Abstract
The cubic phase of monoolein has successfully been used for crystallization of a number of membrane proteins. However, the mechanism of protein crystallization in the cubic phase is still unknown. It was hypothesized, that crystallization occurs at locally formed patches of bilayers. To get insight into the stability of the cubic phase, we investigated the effect of different phospholipids and a model transmembrane peptide on the lipid organization in mixed monoolein systems. Deuterium-labeled 1-oleoyl-rac-[2H5]-glycerol was used as a selective probe for 2H NMR. The phase behavior of the phospholipids was followed by 31P NMR. Upon incorporation of phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, or phosphatidic acid, the cubic phase of monoolein transformed into the Lα or HII phase depending on the phase preference of the phospholipid and its concentration. The ability of phospholipids to destabilize the cubic phase was found to be dependent on the phospholipid packing properties. Electrostatic repulsion facilitated the cubic-to-Lα transition. Incorporation of the transmembrane peptide KALP31 induced formation of the Lα phase with tightly packed lipid molecules. In all cases when phase separation occurs, monoolein and phospholipid participate in both phases. The implications of these findings for protein crystallization are discussed.
INTRODUCTION
Monoglycerides are amphiphatic neutral lipid molecules in which a hydrophobic fatty acid is attached at the rac-1 position of a hydrophilic glycerol backbone via an ester bond. Despite their relatively simple chemical structure, monoglycerides can form various phases found in membrane phospholipid/water systems. The ability of monoglycerides to form bilayer as well as nonbilayer structures offers many interesting opportunities for studies of membrane lipid organization (Boots et al., 1999, 2001; Chupin et al., 2001, 2002).
Recently a novel approach has been devised for the crystallization of membrane proteins by using monoolein cubic phases (Landau and Rosenbusch, 1996; Pebay-Peyroula et al., 1997; Rummel et al., 1998; Chiu et al., 2000). These phases are ordered, three-dimensional water-lipid systems, in which lipids are organized in a highly curved bicontinuous bilayer network (Hyde and Andersson, 1984; Lindblom and Rilfors, 1989). Such matrices support growth of crystals by lateral diffusion of protein molecules in the bilayer network. The mechanism of protein crystallization in the cubic phase is still not known. Recently, it was hypothesized, that protein crystallization occurs in locally formed liquid-crystalline lamellar Lα phases (Caffrey, 2000; Nollert et al., 2001). One indication for that is a lamellar-type packing arrangement of protein molecules within crystals. In addition, the cubic-to-Lα phase transition can be induced by different additives, which are always present during crystallization. For example, membrane protein samples contain phospholipids and detergents (Nollert et al., 2001). Potentially, phospholipids, detergents, and proteins can change the lipid organization in the cubic phase of monoolein and cause a transition to a liquid crystalline bilayer, thereby modulating the crystallization process. The effect of detergents on the cubic phase of monoolein was reported previously (Ai and Caffrey, 2000). However, little is known about how phospholipids and proteins interact with the cubic phase of monoolein. To analyze a possible effect of phospholipids and proteins we investigated the lipid organization of monoolein in the presence of different phospholipids and in the presence of a model transmembrane peptide.
To allow systematic analysis of the effect of different phospholipids we used phospholipids with the same acyl chain as monoolein, but with the headgroups differing in size and electrical charge: 1,2-dioleoylphosphatidylcholine (DOPC), 1,2-dioleoylphosphatidylethanolamine (DOPE), 1,2-dioleoylphosphatidylglycerol (DOPG), and 1,2-dioleoylphosphatidylphosphatidic acid (DOPA). DOPC and DOPE are zwitterionic and electrically neutral, whereas DOPG and DOPA are negatively charged at neutral pH. DOPC and DOPG are typical bilayer-forming phospholipids with relatively large headgroups, whereas DOPE prefers to organize in nonbilayer structures like the inverted hexagonal HII phase (de Kruijff, 1997a). DOPA displays intermediate behavior and can form bilayer and inverted structures depending on a range of factors, including pH and the presence of cations (Verkleij et al., 1982; Lindblom et al., 1991). To mimic the situation when membrane spanning proteins are present we used a model peptide, KALP31, with a hydrophobic core of alternating leucine and alanine, flanked by lysine residues. This peptide forms a transmembrane α-helix in lipid bilayers (de Planque et al., 1999).
The lipid organization of these mixed systems was studied by means of solid state 2H and 31P NMR spectroscopy. Deuterium-labeled monoolein 1-oleoyl-rac-[2H5]-glycerol ([2H5]-MO) (Fig. 1) with a fully deuterated headgroup was used as a selective probe for 2H NMR. Such labels can provide not only information on the phase behavior, but also on the lipid/water interface and glycerol conformation in monoglyceride systems. The behavior of phospholipids was followed by 31P NMR. In this approach, the behavior of individual molecular components can be monitored in the same sample, which is important because monoglyceride/water systems are known to form long-living metastable phases.
FIGURE 1.
Deuterium-labeled 1-monooleoyl-rac-[2H5]-glycerol ([2H5]-MO) and signal assignment in the 2H NMR spectrum of [2H5]-MO in the liquid-crystalline phase at 5°C (85 wt % lipid). The undercooled liquid-crystalline phase was obtained by subsequent heating up to 50°C and cooling down to 5°C of the sample.
