Abstract
A multiparameter selection of Helicobacter pylori antigens for vaccine development identified 15 candidates, 6 of which are known protective antigens. Two novel antigens with low homology to other organisms (HP0231 and HP0410) were overexpressed and purified with high yields. Both confer protective immunity in the mouse Helicobacter infection model.
The gram-negative bacterium Helicobacter pylori is a widespread human pathogen that can cause gastritis, gastric and duodenal ulcers, and gastric cancer. In various preclinical animal models, vaccination has been shown to protect against a Helicobacter challenge infection (7). Most of the vaccines that have been tested contain only one or two antigens, but the results of recent studies suggest that combining several protective antigens can substantially increase vaccine efficacy (11, 22, 32). The two sequenced H. pylori genomes contain some 1,600 genes (2, 36), and appropriate parameters are needed to select a practical number of novel antigen candidates. In one study (10), more than 400 putative membrane- or surface-associated antigens were overexpressed, and about 100 of these could be obtained in sufficient yield and purity. When tested in the mouse Helicobacter infection model, 10 antigens were found to be protective, several of which had previously been identified by empirical approaches (10), suggesting that antigens can be identified in silico, although putative surface localization selection is a rather poor predictive parameter.
The results of studies with mice suggest that CD4+ T cells are essential for protection against an H. pylori infection, while both CD8+ T cells and antibodies appear to be dispensable (4, 9). Several parameters have been suggested as predictive indicators for the ability of a given antigen to induce potent CD4+ T-cell responses, but in most cases, little experimental data exist to directly support such assumptions for H. pylori proteins.
T-cell responses are dose dependent (37), suggesting that abundant H. pylori proteins may be appropriate antigen candidates. Abundant proteins in H. pylori in vitro cultures have recently been identified by proteome analysis (16). However, the in vitro conditions are not likely to accurately reproduce the relevant in vivo situation despite the finding that for three specific genes, relative protein abundance in vitro parallels transcript levels in human stomach biopsy specimens (16, 29). Qualitative information about antigen expression in vivo can be obtained from immunoproteomics (13, 19, 23). Specific recognition of a Helicobacter antigen by sera from infected patients or animals suggests that this antigen is expressed in vivo and is accessible to the immune system.
The localization of a bacterial antigen can influence specific T-cell responses. In a number of pathogens, surface-exposed antigens are thought to be more efficient in inducing a cellular immune response than cytoplasmic antigens (17, 34). H. pylori colonizes the mucous layer and the apical side of gastric epithelia cells, whereas CD4+ T cells that mediate protection reside in the mucosa. Secreted Helicobacter proteins and surface-associated proteins that are sequestered by vesicle budding are more likely to reach antigen-presenting cells in the mucosa for T-cell restimulation, as previously demonstrated for the best-characterized protective antigen urease (21). Indeed, the majority of known protective Helicobacter antigens are apparently surface exposed or secreted (35), and this property has been used with some success to predict novel antigens (10). Sixty-four putative surface-exposed proteins have been theoretically predicted for H. pylori (1). In addition, selective labeling followed by proteome analysis revealed 18 surface-associated proteins (30), and analysis of culture supernatants revealed 23 secreted proteins (5).
Isolates of H. pylori are genetically diverse (3, 31), and vaccines should preferably contain antigens that are highly conserved among different strains. The complete genome sequences of two independent strains and genetic information about various specific loci in multiple strains provide the necessary information to select conserved antigens.
The binding affinities of peptides to major histocompatibility complex class II molecules on antigen-presenting cells can be predicted on the basis of empirical data sets containing known T-cell epitopes (6, 24). Proteins that contain peptides with high theoretical T-cell epitope scores are likely to induce potent CD4+ T-cell responses.
