenascin-C–based substrates. Despite this level of cytoskeletal organization, FAK-null cells showed a significant reduction in contraction, suggesting that the presence of stress fibers is insufficient to drive matrix contraction. Treatment of wild-type fibroblasts with phosphatase inhibitors prevents the dephosphorylation of FAK that occurs in the presence of tenascin-C and allows these cells to partially contract matrices. Maximal levels of matrix contraction were seen in cells treated with both LPA and phosphatase inhibitors. Because this activation is additive, it appears that FAK and RhoA are acting through different pathways. It seems likely that there may be cross-talk between these two pathways, because the activities of FAK and RhoA appear to be closely linked. FAK regulates focal adhesion turnover via a RhoA-dependent pathway (Ren et al., 2000), and manipulation of the activity of RhoA can influence the activation of FAK (Sinnett-Smith et al., 2001). Our results indicate that both FAK activity in focal adhesions and RhoA GTPase-mediated reorganization of the actin cytoskeleton are required for cells to contract the surrounding matrix. Tenascin-C suppresses the activity of both these signaling molecules, resulting in a complete inhibition of matrix contraction.
Fibroblasts on a fibrin-FN matrix assemble actin stress fibers and focal adhesions. Inclusion of tenascin-C promotes a distinct morphology with cortical actin filaments and actin-filled filopodia. In these cells, actin filaments show significant colocalization with the cytoskeletal protein cortactin. Cortactin as a c-Src substrate is found primarily at sites of dynamic actin assembly and acts to regulate the formation and stabilization of a filamentous actin network (Weaver et al., 2001). Cortactin plays a role in cell migration by mediating localized cross-linking of actin at the leading edge of migrating cells (Bowden et al., 1999; Bourguignon et al., 2001), and overexpression of cortactin in NIH 3T3 fibroblasts increases cell motility in vitro (Patel et al., 1998). Tenascin-C has also been shown to stimulate cell migration (Chung et al., 1996). Increased migration may occur through tenascin-C–mediated reduction in FAK phosphorylation and focal adhesion formation as shown in this report and by suppression of RhoA activation as shown previously (Wenk et al., 2000). The loss of a stationary cell phenotype and intracellular effects that correlate with cell motility in cells on fibrin-FN+tenascin-C matrix may reflect the ability of tenascin-C to promote cell migration at sites of tissue repair.
Tenascin-C expression is tightly regulated in adult tissues. It appears ∼2 d after wounding at sites of tissue injury and shows a large increase in expression before wound contraction (Betz et al., 1993; Forsberg et al., 1996). Levels of tenascin-C are highest adjacent to the wound bed (Mackie et al., 1988). This is a region of highly controlled cell movement, because macrophages, fibroblasts, and endothelial cells migrate into the wound bed and epithelial cells migrate across the defect in order to reepithelialize the wound (Clark, 1996). This localization of tenascin-C implicates it in the coordination of cell motility and matrix contraction during the healing process. Its presence and clearance may represent an important temporal or spatial switch to prevent and then induce wound contraction. This may help to explain how the differences in the timing of expression of tenascin-C in fetal and adult wounds gives rise to differences in the extent of scar formation. Early expression of tenascin-C in the fetus correlates with optimal contraction and minimal scarring, but delayed expression in adults correlates with delayed contraction and increased scarring (Whitby et al., 1991).
As part of the process of laying down new tissue, fibroblasts assemble an FN matrix that acts as a framework for wound repair. During wound healing, the FN matrix influences directed cell migration by establishing chemotactic gradients in and around the wound space (Clark et al., 1988). Similarly angiogenesis relies on appropriate matrix-derived cues, and the extent of postwound scarring is determined by the amount of matrix deposited (Welch et al., 1990; Madri and Marx, 1992). It has been shown that Rho-mediated contractility induces matrix assembly by exposing a cryptic site in FN (Zhong et al., 1998). We have found that tenascin-C causes a dramatic reduction in fibroblast assembly of a FN matrix by regulating the activity of RhoA.
Taken together, our results suggest a new function for tenascin-C, to limit the boundaries of the wound bed. Within these boundaries toward the center of the wound, where tenascin-C is absent, the cells primarily contact a fibrin-FN matrix. The fibrin-FN matrix promotes cell adhesion and formation of stress fibers and focal adhesions. These firm attachments allow the cells to exert mechanical force, thus contracting the matrix. Upregulation of tenascin-C at the edges of the injured tissue induces a motile cell phenotype, with loose connections to the matrix. The function of tenascin-C at this site would be to inhibit matrix contraction and prevent extensive deposition of newly synthesized FN matrix. Clearance of tenascin-C as healing progresses would then allow the development of stable cell–matrix interactions and the deposition of new FN matrix. Tenascin-C programs intracellular pathways through modulation of RhoA and FAK activation, leading to downstream effects on the actin cytoskeleton. In this way, tenascin-C plays an important role in regulating key events during tissue repair and regeneration.