MATERIALS AND METHODS
Materials
Oleic acid was obtained from Sigma Chemical Company (St. Louis, MO). Deuterated glycerol-d5 and deuterium-depleted water were obtained from Cambridge Isotope Laboratories (Cambridge, MA). 1,2–Dioleoyl-sn-glycerophosphocholine, 1,2–dioleoyl-sn-glycerophosphoethanolamine, 1,2–dioleoyl-sn-glycerophospho-rac-glycerol and 1,2–dioleoyl-sn-glycerophosphate were purchased from Avanti Polar Lipids (Alabaster, AL). 1-Oleoyl-rac-[2H5]-glycerol was synthesized via acylation of 1,2-isopropylidene-[2H5]-glycerol, and removal of the protective group. Monoglyceride was purified on a silica column and crystallized from acetone. The KALP31 peptide (Ac-GKK(LA)12LKKA-amide) was synthesized on an automatic ABI 433A peptide synthesizer using the ABI FastMoc 0.25 mmol protocols (de Planque et al., 1999).
Sample preparation
Samples were prepared by mixing known amounts of [2H5]-MO and phospholipid stock solutions in CHCl3/MeOH (3:1). KALP31 was dissolved in TFE and added to a lipid solution. The solvents were evaporated and residual solvent was removed under high vacuum for at least 12 h. The lipids were subsequently hydrated by adding buffer at room temperature (20 mM Tris, pH 7, prepared with deuterium-depleted water). The samples consisted of 70–100 μmol lipid. A lipid-to-buffer ratio of 3/2 (wt/wt) was used. To study pure [2H5]-MO in the Lα phase, a water content of 15 wt % was used. Above 35 wt % water, MO can form only the cubic and crystalline phase (Qiu and Caffrey, 2000). Before heating the sample of [2H5]-MO was incubated at −20°C during 24 h to obtain the thermodynamically stable crystalline phase (Qiu and Caffrey, 2000). In the NMR probe, the sample was incubated for 10 min at each temperature and then measured for 20 min.
NMR measurements
NMR spectra were recorded on Bruker MSL 300 and Avance 500 WB spectrometers. 2H NMR spectra were obtained using a quadrupolar echo technique (Davis et al., 1976). The recycling delay was 200 ms. 31P NMR spectra were recorded using a high resolution 10 mm broad-band probe with broad-band gated proton decoupling. The recycling delay was 1.5 s and the π/4 pulse width 7 μs. An exponential multiplication with a line-broadening factor of 100 Hz was used before performing the Fourier transformation. All 2H NMR spectra were symmetrized. Chemical shifts in 31P NMR spectra were measured relatively to the isotropic signal.
Theory and application of 2H NMR (Davis, 1983; Seelig and MacDonald, 1987; Smith, 1989) and 31P NMR (Seelig, 1978; Smith and Ekiel, 1984) spectroscopy in lipid systems are described in the literature.
RESULTS
Phase characterization of deuterium headgroup labeled monoglycerides by 2H NMR
We previously demonstrated that 2H NMR spectroscopy on deuterated acyl chain monoglycerides is a convenient technique to monitor phase transitions in monoglyceride-water systems (Chupin et al., 2001), because all different phases give rise to characteristic features in the 2H NMR spectra. In this study, the headgroup-deuterated [2H5]-MO was chosen to investigate not only the phase behavior but also the properties of the interface and glycerol conformation in mixed monoglyceride/phospholipid systems. However, the line shape of 2H NMR spectra of headgroup-labeled monoglycerides in different phases is not known. Therefore we first characterized different phases of [2H5]-MO by 2H NMR.
Signal assignment
In deuterated glycerol of chiral [2H5]-MO, all five deuterons are chemically nonequivalent (Fig. 1) and can give five different resonances in a 2H NMR spectrum. The two enantiomers present in the racemic mixture cannot be distinguished in the NMR spectra. The signal assignment in the hydrated liquid-crystalline Lα phase of [2H5]-MO is shown in Fig. 1. The mobility of the deuterium labels increases in the direction from the rac-1 position to the rac-3 in the glycerol moiety due to intramolecular rotations around C C bonds and glycerol wobbling as it was shown for headgroup-deuterated phosphatidylglycerol (Wohlgemuth et al., 1980). As a result, three groups of resonances are present in the spectrum (Fig. 1). The signal with the largest quadrupolar splitting of 22 kHz corresponds to two nonequivalent deuterons at the rac-1 position, which are not resolved in this spectrum. The signal at the rac-2 position exhibits a reduced quadrupolar splitting of 8 kHz, indicating motional averaging compared with the labels at the rac-1 position, which is mainly due to rotation around the C(1)
C bonds and glycerol wobbling as it was shown for headgroup-deuterated phosphatidylglycerol (Wohlgemuth et al., 1980). As a result, three groups of resonances are present in the spectrum (Fig. 1). The signal with the largest quadrupolar splitting of 22 kHz corresponds to two nonequivalent deuterons at the rac-1 position, which are not resolved in this spectrum. The signal at the rac-2 position exhibits a reduced quadrupolar splitting of 8 kHz, indicating motional averaging compared with the labels at the rac-1 position, which is mainly due to rotation around the C(1) C(2) bond. Deuterium labels at the rac-3 position give two resolved resonances with further decreased quadrupolar splittings of 1.3 kHz and less than 0.4 kHz. The quadrupolar splittings of the headgroup-deuterated lipids are also dependent on the conformation of the headgroup and can differ within the same phase (Scherer and Seelig, 1989; Marassi and Macdonald, 1991) as will be shown below for mixed [2H5]-MO/phospholipid systems.
C(2) bond. Deuterium labels at the rac-3 position give two resolved resonances with further decreased quadrupolar splittings of 1.3 kHz and less than 0.4 kHz. The quadrupolar splittings of the headgroup-deuterated lipids are also dependent on the conformation of the headgroup and can differ within the same phase (Scherer and Seelig, 1989; Marassi and Macdonald, 1991) as will be shown below for mixed [2H5]-MO/phospholipid systems.