As most of the various selection parameters for protective H. pylori antigens are rather tentative, we combined them to select potential antigen candidates, assuming that most of the criteria have at least some relevance. At least 59 antigens are recognized by H. pylori-infected patients (13, 19, 23), and 48 of these antigens have a staining intensity that is higher than an arbitrary cutoff equivalent to 0.1% of the total staining intensity (16) (Table 1). Among the 48 seroreactive and abundant antigens, 15 appear to be secreted or surface associated (1, 5, 30), and almost all are present in all 15 isolates analyzed (except Cag26) and contain at least one putative T-cell epitope (Table 1). Interestingly, this set of 15 potential Helicobacter antigens contains six proteins that have already been shown to be highly protective in the mouse infection model, supporting the utility of our selection strategy. Immunization trials with a large set of antigen candidates will be required to validate each of the presently used and other potential selection parameters to further improve the approach. Moreover, the different data sets are still incomplete, and there are probably more antigen candidates. However, the already achieved high selection success rate motivated us to further characterize some of the new candidates.
TABLE 1.
Multiparameter selection of H. pylori antigens
| Antigen | Genea | Seroreactivityb,c | Staining intensityc,d | Surface localizationc,e | Distribution in 15 isolatesc,f | Predicted T-cell epitopesc,g | Homology to other organismsh | Protection |
|---|---|---|---|---|---|---|---|---|
| HP0010 | groEL | + | 5,596 | Surf. | All genomes | 5 | Strong (1.6e − 193) | Yes (11) |
| HP0011 | groES | + | 1,477 | ND | NA | NA | NA | NA |
| HP0027 | icd | + | 683 | ND | NA | NA | NA | NA |
| HP0072 | ureB | + | 3,544 | Surf. | All genomes | 3 | Strong (6.4e − 158) | Yes (25) |
| HP0073 | ureA | + | 1,774 | Surf. | All genomes | 2 | Strong (3e − 199) | Yes (25) |
| HP0109 | dnaK | + | 177 | Surf. | All genomes | 8 | Strong (4.9e − 57) | NA |
| HP0115 | flaB | + | <100 | ND | NA | NA | NA | NA |
| HP0153 | recA | + | 209 | ND | NA | NA | NA | NA |
| HP0154 | eno | + | 214 | ND | NA | NA | NA | NA |
| HP0175 | cell binding factor 2 gene | + | 655 | Sec., Surf. | All genomes | 3 | Strong (9.3e − 31) | NA |
| HP0177 | efp | + | 340 | ND | NA | NA | NA | NA |
| HP0192 | frdA | + | 264 | ND | NA | NA | NA | NA |
| HP0231 | Hypo. ORF | + | 531 | Sec., Surf. | All genomes | 3 | Weak (1.8e − 06) | Yes (this study) |
| HP0243 | napA | + | 673 | Surf. | All genomes | 1 | Strong (3.5e − 22) | Yes (33) |
| HP0264 | clpB | + | 241 | ND | NA | NA | NA | NA |
| HP0305 | Hypo. ORF | + | 320 | ND | NA | NA | NA | NA |
| HP0318 | Cons. hypo. ORF | + | 227 | ND | NA | NA | NA | NA |
| HP0371 | fabE | + | 340 | ND | NA | NA | NA | NA |
| HP0400 | lytB | + | 130 | ND | NA | NA | NA | NA |
| HP0410 | hpaA homologue | + | 415 | Surf. | All genomes | 4 | None | Yesi (this study) |
| HP0512 | glnA | + | 397 | ND | NA | NA | NA | NA |
| HP0522 | cag3 | + | 93 | ND | NA | NA | NA | NA |
| HP0537 | cag16 | + | 76 | ND | NA | NA | NA | NA |
| HP0547 | cag26 | + | 1,194 | Sec. | 11 of 15 | 6 | Weak (0.00012) | Yes (22) |
