Abstract
Using a mean-field analysis we derive a consistent model for the perturbation of a symmetric polymeric bilayer due to the incorporation of transmembrane proteins, as a function of the polymer molecular weight and the protein dimensions. We find that the mechanism for the inhibition of protein incorporation in polymeric bilayers differs from that of their inclusion in polymer-carrying lipid vesicles; in polymersomes, the equilibrium concentration of transmembrane proteins decreases as a function of the thickness mismatch between the protein and the bilayer core, whereas in liposomes the presence of polymer chains affects the protein adsorption kinetics. Despite the increased stiffness of polymer bilayers (when compared to lipid ones), their perturbation decay length and range of protein-protein interaction is found to be relatively long. The energetic penalty due to protein adsorption increases relatively slowly as a function of the polymer chain length due to the self-assembled nature of the polymer bilayer. As a result, we predict that transmembrane proteins may be incorporated in significant numbers even in bilayers where the thickness mismatch is large.
INTRODUCTION
To succeed, innovative drug therapies require both effective drugs and a reliable delivery mechanism. One such vehicle is lipid-based vesicles, or liposomes, where an internal aqueous core solubilizing hydrophilic drugs is protected from the environment by a relatively impermeable amphiphile bilayer. Delivery characteristics such as drug release rate (and possibly site) are related to the bilayer properties, and may therefore be controlled to some degree by the type of lipid used.
Proteins are the active component of biomembranes (see, for example, Gennis, 1989). Their inclusion in synthetic membranes such as those of vesicle-based drug carriers imparts favorable, or unfavorable, functionalization. For example, the adsorption of immunoproteins (e.g. immunoglobulins) must be suppressed, since they enable recognition by reticuloendothelial cells which mediate the clearance process (Devine and Marjan, 1997; Semple et al., 1998). On the other hand, the stable incorporation of polyethylene glycol (PEG) carrying lipids is favored, since it has been shown to greatly enhance their efficacy (Klibanov et al., 1990; Allen et al., 1991; Mori et al., 1991; Maruyama et al., 1991; Semple et al., 1998).
Several studies examined the incorporation of proteins or large molecules into lipid bilayers (see, for example, Cladera et al., 1997; Rigaud et al., 1988; Parmar et al., 1999; Zhelev et al., 2001; Kahya et al., 2001). The equilibrium concentration of proteins in the bilayer, their arrangement (aggregation/dispersion) and their functions was shown to depend on the bilayer characteristics. For example, Keller et al. (1993) and Chen et al. (2002) found that transport through alamethicin ion channels is significantly affected by the bilayer composition. The interactions between membrane proteins and their aggregation behavior have been linked to the membrane properties (see, for example, Mouritsen, 1998 and references within). The equilibrium concentration and interactions between inclusions also depend on the inclusion properties. For example, the chain length of polyethylene glycol (PEG) has been found to significantly affect the saturation concentration of PEG-lipids in lipid bilayers (Shimada et al., 2000; Bradley et al., 1998; Montesano et al., 2001).
Theoretical analysis of protein or large molecule incorporation in self-assembled lipid bilayers has shown that the inclusions perturb the local bilayer structure, thereby giving rise to an energetic penalty whose magnitude depends on the induced deformation, as well as on the bilayer stiffness and curvature moduli (see, for example, Dan et al., 1993; Dan and Safran, 1995; Fattal and Ben-Shaul, 1995; Aranda-Espinoza et al., 1996; Cantor, 1997, 2002; May, 2000; Bezrukov, 2000; Maddox and Longo, 2002).