Phase characterization
According to the phase diagram of MO (Qiu and Caffrey, 2000), different phases can be obtained by varying the temperature and/or water content. As an example, the spectra of [2H5]-MO at 15 wt % water concentration are shown in Fig. 2 recorded upon subsequent heating (Fig. 2, A–C) and cooling (Fig. 2 D). In the crystalline phase of [2H5]-MO, the spectrum consists of a broad powder pattern with a quadrupolar splitting of ∼120 kHz (Fig. 2 A), which is characteristic for an immobilized headgroup with nonresolved signals from the different labeled sites. In the Lα phase at 40°C, the spectrum of [2H5]-MO shows three group of resolved signals with reduced quadrupolar splittings of 14 kHz, 4, and 1.2/0.2 kHz from the labeled sites rac-1, rac-2, and rac-3 (Fig. 2 B), indicating motional averaging in this phase as compared to the crystalline phase. In the cubic phase, the 2H NMR spectrum of [2H5]-MO shows a narrow signal originating from isotropically moving lipid molecules (Fig. 2 C). Once heated above the crystalline-to-liquid crystalline transition temperature, [2H5]-MO at 5°C forms the undercooled Lα phase (Fig. 2 D), which is in line with the x-ray data (Qiu and Caffrey, 2000). The increased quadrupolar splitings in the Lα phase at 5°C (see also Fig. 1) as compared to 40°C reflects the decrease in motion of the [2H5]-MO at the lower temperature.
FIGURE 2.

2H NMR spectra representing the different phases of [2H5]-MO (85 wt % lipid) crystalline phase. (A) Liquid-crystalline phase. (B) Cubic phase of [2H5]-MS. (C) Undercooled liquid crystalline phase of [2H5]-MO. (D) The thermodynamically stable crystalline phase was obtained by incubation of the sample at −20°C during 24 h.
The data presented above demonstrate that 2H NMR on deuterium-headgroup-labeled monoglycerides is a sensitive technique to monitor lipid organization in monoglyceride systems.
Effect of phospholipids on phase behavior of monoolein
To elucidate a possible effect of phospholipids on the cubic phase of MO we investigated the lipid organization in mixtures with [2H5]-MO/phospholipid ratio's of 1:0, 9:1, 7:3, 1:1, 3:7, 1:9, and 0:1 (wt/wt) by 2H and 31P NMR. The weight concentration was used rather than molar ratio because this is commonly accepted for phase diagrams in monoglyceride systems. All experiments were performed at a lipid/water ratio of 3/2 (wt/wt) and at a temperature of 20°C, which are the same conditions that were used for protein crystallization from the cubic phase (Nollert et al., 2001). Under these conditions, pure [2H5]-MO forms a cubic phase.
Phosphatidylcholine
Phosphatidylcholine is a bilayer preferring lipid (de Kruijff, 1997a). The 31P NMR spectrum of DOPC (Fig. 3 A) exhibits a line shape with a high field peak and a low field shoulder, which is characteristic of the Lα phase. The isotropic signal of the pure [2H5]-MO cubic phase is shown in Fig. 3 L. At concentrations of DOPC below 50 wt %, 31P, and 2H NMR spectra show isotropic signals (Fig. 3, E, F, J, and K), indicating that both phospholipid and monoglyceride molecules are organized in the isotropic cubic phase. At a concentration of DOPC of 50 wt %, both 31P and 2H NMR spectra show a superposition of isotropic and anisotropic signals (Fig. 3, D and I). The line shapes of the anisotropic signals indicate formation of the Lα phase. The 31P NMR spectrum (Fig. 3 D) shows a peak at the high field like pure DOPC (compare with Fig. 3 A). In the 2H NMR spectrum (Fig. 3 I), the signal of the rac-1 labels exhibits a quadrupolar splitting of 21 kHz, which corresponds to that of the Lα phase of pure [2H5]-MO. Thus, at this concentration phase separation occurs between an Lα and a cubic phase, with both lipids participating in both phases. At concentrations of DOPC higher than 50 wt %, both 31P and 2H NMR spectra (Fig. 3, B, C, G, and H) show a line shape, which is characteristic of the Lα phase. The phase behavior of the [2H5]-MO/DOPC mixture at 28°C (not shown) is in line with the phase diagram reported previously for MO/DOPC/water mixtures, obtained by using 31P NMR on DOPC and 2H NMR on D2O (Lindblom and Rilfors, 1989). However, in our approach we can follow the behavior of both DOPC and [2H5]-MO. No lipid separation of DOPC and [2H5]-MO was detected indicating that both phospholipid and monoglyceride are homogeneously mixed in the cubic and Lα phase.
FIGURE 3.
31P NMR spectra (left) and 2H NMR (right) of [2H5]-MO/DOPC at different [2H5]-MO/phospholipid ratios, as indicated in the figure.
Phosphatidylethanolamine
In contrast to phosphatidylcholine, phosphatidylethanolamine has a strong propensity to form inverted structures with a negative curvature (de Kruijff, 1997a). At 20°C, DOPE forms a hexagonal HII phase, as indicated by the 31P NMR spectrum with a low field peak and a high field shoulder and a twofold-reduced chemical shift anisotropy (Fig. 4 A). At concentrations of DOPE up to 50 wt % the [2H5]-MO:DOPE mixture still forms the HII phase as evidenced by the 31P NMR spectra (Fig. 4, B–D). In the corresponding 2H NMR spectra (Fig. 4, F–H), the quadrupolar splitting of the rac-1 labels of [2H5]-MO is reduced by a factor of ∼2 as compared with that in the Lα phase of [2H5]-MO/DOPC mixtures. This is typical for an organization of [2H5]-MO in the HII phase, in which additional fast rotation of lipid molecules around the cylinders of the HII phase causes a twofold decrease in the quadrupolar splitting (Davis, 1983; Sternin et al., 1988). At DOPE content of 30% (Fig. 4, E and I) and below (not shown), [2H5]-MO/DOPE mixtures show isotropic signals in 31P and 2H NMR spectra, indicating that both lipids are co-organized in the isotropic phase.