| HP0589 | Ferredoxin oxidoreductase/gene | + | 263 | ND | NA | NA | NA | NA |
| HP0599 | hylB | + | 302 | ND | NA | NA | NA | NA |
| HP0601 | flaA | + | 450 | Surf. | All genomes | 3 | Strong (4.7e − 91) | NA |
| HP0649 | aspA | + | 340 | ND | NA | NA | NA | NA |
| HP0691 | yxjD | + | 237 | ND | NA | NA | NA | NA |
| HP0752 | fliD | + | 230 | ND | NA | NA | NA | NA |
| HP0779 | acnB | + | 843 | ND | NA | NA | NA | NA |
| HP0794 | clpP | + | 237 | ND | NA | NA | NA | NA |
| HP0795 | tig | + | 450 | ND | NA | NA | NA | NA |
| HP0829 | quaB | + | 212 | ND | NA | NA | NA | NA |
| HP0875 | Catalase gene | + | 1,021 | Surf. | All genomes | 2 | Strong (9.4e − 157) | Yes (28) |
| HP0900 | hypB | + | 341 | ND | NA | NA | NA | NA |
| HP0912 | omp20 | + | 150 | Surf. | NA | NA | NA | NA |
| HP1018 | Hypo. ORF | + | 150 | ND | NA | NA | NA | NA |
| HP1019 | htrA | + | 603 | Sec., Surf. | All genomes | 4 | Strong (2.3e − 91) | NA |
| HP1037 | pepO | + | 213 | ND | NA | NA | NA | NA |
| HP1098 | Cons. hypo. secreted ORF | + | 646 | Surf. | All genomes | 6 | Strong (1.7e − 36) | NA |
| HP1110 | Pyruvate ferredoxin oxidoreductase gene | + | 250 | ND | NA | NA | NA | NA |
| HP1125 | omp18 | + | 900 | Surf. | All genomes | 1 | Strong (1.3e − 23) | NA |
| HP1132 | atpD | + | 171 | ND | NA | NA | NA | NA |
| HP1134 | atpA | + | 162 | ND | NA | NA | NA | NA |
| HP1152 | ffh | + | 124 | ND | NA | NA | NA | NA |
| HP1199 | rpl7/l12 | + | 915 | ND | NA | NA | NA | NA |
| HP1201 | rlpA | + | 398 | ND | NA | NA | NA | NA |
| HP1205 | tufB | + | 727 | ND | NA | NA | NA | NA |
| HP1285 | Cons. hypo. ORF | + | 59 | ND | NA | NA | NA | NA |
| HP1293 | rpoA | + | 273 | ND | NA | NA | NA | NA |
| HP1302 | recA | + | 603 | ND | NA | NA | NA | NA |
| HP1307 | rplE | + | 397 | ND | NA | NA | NA | NA |
| HP1350 | Protease gene | + | 154 | ND | NA | NA | NA | NA |
| HP1555 | tsf | + | 251 | ND | NA | NA | NA | NA |
| HP1563 | tsaA | + | 2,122 | ND | NA | NA | NA | NA |
| HP1564 | omp | + | 434 | Surf. | All genomes | 3 | Strong (5.4e − 58) | NA |
| HP1582 | pdxJ | + | 355 | ND | NA | NA | NA | NA |
Abbreviations: Hypo., hypothetical; ORF, open reading frame; Cons., conserved.
The particular criteria matching proteins are boxed.
Arbitrary units as determined from spot intensities of H. pylori two-dimensional gels using the TOPSPOT program (16). An arbitrary threshold of 175 equivalent to 0.1% of the total staining intensity was used.
Data from references 1, 5, 15, 26, 27, and 30. Abbreviations: Sec., secreted; Surf., surface; ND, not detected.
Presence as determined by DNA-microarray hybridization (31). NA, not analyzed.
Murine H-2d major histocompatibility complex class II-restricted T-cell epitopes were predicted by comparing the amino acid sequence to those of known T-cell epitopes (20); the number of nonoverlapping epitopes with a predicted score equal or higher than that of the well-defined ovalbumin T-cell epitope (amino acids 323 to 339) are given. NA, not analyzed.
Homology (P value) for the closest non-Helicobacter homologue in the Comprehensive Microbial Database (at http://www.tigr.org). A P value below 1.0 × 10−20 is called strong homology. A P value of 0.001 to 1.0 × 10−20 is called weak homology. NA, not analyzed.