Recently, interest has focused on polymeric vesicles, composed of hydrophobic-hydrophilic diblock copolymers, as drug delivery vehicles (Cho and Kim, 1998; Discher et al., 1999, 2000; Brown et al., 2000; Dufes et al., 2000; Wang et al., 2001; Bermudez et al., 2002). The advantages of these polymersomes, as compared to liposomes, include enhanced mechanical stability and greater flexibility to tailor bilayer characteristics such as thickness and chemical composition (Discher et al., 1999, 2002; Lee et al., 2001; Discher and Eisenberg, 2002; Dimova et al., 2002). Moreover, it has been speculated that protein (and ligand) interactions with the polymeric bilayers will greatly differ from their interactions with lipid ones, thereby affecting drug delivery characteristics such as circulation time in vivo. This is based on observations made for lipid bilayers carrying PEG chains (stealth liposomes), where the polymer was shown to slow the kinetics of protein adsorption (Needham et al., 1992; Woodle et al., 1994; Storm et al., 1995; Szleifer, 1997a,b,c; Satulovsky et al., 2000, Efremova et al., 2000); thereby increasing the liposome circulation time in vivo (Storm and Crommelin, 1998; Allen et al., 1991; Blume and Cevc, 1990; Klibanov et al.,1990). However, to date little is known regarding protein adsorption into purely polymeric bilayers.
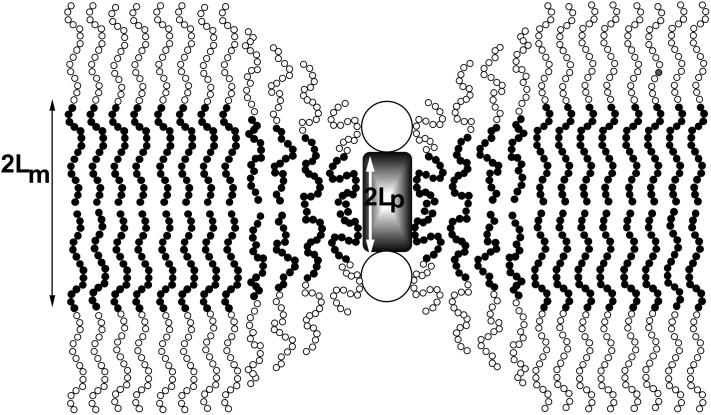
In this article we develop a model for transmembrane protein adsorption into polymeric bilayers composed of symmetric diblock copolymers, as a function of the polymer chain length and the protein dimensions. In the dilute limit, where direct protein-protein interactions are negligible, the fraction of proteins in the membrane depends both on protein hydrophobicity (equivalent to the bare surface adsorption energy) and on membrane-protein coupling. The latter arises from the protein-imposed perturbation of local membrane structure, due to the thickness mismatch between the hydrophobic protein regions and the unperturbed membrane (see Fig. 1).
FIGURE 1.
Conformation of polymer chains near an inclusion in a polymeric bilayer: 2Lm in the thickness of a flat bilayer, 2Lp the inclusion thickness and z is the distance from the inclusion boundary. The chains in the unperturbed bilayer are highly stretched (LmaN2/3, where N is the number of segments and a the segment length). As a result, the requirement to match the thickness of much shorter proteins can be achieved without undergoing significant compression when compared to the free chain radius of gyration. For example, a polymer chain with N = 1000 will have an unperturbed radius of gyration that scales as 30a, but can stretch to order 100a in the bilayer. Thus, matching a protein whose thickness is half that of the bilayer (50a) is easily obtained.
Why should the incorporation of transmembrane proteins in polymeric bilayers differ from their incorporation in lipid, or polymer-carrying lipid, bilayers? Nature has engineered the dimensions of transmembrane proteins to match the core thickness of the lipid bilayers, i.e., of order 4 nm. As a result, the presence of transmembrane proteins does not significantly perturb the hydrophobic lipid bilayer core, whether hydrophilic polymer chains are attached or not. The observed inhibition of protein adsorption onto stealth liposomes (Needham et al., 1992; Woodle et al., 1994; Storm et al., 1995; Szleifer, 1997a,b,c; Satulovsky et al., 2000, Efremova et al., 2000) is due to a polymer-induced slowing of adsorption kinetics.
On the other hand, the thickness of polymeric bilayers is several times that of lipid ones (see, for example, Bermudez et al., 2002), so that transmembrane proteins may deform the bilayer significantly (see Fig. 1). The energetic penalty arising from this deformation is expected to reduce the equilibrium concentration of transmembrane proteins in the bilayer (note that, because of the presence of the hydrophilic block in these systems, polymeric bilayers are expected to display a slowdown of adsorption kinetics similar to that of stealth liposomes).