FIGURE 4.
31P NMR spectra (left) and 2H NMR (right) of [2H5]-MO/DOPE at different [2H5]-MO/phospholipid ratios, as indicated in the figure.
Phosphatidic acid
Phosphatidic acid is a minor but very important membrane lipid as key intermediate in lipid biosynthesis and because it is involved in different cellular functions (Schmidt et al., 1999; Weigert et al., 1999; Barr and Shorter, 2000). Phosphatidic acid shows a rich polymorphism (Verkleij et al., 1982; Lindblom et al., 1991). At neutral pH and in the absence of divalent cations, DOPA forms a liquid crystalline bilayer at 20°C, as indicated by the 31P NMR spectrum (Fig. 5 A). At a DOPA content of 90 wt %, the 2H and 31P NMR spectra show a line shape of the liquid-crystalline bilayer (Fig. 5, B and F). In the presence of 30 wt % [2H5]-MO phase separation occurs between an Lα and an isotropic phase, in which both molecules participate (Fig. 5, C and G). Interestingly, upon further decreasing the DOPA content, the mixture with a [2H5]-MO/DOPA ratio of 1:1 (wt/wt) transforms into a mixture of the HII and isotropic phase as indicated by a twofold decrease of the quadrupolar splitting and chemical shift anisotropy of the anisotropic signal in 2H NMR and 31P NMR spectra (Fig. 5, D and H). A further decrease of the DOPA content to 30 wt % and lower (not shown) again leads to isotropic signals in both 31P NMR and 2H NMR spectra of [2H5]-MO/DOPA (Fig. 5, E and I). Subsequent transformation from the Lα to the HII and then to the cubic phase as observed upon increasing the [2H5]-MO content is very unusual, because normally the isotropic phase is intermediate between the Lα and HII phase.
FIGURE 5.
31P NMR spectra (left) and 2H NMR (right) of [2H5]-MO/DOPA at different [2H5]-MO/phospholipid ratios, as indicated in the figure.
Phosphatidylglycerol
Phosphatidylglycerol is a common anionic bacterial lipid, which organizes by itself in a lamellar phase (Fig. 6 A). Up to 50 wt % of [2H5]-MO can be homogeneously incorporated into the Lα phase of DOPG (Fig. 6, B–D, G–I). At 30 wt % of DOPG, phase separation occurs between the Lα and an isotropic phase but the lamellar phase prevails (Fig. 6, E and J). At a [2H5]-MO/DOPG ratio of 9:1 (wt/wt), 31P and 2H NMR spectra show isotropic signals (Fig. 6, F and K). Comparison with Fig. 3 reveals that DOPG is much more effective in stabilization of the Lα phase of [2H5]-MO/PL as compared to DOPC.
FIGURE 6.
31P NMR spectra (left) and 2H NMR (right) of [2H5]-MO/DOPG at different [2H5]-MO/phospholipid ratios, as indicated in the figure.
Another interesting difference between the [2H5]-MO/DOPC and [2H5]-MO/DOPG systems is seen in the line shape of the 2H NMR spectra of [2H5]-MO in the Lα phase (PL/[2H5]-MO, 9:1 wt/wt) (Fig. 3 G and Fig. 6 G). The expanded spectra are shown in Fig. 7. The quadrupolar splittings of ∼19 kHz of rac-1 labels of [2H5]-MO are identical in both [2H5]-MO/DOPC and [2H5]-MO/DOPG bilayers. However, the quadrupolar splitting of the rac-2 label in DOPC bilayer is 5 kHz as compared to 6 kHz in DOPG. For labels at the rac-3 position the effect is opposite. The quadrupolar splitting in the DOPC bilayer (2 kHz) is much larger compared to the value of 0.6 kHz in the DOPG bilayer. In the [2H5]-MO/DOPA bilayer, the quadrupolar splitting of the rac-2 label (7.9 kHz) is even more increased, whereas quadrupolar splittings of the rac-3 labels (0 and 0.6 kHz) are decreased compared with these in the DOPC and DOPG bilayers. Such changes strongly suggest (Seelig and Macdonald, 1987; Scherer and Seelig, 1989) that the orientation of the glycerol headgroup of [2H5]-MO with respect to the bilayer surface is different in DOPC and DOPG bilayers.
FIGURE 7.
2H NMR spectra of [2H5]-MO/DOPC (A) and [2H5]-MO/DOPG (B) in the Lα phase. [2H5]-MO/phospholipid ratio 1:9 (wt/wt).