If present in challenge strain.
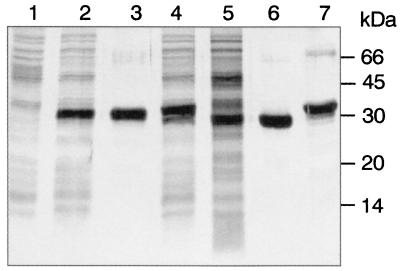
To identify novel attractive antigens with minimal cross-reactivity, we selected three candidates with weak homology to other organisms (Table 1) (homology derived from the Comprehensive Microbial Database at http://www.tigr.org): the hypothetical protein HP0231, the putative neuraminyllactose-binding hemagglutinin HpaA homologue HP0410, and the hypothetical secreted protein HP1098 that was later found to have a homologue with high similarity in Magnetococcus sp. strain MC-1. The corresponding genes were PCR amplified from chromosomal DNA from strain P76 (12) using the primers shown in Table 2, and cloned into pET15b (Novagen). The His6-tagged proteins were overexpressed in Escherichia coli BL21(DE3) and purified by cobalt affinity chromatography. HP0231 and HP0410 could be recovered from inclusion bodies of induced E. coli cultures at high purity and yields (Fig. 1). Interestingly, a soluble form of HP0410 was also recovered from culture supernatants. The soluble form has a somewhat lower apparent molecular weight compared to that of the insoluble form and may have been processed by signal peptide cleavage. In contrast to HP0231 and HP0410, HP1098 was only weakly expressed in E. coli even under inducing conditions and was therefore not investigated further.
TABLE 2.
Oligonucleotide primers used
| Antigen | Primer direction | Sequence (5′ to 3′)a |
|---|---|---|
| HP0231 | Forward | TTAGGAGTTCATATGATATTAAGAGC |
| Reverse | GCGATATCGGATCCGTCGACTAATGATGATGATGATGATGTGCCTTATAATGGTATAAGAAA | |
| HP0410 | Forward | GAAAGGAATCATATGAAAAAAGGT |
| Reverse | GCGATATCGGATCCGTCGACTAATGATGATGATGATGATGCTTTCGTTTTTTCATTTCAC | |
| HP1098 | Forward | AGGAGATACCATATGTTAGAAAATGTC |
| Reverse | GCGATATCGGATCCGTCGACTAATGATGATGATGATGATGAACTTTGATCTTAAGCTGCTT |
Underlined sequence regions indicate NdeI and BamHI recognition sites used for cloning.
FIG. 1.
Expression and purification of recombinant H. pylori antigens as analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining. Lane 1, E. coli BL21; lane 2, E. coli BL21 expressing HP0231; lane 3, purified HP0231 from inclusion bodies; lane 4, E. coli BL21 expressing HP0410; lane 5, supernatant of E. coli BL21 expressing HP0410; lane 6, purified HP0410 from supernatant; lane 7, purified HP0410 from inclusion bodies. The positions of molecular mass markers (in kilodaltons) are indicated to the right of the gel.