Indeed, we find that there are qualitative differences between transmembrane protein incorporation in lipid bilayers and polymeric ones. The most significant one is that, as speculated, there is an increase in the perturbation energy of the bilayer (for a given protein) as a function of the bilayer molecular weight. However, this increase depends on the chain length in a weaker fashion than expected, due to the self-assembled nature of the polymeric bilayer. We also find that the range of the perturbation profile away from the protein increases with chain length, despite the fact that so does the bending modulus, and that, despite our use of a linear perturbation model the penalty for bilayer perturbation is asymmetric with respect to the degree of perturbation. The deformation energy for stretching is smaller than for comparable compression. The concentration of any given protein is predicted to be maximal in bilayers where there is no thickness mismatch. However, due to the protein/solution interactions, in any given bilayer the protein whose concentration is highest is one whose thickness exceeds that of the bilayer.
MODEL
We focus here on systems where the concentration of transmembrane proteins embedded in the polymeric membrane is low, so that direct interactions between the proteins may be neglected. Consider a membrane section containing a protein (Fig. 1). The coupling between the hydrophobic protein core and the bilayer can be translated into a geometrical boundary condition for the perturbation on the bilayer thickness at the protein/bilayer boundary. For simplicity we assume that the protein width is relatively large, thereby allowing us to apply a one-dimensional analysis. Although this assumption is obviously unrealistic, previous analysis has shown that the one-dimensional model yields qualitatively and quantitatively similar results to that of the two-dimensional one in liposome-protein systems (Aranda-Espinoza et al., 1996). By symmetry, the two monolayers constituting the bilayer are equivalent, so that we may limit our analysis to one monolayer. Also, we assume that the system contains only one type of polymer, and no cosurfactants. The polymer is taken to be symmetric, so that the number and length of the hydrophilic segments is identical to that of the hydrophobic ones thereby allowing us to assume the spontaneous curvature is zero (see, for example, Wang and Safran, 1991). Although this is a clearly idealized case, it captures all the significant features of diblock copolymer assemblies (see Halperin et al., 1992) and is not expected to affect, qualitatively, transmembrane protein incorporation (Dan and Safran, 1995). The free energy of a protein embedded in a membrane has two contributions. The first one is associated with the transition of the protein from the aqueous to the membrane environment, similar to the local, chemical adsorption energy of a molecule on a solid surface. It is a function of the inclusion hydrophobicity and possible changes in inclusion conformation in the membrane environment. The second contribution arises from the membrane-protein coupling. When a molecule adsorbs on a solid surface, the surface structure remains, to a large extent, unchanged. However, the membrane is a self-assembled structure, so that the membrane deformation and subsequent energy gain or loss due to the protein-induced deformation must be taken into account.
The equilibrium bilayer is locally flat and is composed of two identical monolayers characterized by a thickness Lm which is coupled to a surface density of 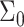 (area per molecule) through an equation of state. In this analysis we take this equation of state to be the condition of incompressibility of the hydrophobic copolymer, so that
(area per molecule) through an equation of state. In this analysis we take this equation of state to be the condition of incompressibility of the hydrophobic copolymer, so that  where
where 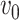 is a segment volume and N is the number of segments. The energy per protein (per unit width) is then given by
is a segment volume and N is the number of segments. The energy per protein (per unit width) is then given by
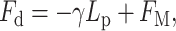 |
(1) |
where γ is the surface tension of the protein hydrophobic region, describing the energy difference (per unit area) between the protein in solution and embedded in the bilayer. Lp is the protein height, and FM is the protein-induced membrane perturbation energy, given by (Dan et al., 1993)
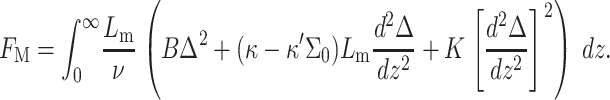 |
(2) |
This energy is a function of the local deformation of the membrane, which is characterized by a dimensionless change in its thickness, relative to the equilibrium thickness. The deformation is defined by  where