Effect of [2H5]-MO on the lipid packing in the Lα phase of [2H5]-MO/PL
The quadrupolar splittings arising from the deuterium labels at the rac-1 position of [2H5]-MO are well resolved and can be used as a measure for the molecular order of the [2H5]-MO headgroup. As shown in Fig. 8, these quadrupolar splittings are dependent on the presence of [2H5]-MO in the Lα phase. Upon increasing the [2H5]-MO content in the [2H5]-MO/DOPC and [2H5]-MO/DOPG bilayers, the quadrupolar splittings also increase, indicating that the packing density of the lipid molecules increases. The results suggest that when a critical packing density level corresponding to the quadrupolar splitting of ∼21.5 kHz is reached, the system transforms from the Lα phase into the cubic phase. The quadrupolar splittings of the rac-1 labels of [2H5]-MO are almost identical in the [2H5]-MO/DOPC and [2H5]-MO/DOPG bilayers with the same [2H5]-MO/PL ratio (Fig. 8). This is in line with the similar packing properties of DOPC and DOPG as can be concluded from their similar gel-to-Lα transition temperatures (March, 1990). The quadrupolar splittings of the rac-1 labels of [2H5]-MO in the [2H5]-MO/DOPA bilayer are larger as compared to the bilayers of [2H5]-MO/DOPC and [2H5]-MO/DOPG (Fig. 8), which is most likely due to the small size of the polar headgroup of DOPA and more tight packing of the lipid molecules in the liquid crystalline bilayers of [2H5]-MO/DOPA .
FIGURE 8.
Quadrupolar splitting DnQ of rac-1 labels of [2H5]-MO in the liquid crystalline lamellar Lα phase of DOPG, DOPC, and DOPA as a function of the [2H5]-MO content.
Effect of a transmembrane α-helical peptide KALP31 on the cubic phase
To get insight into effects of proteins on the cubic phase, we investigated the lipid organization of a [2H5]-MO/DOPC, 7:3 (w/w) mixture in the presence of a model peptide KALP31. KALP31 is a hydrophobic peptide, which forms a transmembrane α-helix in lipid bilayers that is interfacially stabilized by the N- and C-terminal lysines (de Planque et al., 1999). The mixture of [2H5]-MO/DOPC, 7:3 (wt/wt), was chosen because it forms a cubic phase by itself but contains the bilayer preferring DOPC.
31P and 2H NMR spectra of a [2H5]-MO/DOPC, 7:3 (w/w) mixture in the presence of increasing amounts of KALP31 are shown in Fig. 9. In each panel, all spectra were normalized to the same height. The 2H NMR spectra on the right panel are shown in the expanded vertical scale. In the absence of KALP31, anisotropic signals were not detected (Fig. 9, top left and right). In the presence of even very low concentration of KALP31, an anisotropic signal was observed in both 31P and 2H NMR spectra with a line shape, which is characteristic of the Lα phase (Fig. 9, bottom three, left and right). The intensity of the anisotropic signal increases upon increasing the KALP31 content (Fig. 9, bottom three, left and right) demonstrating that KALP31 stabilizes the Lα phase. The quadrupolar splitting of 22 kHz of the rac-1 labels in the anisotropic 2H NMR signals indicates that lipid molecules are tightly packed in the Lα phase (compare with Fig. 8).
FIGURE 9.
31P NMR and 2H NMR spectra of [2H5]-MO/DOPC, 7:3 (wt/wt) mixture at different KALP31/lipid ratios, as indicated in the figure.
DISCUSSION
Upon incorporation of increasing amounts of different phospholipids, the cubic phase of monoolein transforms into the Lα or HII phase depending on the type of phospholipid and its concentration. The ability of phospholipids to rearrange the lipid organization in the cubic phase of monoolein is correlated with the phospholipid headgroup size and charge, which both contribute to the lipid packing in the mixed [2H5]-MO/PL systems.
Effect of phospholipids on phase behavior of MO
Lipid organization can be understood by relating the packing properties of lipid molecules to their shape via a packing parameter p (Israelachvili et al., 1977):
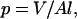 |
(1) |
where V is the acyl chains volume, A is the area of the lipid headgroup at the hydrocarbon-water interface, and l is the length of the acyl chains
If p ≅ 1, lipid molecules form a bilayer. If p > 1, they tend to form structures with a negative curvature like the HII phase and if p < 1, they tend to form structures with a positive curvature, such as micelles. Cubic phases can be considered as intermediate between the bilayer and HII phase.
Zwitterionic DOPC and DOPE are electrically neutral but differ in the size of the polar headgroup. DOPE has a small headgroup, which increases p (Eq. 1). As a result, incorporation of the inverted phase forming DOPE in [2H5]-MO destabilizes the cubic phase-inducing formation of the HII phase. In contrast to DOPE, DOPC has a large headgroup, which decreases p (Eq. 1). DOPC transforms the cubic phase of [2H5]-MO into the Lα phase.
Like DOPC, negatively-charged DOPG is a bilayer-forming phospholipid with a relatively large headgroup (de Kruijff, 1997a). Moreover, for the same fatty acid composition the acyl chain packing in the liquid crystalline bilayers of phosphatidylglycerols is similar to that of phosphatidylcholines, as indicated by the remarkably close values of the gel-to-liquid crystalline transition temperatures of these phospholipids (March, 1990). Therefore, the effect of electrostatic interactions on the stability of the cubic phase of monoolein can be derived by comparison of the [2H5]-MO/DOPC and [2H5]-MO/DOPG systems. Both DOPC and DOPG destabilize the cubic phase of [2H5]-MO. However, despite the similar packing properties of DOPC and DOPG, the cubic-to-Lα phase transition occurs at a significantly lower content of DOPG as compared to that of DOPC (Fig. 6). This higher efficiency of DOPG to destabilize the cubic phase of [2H5]-MO indicates that electrostatic interactions play an important role. A likely possibility is that electrostatic repulsion between the negatively-charged headgroup of DOPG induces an increase in the diameter of the water channels of the cubic phase as observed for MO/PA (Shu et al., 2001), leading to subsequent disruption of the cubic phase, which transforms in the liquid crystalline bilayer.