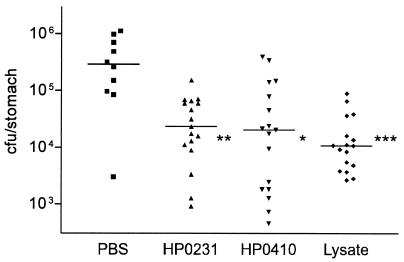
The HP0231 and HP0410 antigens were individually tested for protective efficacy in groups of 5 to 10 female 6- to 8-week-old female BALB/c mice with specific-pathogen-free health status using four orogastric administrations (days 0, 21, 28, and 35) of 100 μg of purified protein in 100 μl of phosphate-buffered saline (PBS) containing 10 μg of the mucosal adjuvant cholera toxin. Four to six weeks after the last immunization, the mice were challenged with one or three orogastric doses of 2 × 108 to 5 × 108 CFU of the mouse-adapted H. pylori strain P76; four to six weeks later, the mice were sacrificed under anesthesia, and H. pylori stomach load and urease activity were determined as described previously (12). Compared to the sham-immunized control group, mice that had received HP0231 or HP0410 were protected against an H. pylori challenge infection (Fig. 2), with levels of protection (median CFU of 8% compared to that for the controls) equivalent to previous results for the best known antigens (8, 11, 14, 18, 25, 28, 32, 35) and approximating those of an immunization control group that had received four doses of 500 μg of P76 lysate, which is generally considered the gold standard for Helicobacter immunization (median CFU of 4% compared to that for the controls) (Fig. 2). There was no significant difference in protective efficacy between soluble and insoluble forms of HP0410 (data not shown). The protective effect of immunization with HP0231 or HP0410 was also evident from determinations of urease activity in the stomach samples (P > 0.0001 [t test] for both proteins; data not shown). Immunization with HP0231, but not with HP0410, induced specific serum antibodies that could be detected both by Western blotting and enzyme-linked immunosorbent assay (P > 0.005 [t test] compared to sham-immunized control group; data not shown). This suggests that serum antibody responses do not correlate with protective efficacy, which is in agreement with the results of previous studies (4, 9).
FIG. 2.
Murine H. pylori stomach loads after oral immunization and an H. pylori P76 challenge. Combined results from two independent vaccination experiments are shown (a total of 10 mice tested for PBS; a total of 17 mice tested (each) for HP0231, HP0410, and lysate). The horizontal lines represent medians. Statistically significant differences compared to the values for the sham-immunized control group (PBS) were analyzed with the t test (∗, P < 0.005; ∗∗, P < 0.001; ∗∗∗, P < 0.0005).
In conclusion, a combination of theoretical and experimental selection parameters predicts protective H. pylori antigens with a success rate (at least 8 of 15 predicted antigens) that is superior to previous attempts. Two novel antigens identified in this study have protective efficacies similar to those of the best previously known antigens and, unlike most other protective antigens, are highly specific for Helicobacter. Further studies will help to validate individual selection parameters for further improvement of the combination approach.
Acknowledgments
We thank T. Aebischer for helpful discussions.
This study was supported in part by the Deutsche Forschungsgemeinschaft (Me 756/6-1).
Editor: E. I. Tuomanen
REFERENCES
- 1.Alm, R. A., J. Bina, B. M. Andrews, P. Doig, R. E. Hancock, and T. J. Trust. 2000. Comparative genomics of Helicobacter pylori: analysis of the outer membrane protein families. Infect. Immun. 68:4155-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 3.Alm, R. A., and T. J. Trust. 1999. Analysis of the genetic diversity of Helicobacter pylori: the tale of two genomes. J. Mol. Med. 77:834-846. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard, T. G., S. J. Czinn, R. W. Redline, N. Sigmund, G. Harriman, and J. G. Nedrud. 1999. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell. Immunol. 191:74-80. [DOI] [PubMed] [Google Scholar]