ACKNOWLEDGMENTS
We thank Dr. Marc Symons for providing Rat-1 cells, Dr. Dusko Ilic for wild-type and FAK-null embryonic fibroblasts, and Nedra Guckert and Rajeev Puri for technical assistance. This work was supported by a grant from the National Institutes of Health.
Abbreviations used:
- FAK
focal adhesion kinase
- FN
fibronectin
- pFN
plasma fibronectin
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–05–0292. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–05–0292.
REFERENCES
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Betz P, Nerlich A, Tubel J, Penning R, Eisenmenger W. Localization of tenascin in human skin wounds—an immunohistochemical study. Int J Legal Med. 1993;105:325–328. doi: 10.1007/BF01222116. [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Zhu H, Shao L, Chen YW. CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid-dependent ovarian tumor cell migration. J Biol Chem. 2001;276:7327–7336. doi: 10.1074/jbc.M006498200. [DOI] [PubMed] [Google Scholar]
- Bowden ET, Barth M, Thomas D, Glazer RI, Mueller SC. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 1999;18:4440–4449. doi: 10.1038/sj.onc.1202827. [DOI] [PubMed] [Google Scholar]
- Brenner KA, Corbett SA, Schwarzbauer JE. Regulation of fibronectin matrix assembly by activated Ras in transformed cells. Oncogene. 2000;19:3156–3163. doi: 10.1038/sj.onc.1203626. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Kalla P, Pearson CA, Beck K, Chiquet M. Tenascin interferes with fibronectin action. Cell. 1988;6:383–390. doi: 10.1016/0092-8674(88)90158-4. [DOI] [PubMed] [Google Scholar]
- Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell. 1996;7:883–892. doi: 10.1091/mbc.7.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA, Lanigan JM, DellaPelle P, Manseau E, Dvorak HF, Colvin RB. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 1982;79:264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- Clark RA, Wikner NE, Doherty DE, Norris DA. Cryptic chemotactic activity of fibronectin for human monocytes resides in the 120-kDa fibroblastic cell-binding fragment. J Biol Chem. 1988;263:12115–12123. [PubMed] [Google Scholar]
- Clark RAF. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1996. [Google Scholar]
- Corbett SA, Lee L, Wilson CL, Schwarzbauer JE. Covalent cross-linking of fibronectin to fibrin is required for maximal cell adhesion to a fibronectin-fibrin matrix. J Biol Chem. 1997;272:24999–25005. doi: 10.1074/jbc.272.40.24999. [DOI] [PubMed] [Google Scholar]
- Corbett SA, Schwarzbauer JE. Requirements for alpha(5)beta(1) integrin-mediated retraction of fibronectin-fibrin matrices. J Biol Chem. 1999;274:20943–20948. doi: 10.1074/jbc.274.30.20943. [DOI] [PubMed] [Google Scholar]
- Corbett SA, Wilson CL, Schwarzbauer JE. Changes in cell spreading and cytoskeletal organization are induced by adhesion to a fibronectin-fibrin matrix. Blood. 1996;88:158–166. [PubMed] [Google Scholar]
- Crossin KL. Tenascin: a multifunctional extracellular matrix protein with a restricted distribution in development and disease. J Cell Biochem. 1996;61:592–598. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C592::AID-JCB13%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Crowley E, Horwitz AF. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J Cell Biol. 1995;131:525–537. doi: 10.1083/jcb.131.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP, Bourdon MA. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Forsberg E, Hirsch E, Frohlich L, Meyer M, Ekblom P, Aszodi A, Werner S, Fassler R. Skin wounds and severed nerves heal normally in mice lacking tenascin-C. Proc Natl Acad Sci USA. 1996;93:6594–6599. doi: 10.1073/pnas.93.13.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohla A, Harhammer R, Schultz G. The G-protein G13 but not G12 mediates signaling from lysophosphatidic acid receptor via epidermal growth factor receptor to Rho. J Biol Chem. 1998;273:4653–4659. doi: 10.1074/jbc.273.8.4653. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Billingham RE, Burgess L. Distribution of fibronectin during wound healing in vivo. J Invest Dermatol. 1981;76:181–189. doi: 10.1111/1523-1747.ep12525694. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hocking DC, Sottile J, Langenbach KJ. Stimulation of integrin-mediated cell contractility by fibronectin polymerization. J Biol Chem. 2000;275:10673–10682. doi: 10.1074/jbc.275.14.10673. [DOI] [PubMed] [Google Scholar]
- Huang W, Chiquet-Ehrismann R, Moyano JV, Garcia-Pardo A, Orend G. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 2001;61:8586–8594. [PubMed] [Google Scholar]
- Ilic D, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Kanner SB, Gilmer TM, Reynolds AB, Parsons JT. Novel tyrosine phosphorylations accompany the activation of pp60c-src during chemical carcinogenesis. Oncogene. 1989;4:295–300. [PubMed] [Google Scholar]