The behavior of [2H5]-MO/DOPA is more complex. Upon increasing the DOPA content in [2H5]-MO/DOPA mixture, the cubic phase subsequently transforms into the HII phase and then into the Lα phase. Such polymorphic behavior is not usual for lipid systems, in which the cubic phases are structures that are intermediate between a bilayer and inverted phase organization (de Kruijff, 1997b). The behavior of the [2H5]-MO/DOPA system can be explained in terms of electrostatic interactions. When the content of DOPA is high, strong electrostatic repulsive forces cause an increase of the area of the lipid headgroup at the lipid-water interface A (Eq. 1), which leads to stabilization of the Lα phase. Upon decreasing the DOPA content in the neutral [2H5]-MO matrix, the electrical charge density is decreased at the lipid/water interface, decreasing the electrostatic repulsion. To some extent, this is equivalent to a screening of the charges of DOPA, which is known to induce the Lα-to-HII transition in pure DOPA (Verkleij et al., 1982; Lindblom et al., 1991). At a [2H5]-MO/DOPA ratio of 1:1 (wt/wt), the electrostatic repulsion is not strong enough to overcome the propensity of DOPA to form a nonbilayer structure and the system transforms into the mixture of the HII and isotropic phase. Upon further decreasing the DOPA content, the system transforms into the cubic phase because the lipid organization now is determined by the packing properties of [2H5]-MO.
Lipid packing and cubic phase formation in [2H5]-MO/PL systems
The combination of 2H NMR on [2H5]-MO and 31P NMR on phospholipids allows to monitor the phase organization of monoolein and phospholipids in the mixed [2H5]-MO/PL systems. In transitions from the cubic-to-Lα, cubic-to-HII, or Lα-to-HII mediated by the lipid composition of [2H5]-MO/PL mixtures, both monoolein and phospholipid are involved. The 2H NMR data also allow to get insight in the lipid packing of the different systems. In the Lα phase of [2H5]-MO/DOPC and [2H5]-MO/DOPG, increasing amounts of [2H5]-MO lead to increase lipid packing density, as indicated by the increase of the quadrupolar splitting of the rac-1 labels (Fig. 8). When a critical packing density is reached the system transforms to the cubic phase. Similarly in [2H5]-MO/DOPA, the Lα phase transforms into the HII phase at the same lipid packing density as the cubic phase is formed in [2H5]-MO/DOPG and [2H5]-MO/DOPC, as indicated by the close values of the quadrupolar splittings of the rac-1 labels (Fig. 8). The molecular order increase in the Lα phase upon incorporation of nonbilayer-forming lipids with a small headgroup has a general character and was also observed for a number of other lipid systems (Lafleur et al., 1990a,b; Killian et al., 1992; Chupin et al., 1994; Oradd et al., 2000).
Conformation of the monoolein headgroup in the Lα phase
In the Lα phase of the MO/water system, the rac-3 hydroxyl group forms an intramolecular hydrogen bond to the carbonyl group, which by addition of DOPC is broken and hydrogen bonding between the hydroxyl groups of MO and phosphate group of DOPC begins to form (Nilsson et al., 1991). Such interaction with phospholipids can change the conformation of the monolein headgroup. In this study, we observed that the quadrupolar splittings of the rac-2 label of [2H5]-MO are decreasing, whereas the quadrupolar splittings of the rac-1 labels are increasing in bilayers of DOPC, DOPG, and DOPA, respectively. This indicates a different conformation of the headgroup of [2H5]-MO in the DOPC, DOPG, and DOPA bilayers. Previously such effect was observed for headgroup-deuterated phosphocholine, in which the headgroup can change its orientation with respect to the bilayer surface due to electrostatic interactions (Seelig and Macdonald, 1987; Scherer and Seelig, 1989). Based on those data, our observation can be interpreted as follows. The tilt of the glycerol headgroup of [2H5]-MO with respect to the plane of the lipid-water interface is increasing in the DOPC < DOPG < DOPA bilayers, which correlates with the geometrical headgroup size of these phospholipids. Most likely, the bulky choline headgroups of DOPC decrease the tilt of the headgroups of [2H5]-MO due to spatial hindrance, which is lower in the DOPG and especially low in the DOPA bilayer.
Implication for protein crystallization
It was proposed, that crystallization of proteins in the cubic phase of monoolein occurs in the locally formed Lα phase (Caffrey, 2000; Nollert et al., 2001). Our results show that the presence of the bilayer-forming phospholipids DOPC and DOPG facilitates formation of the liquid crystalline bilayer in the cubic phase of monoolein. Moreover, the effect of DOPG is larger compared to that of DOPC, indicating that electrostatic repulsion is important for the bilayer formation. The presence of proteins can also destabilize the cubic phase of monoolein, as concluded from the effect of the transmembrane KALP31 peptide, which induces formation of a bilayer coexisting with a cubic phase. Within this bilayer, the presence of the nonbilayer-forming monoolein creates a tight packing of the lipid molecules, as indicated by the quadrupolar splitting of the rac-1 labels of [2H5]-MO. One could speculate that as a result of this, protein molecules are forced to aggregate, which also can facilitate crystallization (de Kruijff, 1997b).
In the case of membrane proteins, which were successfully crystallized from the cubic phase, the crystallization conditions can facilitate formation of the Lα phase. For example, crystallization of bacteriorhodopsin in the cubic phase of monoolein always occurs in the presence of anionic lipids of purple membrane, phosphatidylglycerophosphate, and phosphatidylglycerosulfate (Nollert et al., 2001), which can facilitate the cubic-to-Lα phase transition. In this study, we demonstrated that the stability of the cubic phase of monoolein can be regulated by the presence of phospholipids. It is possible that crystallization of different membrane proteins can be facilitated by incorporation in the cubic phase of lipids, which can facilitate formation of the liquid crystalline bilayer, thereby creating crystallization sites.