- 5.Bumann, D., S. Aksu, M. Wendland, K. Janek, U. Zimny-Arndt, N. Sabarth, T. F. Meyer, and P. R. Jungblut. 2002. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun. 70:3396-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davenport, M. P., I. A. Ho Shon, and A. V. Hill. 1995. An empirical method for the prediction of T-cell epitopes. Immunogenetics 42:392-397. [DOI] [PubMed] [Google Scholar]
- 7.Del Giudice, G., A. Covacci, J. L. Telford, C. Montecucco, and R. Rappuoli. 2001. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 19:523-563. [DOI] [PubMed] [Google Scholar]
- 8.Dunkley, M. L., S. J. Harris, R. J. McCoy, M. J. Musicka, F. M. Eyers, L. G. Beagley, P. J. Lumley, K. W. Beagley, and R. L. Clancy. 1999. Protection against Helicobacter pylori infection by intestinal immunisation with a 50/52-kDa subunit protein. FEMS Immunol. Med. Microbiol. 24:221-225. [DOI] [PubMed] [Google Scholar]
- 9.Ermak, T. H., P. J. Giannasca, R. Nichols, G. A. Myers, J. Nedrud, R. Weltzin, C. K. Lee, H. Kleanthous, and T. P. Monath. 1998. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J. Exp. Med. 188:2277-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrero, R. L., and A. Labigne. 2001. Helicobacter pylori vaccine development in the post-genomic era: can in silico translate to in vivo? Scand. J. Immunol. 53:443-448. [DOI] [PubMed] [Google Scholar]
- 11.Ferrero, R. L., J. M. Thiberge, I. Kansau, N. Wuscher, M. Huerre, and A. Labigne. 1995. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc. Natl. Acad. Sci. USA 92:6499-6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Duarte, O. G., B. Lucas, Z. X. Yan, K. Panthel, R. Haas, and T. F. Meyer. 1998. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine 16:460-471. [DOI] [PubMed] [Google Scholar]
- 13.Haas, G., G. Karaali, K. Ebermayer, W. G. Metzger, S. Lamer, U. Zimny-Arndt, S. Diescher, U. B. Goebel, K. Vogt, A. B. Roznowski, B. J. Wiedenmann, T. F. Meyer, T. Aebischer, and P. Jungblut. 2002. Immunoproteomics of Helicobacter pylori infection and relation to gastric disease. Proteomics 2:313-324. [DOI] [PubMed] [Google Scholar]
- 14.Hocking, D., E. Webb, F. Radcliff, L. Rothel, S. Taylor, G. Pinczower, C. Kapouleas, H. Braley, A. Lee, and C. Doidge. 1999. Isolation of recombinant protective Helicobacter pylori antigens. Infect. Immun. 67:4713-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huesca, M., S. Borgia, P. Hoffman, and C. A. Lingwood. 1996. Acidic pH changes receptor binding specificity of Helicobacter pylori: a binary adhesion model in which surface heat shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect. Immun. 64:2643-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jungblut, P. R., D. Bumann, G. Haas, U. Zimny-Arndt, P. Holland, S. Lamer, F. Siejak, A. Aebischer, and T. F. Meyer. 2000. Comparative proteome analysis of Helicobacter pylori. Mol. Microbiol. 36:710-725. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann, S. H., and J. Hess. 1999. Impact of intracellular location of and antigen display by intracellular bacteria: implications for vaccine development. Immunol. Lett. 65:81-84. [DOI] [PubMed] [Google Scholar]
- 18.Kim, B. O., S. S. Shin, Y. H. Yoo, and S. Pyo. 2001. Peroral immunization with Helicobacter pylori adhesin protein genetically linked to cholera toxin A2B subunits. Clin. Sci. 100:291-298. [PubMed] [Google Scholar]
- 19.Kimmel, B., A. Bosserhoff, R. Frank, R. Gross, W. Goebel, and D. Beier. 2000. Identification of immunodominant antigens from Helicobacter pylori and evaluation of their reactivities with sera from patients with different gastroduodenal pathologies. Infect. Immun. 68:915-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas, B., D. Bumann, A. Walduck, J. Koesling, L. Develioglu, T. F. Meyer, and T. Aebischer. 2001. Adoptive transfer of CD4+ T cells specific for subunit A of Helicobacter pylori urease reduces H. pylori stomach colonization in mice in the absence of interleukin-4 (IL-4)/IL-13 receptor signaling. Infect. Immun. 69:1714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mai, U. E., G. I. Perez-Perez, J. B. Allen, S. M. Wahl, M. J. Blaser, and P. D. Smith. 1992. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J. Exp. Med. 175:517-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchetti, M., B. Arico, D. Burroni, N. Figura, R. Rappuoli, and P. Ghiara. 1995. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science 267:1655-1658. [DOI] [PubMed] [Google Scholar]