- Kranenburg O, Poland M, Gebbink M, Oomen L, Moolenaar WH. Dissociation of LPA-induced cytoskeletal contraction from stress fiber formation by differential localization of RhoA. J Cell Sci. 1997;110:2417–2427. doi: 10.1242/jcs.110.19.2417. [DOI] [PubMed] [Google Scholar]
- Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- Laufs U, Marra D, Node K, Liao JK. 3-Hydroxy-3-methylglutaryl-CoA reductase inhibitors attenuate vascular smooth muscle proliferation by preventing rho GTPase-induced down-regulation of p27(Kip1) J Biol Chem. 1999;274:21926–21931. doi: 10.1074/jbc.274.31.21926. [DOI] [PubMed] [Google Scholar]
- Luczak JA, Redick SD, Schwarzbauer JE. A single cysteine, Cys-64, is essential for assembly of tenascin-C hexabrachions. J Biol Chem. 1998;273:2073–2077. doi: 10.1074/jbc.273.4.2073. [DOI] [PubMed] [Google Scholar]
- Mackie EJ, Halfter W, Liverani D. Induction of tenascin in healing wounds. J Cell Biol. 1988;107:2757–2767. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie EJ, Tucker RP. The tenascin-C knockout revisited. J Cell Sci. 1999;112:3847–3853. doi: 10.1242/jcs.112.22.3847. [DOI] [PubMed] [Google Scholar]
- Madri JA, Marx M. Matrix composition, organization and soluble factors: modulators of microvascular cell differentiation in vitro. Kidney Int. 1992;41:560–565. doi: 10.1038/ki.1992.82. [DOI] [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function, and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Lightner VA, Aukhil I, Yan YZ, Erickson HP, Hook M. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J Cell Biol. 1991;115:1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orend G, Chiquet-Ehrismann R. Adhesion modulation by antiadhesive molecules of the extracellular matrix. Exp Cell Res. 2000;261:104–110. doi: 10.1006/excr.2000.5041. [DOI] [PubMed] [Google Scholar]
- Patel AS, Schechter GL, Wasilenko WJ, Somers KD. Overexpression of EMS1/cortactin in NIH3T3 fibroblasts causes increased cell motility and invasion in vitro. Oncogene. 1998;16:3227–3232. doi: 10.1038/sj.onc.1201850. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113:3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- Retta SF, Barry ST, Critchley DR, DeFilippi P, Silengo L, Tarone G. Focal adhesion and stress fiber formation is regulated by tyrosine phosphatase activity. Exp Cell Res. 1996;229:307–317. doi: 10.1006/excr.1996.0376. [DOI] [PubMed] [Google Scholar]
- Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- Schwarzbauer JE. Alternative splicing of fibronectin: three variants, three functions. Bioessays. 1991;13:527–533. doi: 10.1002/bies.950131006. [DOI] [PubMed] [Google Scholar]
- Sechler JL, Rao H, Cumiskey AM, Vega-Colon I, Smith MS, Murata T, Schwarzbauer JE. A novel fibronectin binding site required for fibronectin fibril growth during matrix assembly. J Cell Biol. 2001;154:1081–1088. doi: 10.1083/jcb.200102034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnett-Smith J, Lunn JA, Leopoldt D, Rozengurt E. Y-27632, an inhibitor of Rho-associated kinases, prevents tyrosine phosphorylation of focal adhesion kinase and paxillin induced by bombesin: dissociation from tyrosine phosphorylation of p130(C.A.S) Exp Cell Res. 2001;266:292–302. doi: 10.1006/excr.2001.5219. [DOI] [PubMed] [Google Scholar]
- Spring J, Beck K, Chiquet-Ehrismann R. Two contrary functions of tenascin: dissection of the active sites by recombinant tenascin fragments. Cell. 1989;59:325–334. doi: 10.1016/0092-8674(89)90294-8. [DOI] [PubMed] [Google Scholar]
- Tang DD, Gunst SJ. Depletion of focal adhesion kinase by antisense depresses contractile activation of smooth muscle. Am J Physiol Cell Physiol. 2001;280:C874–C883. doi: 10.1152/ajpcell.2001.280.4.C874. [DOI] [PubMed] [Google Scholar]
- Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001;11:370–374. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- Welch MP, Odland GF, Clark RA. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990;110:133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk MB, Midwood KS, Schwarzbauer JE. Tenascin-C suppresses Rho activation. J Cell Biol. 2000;150:913–920. doi: 10.1083/jcb.150.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby DJ, Longaker MT, Harrison MR, Adzick NS, Ferguson MW. Rapid epithelialisation of fetal wounds is associated with the early deposition of tenascin. J Cell Sci. 1991;99:583–586. doi: 10.1242/jcs.99.3.583. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Schwarzbauer JE. The alternatively spliced V region contributes to the differential incorporation of plasma and cellular fibronectins into fibrin clots. J Cell Biol. 1992;119:923–933. doi: 10.1083/jcb.119.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–1426. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8:1415–1425. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]