Acknowledgments
This work was supported by the Netherlands Organization for Scientific Research (NWO), CW/STW project 349-4608.
References
- Ai, X., and M. Caffrey. 2000. Membrane protein crystallization in lipidic mesophases: detergent effects. Biophys. J. 79:394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, F. A., and J. Shorter. 2000. Membrane traffic: do cones mark sites of fission? Curr. Biol. 10:R141–R144. [DOI] [PubMed] [Google Scholar]
- Boots, J.-W. P., V. Chupin, J. A. Killian, R. A. Demel, and B. de Kruijff. 1999. Interaction mode specific reorganization of gel phase monoglyceride bilayers by beta-lactoglobulin. Biochim. Biophys. Acta. 1420:241–251. [DOI] [PubMed] [Google Scholar]
- Boots, J.-W. P., V. Chupin, J. A. Killian, R. A. Demel, and B. de Kruijff. 2001. The specificity of monoglyceride-protein interactions and mechanism of the protein induced L(β) to coagel phase transition. Biochim. Biophys. Acta. 1510:401–413. [DOI] [PubMed] [Google Scholar]
- Caffrey, M., 2000. A lipid's eye view of membrane protein crystallization in mesophases. Curr. Opin. Struct. Biol. 10:486–497. [DOI] [PubMed] [Google Scholar]
- Chiu, M. L., P. Nollert, M. C. Loewen, H. Belrhali, E. Pebay-Peyroula, J. P. Rosenbusch, and E. M. Landau. 2000. Crystallization in cubo: general applicability to membrane proteins. Acta Crystallogr. D Biol. Crystallogr. 56:781–784. [DOI] [PubMed] [Google Scholar]
- Chupin, V., J.-W. P. Boots, J. A. Killian, R. A. Demel, and B. de Kruijff. 2001. Lipid organization and dynamics of the monostearoylglycerol-water system. A 2H NMR study. Chem. Phys. Lipids. 109:15–28. [DOI] [PubMed] [Google Scholar]
- Chupin V., J.-W. P. Boots, J. A. Killian, R. A. Demel, and B. de Kruijff. 2002. Thermotropic phase behavior of monoglyceride-dicetylphosphate dispersions and interactions with proteins: a 2H and 31P NMR study. Biophys. J. 82:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chupin, V., R. van 't Hof, and B. de Kruijff. 1994. The transit sequence of a chloroplast precursor protein reorients the lipids in monogalactosyl diglyceride containing bilayers. FEBS Lett. 350:104–108. [DOI] [PubMed] [Google Scholar]
- Davis, J. H. 1983. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim. Biophys. Acta. 737:117–171. [DOI] [PubMed] [Google Scholar]
- Davis, J. A., K. R. Jeffrey, M. Bloom, M. I. Valic, and T. P. Higgs. 1976. Quadrupolar echo deuterium magnetic resonance spectroscopy in ordered hydrocarbon chains. Chem. Phys. Lett. 42:390–394. [Google Scholar]
- de Kruijff, B. 1997a. Lipid polymorphism and biomembrane function. Curr. Opin. Chem. Biol. 1:564–569. [DOI] [PubMed] [Google Scholar]
- de Kruijff, B. 1997b. Lipids beyond the bilayer. Nature. 386:129–130. [DOI] [PubMed] [Google Scholar]
- De Planque, M. R., J. A. Kruijtzer, R. M. Liskamp, D. Marsh, D. V. Greathouse, R. E. Koeppe 2nd, B. de Kruijff, and J. A. Killian. 1999. Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane α-helical peptides. J. Biol. Chem. 274:20839–20846. [DOI] [PubMed] [Google Scholar]
- Hyde, S. T., and S. Andersson. 1984. A cubic structure consisting of a lipid bilayer forming an infinite periodic minimum surface of the gyroid type in the glycerolmonooleat-water system. Z. Kristallogr. 168:213–219. [Google Scholar]
- Israelachvili, J. N., D. J. Mitchell, and B. W. Ninham. 1977. Theory of self-assembly of lipid bilayers and vesicles. Biochim. Biophys. Acta. 470:185–201. [DOI] [PubMed] [Google Scholar]
- Killian, J. A., C. H. J. P. Fabrie, W. Baart, S. Morein, and B. de Kruijff. 1992. Effects of temperature variation and phenethyl alcohol addition on acyl chain order and lipid organization in Escherichia coli derived membrane systems. A 2H- and 31P-NMR study. Biochim. Biophys. Acta. 1105:253–262. [DOI] [PubMed] [Google Scholar]
- Lafleur, M., M. Bloom, and P. R. Cullis. 1990a. Lipid polymorphism and hydrocarbon order. Biochem. Cell. Biol. 68:1–8. [DOI] [PubMed] [Google Scholar]
- Lafleur, M., P. R. Cullis, and M. Bloom. 1990b. Modulation of the orientational order profile of the lipid acyl chain in the Lα phase. Eur. Biophys. J. 19:55–62. [DOI] [PubMed] [Google Scholar]
- Landau, E. M., and J. P. Rosenbusch. 1996. Lipidic cubic phases: a novel concept for the crystallization of membrane proteins. Proc. Natl. Acad. Sci. USA. 93:14532–14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblom, G., and L. Rilfors. 1989. Cubic phases and isotropic phases formed by membrane lipids—possible biological relevance. Biochim. Biophys. Acta. 988:221–256. [Google Scholar]
- Lindblom, G., L. Rilfors, J. B. Hauksson, I. Brentel, M. Sjolund, and B. Bergenstahl. 1991. Effect of headgroup structure and counterion condensation on phase equilibria in anionic phospholipid-water systems studied by 2H, 23Na, and 31P NMR and x-ray diffraction. Biochemistry. 30:10938–10948. [DOI] [PubMed] [Google Scholar]
- Marassi, F. M., and P. M. Macdonald. 1991. Response of the headgroup of phosphatidylglycerol to membrane surface charge as studied by deuterium and phosphorus-31 nuclear magnetic resonance. Biochemistry. 30:10558–10566. [DOI] [PubMed] [Google Scholar]
- March, D. 1990. Handbook of Lipid Bilayers. CRC Press, Boca Raton, Ann Arbor, and Boston. pp. 135–158.