- 23.McAtee, C. P., M. Y. Lim, K. Fung, M. Velligan, K. Fry, T. Chow, and D. E. Berg. 1998. Identification of potential diagnostic and vaccine candidates of Helicobacter pylori by two-dimensional gel electrophoresis, sequence analysis, and serum profiling. Clin. Diagn. Lab. Immunol. 5:537-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meister, G. E., C. G. Roberts, J. A. Berzofsky, and A. S. De Groot. 1995. Two novel T cell epitope prediction algorithms based on MHC-binding motifs: comparison of predicted and published epitopes from Mycobacterium tuberculosis and HIV protein sequences. Vaccine 13:581-591. [DOI] [PubMed] [Google Scholar]
- 25.Michetti, P., I. Corthesy-Theulaz, C. Davin, R. Haas, A. C. Vaney, M. Heitz, J. Bille, J. P. Kraehenbuhl, E. Saraga, and A. L. Blum. 1994. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology 107:1002-1011. [DOI] [PubMed] [Google Scholar]
- 26.Namavar, F., M. Sparrius, E. C. Veerman, B. J. Appelmelk, and C. M. Vandenbroucke-Grauls. 1998. Neutrophil-activating protein mediates adhesion of Helicobacter pylori to sulfated carbohydrates on high-molecular-weight salivary mucin. Infect. Immun. 66:444-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phadnis, S. H., M. H. Parlow, M. Levy, D. Ilver, C. M. Caulkins, J. B. Connors, and B. E. Dunn. 1996. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect. Immun. 64:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radcliff, F. J., S. L. Hazell, T. Kolesnikow, C. Doidge, and A. Lee. 1997. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect. Immun. 65:4668-4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rokbi, B., D. Seguin, B. Guy, V. Mazarin, E. Vidor, F. Mion, M. Cadoz, and M. J. Quentin-Millet. 2001. Assessment of Helicobacter pylori gene expression within mouse and human gastric mucosae by real-time reverse transcriptase PCR. Infect. Immun. 69:4759-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabarth, N., D. Bumann, S. Lamer, U. Zimny-Arndt, P. Jungblut, and T. F. Meyer. 2002. Identification of surface-exposed proteins of Helicobacter pylori by selective biotinylation, affinity purification, and two-dimensional gel electrophoresis. J. Biol. Chem. 277:27896-27902. [DOI] [PubMed] [Google Scholar]
- 31.Salama, N., K. Guillemin, T. K. McDaniel, G. Sherlock, L. Tompkins, and S. Falkow. 2000. A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc. Natl. Acad. Sci. USA 97:14668-14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez, V., S. Gimenez, J. Haensler, C. Geoffroy, B. Rokbi, D. Seguin, L. Lissolo, B. Harris, F. Rizvi, H. Kleanthous, T. Monath, M. Cadoz, and B. Guy. 2001. Formulations of single or multiple H. pylori antigens with DC Chol adjuvant induce protection by the systemic route in mice. Optimal prophylactic combinations are different from therapeutic ones. FEMS Immunol. Med. Microbiol. 30:157-165. [DOI] [PubMed] [Google Scholar]
- 33.Satin, B., G. Del Giudice, V. Della Bianca, S. Dusi, C. Laudanna, F. Tonello, D. Kelleher, R. Rappuoli, C. Montecucco, and F. Rossi. 2000. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J. Exp. Med. 191:1467-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen, H., J. F. Miller, X. Fan, D. Kolwyck, R. Ahmed, and J. T. Harty. 1998. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell 92:535-545. [DOI] [PubMed] [Google Scholar]
- 35.Sutton, P. 2001. Progress in vaccination against Helicobacter pylori. Vaccine 19:2286-2290. [DOI] [PubMed] [Google Scholar]
- 36.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 37.Zinkernagel, R. M., S. Ehl, P. Aichele, S. Oehen, T. Kundig, and H. Hengartner. 1997. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol. Rev. 156:199-209. [DOI] [PubMed] [Google Scholar]