- Nilsson, A., A. Holmgren, and G. Lindblom. 1991. Fourier-transform infrared spectroscopy study of dioleoylphosphatidylcholine and monooleoylglycerol in lamellar and cubic liquid crystals. Biochemistry. 30:2126–2133. [DOI] [PubMed] [Google Scholar]
- Nollert, P., H. Qiu, M. Caffrey, J. P. Rosenbusch, and E. M. Landau. 2001. Molecular mechanism for the crystallization of bacteriorhodopsin in lipidic cubic phases. FEBS Lett. 504:179–186. [DOI] [PubMed] [Google Scholar]
- Oradd, G., A.-S. Andersson, L. Rilfors, G. Lindblom, E. Strandberg, and P. Andrén. 2000. α-Methylene ordering of acyl chains differs in glucolipids and phosphatidylglycerol from Acholeplasma laidlawii membranes—2H NMR quadrupole splittings from individual lipids in mixed bilayers. Biochim. Biophys. Acta. 1468:329–344. [DOI] [PubMed] [Google Scholar]
- Pebay-Peyroula, E., G. Rummel, J. P. Rosenbusch, and E. M. Landau. 1997. X-ray structure of bacteriorhodopsin at 2.5 Å from microcrystals grown in lipidic cubic phases. Science. 277:1676–1681. [DOI] [PubMed] [Google Scholar]
- Qiu, H., and M. Caffrey. 2000. The phase diagram of the monoolein/water system. Equilibrium and metastability aspects. Biomaterials. 21:223–234. [DOI] [PubMed] [Google Scholar]
- Rummel, G., A. Hardmeyer, C. Widmer, M. L. Chiu, P. Nollert, K. P. Locher, I. I. Pedruzzi, E. M. Landau, and J. P. Rosenbusch. 1998. Lipidic cubic phases: new matrices for the three-dimensional crystallization of membrane proteins. J. Struct. Biol. 121:82–91. [DOI] [PubMed] [Google Scholar]
- Scherer, P. G., and J. Seelig. 1989. Electric charge effects on phospholipid headgroups. Phosphatidylcholine in mixtures with cationic and anionic amphiphiles. Biochemistry. 28:7720–7728. [DOI] [PubMed] [Google Scholar]
- Seelig, J. 1978. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim. Biophys. Acta. 515:105–140. [DOI] [PubMed] [Google Scholar]
- Seelig, J., and D. M. Macdonald. 1987. Phospholipids and proteins in biological membranes. 2H-NMR as method to study structures, dynamics and interactions. Acc. Chem. Res. 20:221–228. [Google Scholar]
- Schmidt, A., M. Wolde, C. Thiele, W. Fest, H. Kratzin, A. V. Podtelejnikov, W. Witke, W. B. Huttner, and H. D. Soling. 1999. Endophilin-I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature. 401:133–141. [DOI] [PubMed] [Google Scholar]
- Shu, J. L., Y. Yamashita, and M. Yamazaki. 2001. Effect of electrostatic interactions on phase stability of cubic phases of membranes of monoolein/dioleoylphosphatidic acid mixtures. Biophys. J. 8:983–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, I. C. P. 1989. Application of solid state NMR to the lipids of model and biological membranes. In NMR: Principal and Applications to Biomedical Research. J. W. Petergrew, editor. Springer-Verlag Inc., New York. pp. 124–156.
- Smith, I. C. P., and I. H. Ekiel. 1984. Phosphorus-31 NMR of phospholipids in membranes. In Phosphorus-31 NMR: Principle and Application. D. Gorenstein, editor. Academic Press, New York. pp. 447–475.
- Sternin, E., B. Fine, M. Bloom, C. P. Tilcock, K. F. Wong, and P. R. Cullis. 1988. Acyl chain orientational order in the hexagonal HII phase of phospholipid-water dispersions. Biophys. J. 54:689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij, A. J., R. de Maagd, J. Leunissen-Bijvelt, and B. de Kruijff. 1982. Divalent cations and chlorpromazine can induce non-bilayer structures in phosphatidic acid-containing model membranes. Biochim. Biophys. Acta. 684:255–262. [DOI] [PubMed] [Google Scholar]
- Weigert, R., M. G. Silletta, S. Spano, G. Turacchio, C. Cericola, A. Colanzi, S. Senatore, R. Mancini, E. V. Polishchuk, M. Salmona, F. Facchiano, K. N. Burger, A. Mironov, A. Luini, and D. Corda. 1999. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 402:429–433. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth, R., N. Waespe-Sarcevic, and J. Seelig. 1980. Bilayers of phosphatidylglycerol. A deuterium and phosphorous nuclear magnetic resonance study of the headgroup region. Biochemistry. 19:3315–3321. [DOI] [PubMed] [Google Scholar]