where 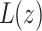 is the local thickness of the perturbed monolayer and z the distance from the protein boundary. Δ0 defines the perturbation at the protein boundary, equal to the normalized difference between the protein and bilayer thickness. ν is the volume of the hydrophobic block, given by Nνo. B is the monolayer compressibility, namely, the energetic cost associated with perturbation of the area per chain from
is the local thickness of the perturbed monolayer and z the distance from the protein boundary. Δ0 defines the perturbation at the protein boundary, equal to the normalized difference between the protein and bilayer thickness. ν is the volume of the hydrophobic block, given by Nνo. B is the monolayer compressibility, namely, the energetic cost associated with perturbation of the area per chain from 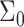 (Each monolayer is assumed to bend with the same sign of the curvature, in distinction to the usual bending modes of a bilayer where the two monolayers have nearly equal and opposite curvatures). The monolayer spontaneous curvature,
(Each monolayer is assumed to bend with the same sign of the curvature, in distinction to the usual bending modes of a bilayer where the two monolayers have nearly equal and opposite curvatures). The monolayer spontaneous curvature, 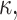 determines the sign and magnitude of the free interface curvature of the monolayer at a water-oil interface. The change in spontaneous curvature as a function of density is given by κ′ = ∂κ/∂Σ, evaluated at the equilibrium bilayer surface density Σ0. K is the bending modulus, i.e., the energetic penalty for bending the monolayer. All energies are given in units of kT, where k is the Boltzmann coefficient and T the temperature, and all length scales are dimensionless, normalized by Lm.
determines the sign and magnitude of the free interface curvature of the monolayer at a water-oil interface. The change in spontaneous curvature as a function of density is given by κ′ = ∂κ/∂Σ, evaluated at the equilibrium bilayer surface density Σ0. K is the bending modulus, i.e., the energetic penalty for bending the monolayer. All energies are given in units of kT, where k is the Boltzmann coefficient and T the temperature, and all length scales are dimensionless, normalized by Lm.
In the case of symmetrical copolymers, the spontaneous curvature and its derivative are zero. B and K are related to the copolymer chain length through the relationship (Milner and Witten, 1988; Dan et al., 1993)
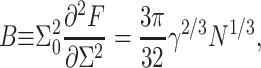 |
(3a) |
 |
(3b) |
where N is the number of segments in each block, a is the segment length, γ is the surface tension between the hydrophilic and hydrophobic regions (assumed to be similar in magnitude to the surface tension between the protein hydrophobic region and the solution, given in Eq. 1), and Σ0 is the equilibrium surface density. Here we adapt the original Milner and Witten (1988) analysis (developed for polymer chains that are end grafted at a fixed density to a solid substrate in the melt state, and assuming that the grafting density is in the relatively high brush regime) to the polymersomes self assembled system. Briefly, using scaling terminology, this is done by considering the free energy of a symmetrical, flat bilayer interface which can be written as γΣ + 2N/Σ2. The first term accounts for the interfacial tension, the second for the polymer stretching energy. Minimization of this energy with respect to Σ will yield the optimal surface area per chain; Σ0 ∼ (N/γ)1/3, the layer thickness scales as aγ1/3N2/3, and the free energy as γΣ0 ∼ γ2/3N1/3.
Does the Milner and Witten (1988) modified model work for polymeric bilayers? To answer this, we can examine the recently published data of Bermudez et al., (2002) who examined the moduli of polymeric bilayers. They find that the force (or tension) per unit area, required to perturb a polymeric bilayer from its equilibrium value is proportional to the surface tension, and is independent of chain length. This is in agreement with our discussion above, where the energy per unit area is predicted to scale as γ(Σ − Σ0)/Σ0. Moreover, Bermudez et al., (2002) find that the hydrophobic core thickness scales as the chain length to a power of order 1/2 (although a fit of a 2/3 power works quite well too), in general agreement with the model predictions.
To calculate the protein-induced bilayer perturbation energy and perturbation profile, the free energy (Eq. 2) must be minimized consistently. Boundary conditions for the system include a thickness match at the protein boundary (namely, Δ = Δ0 at z = 0), and that the perturbation decays to zero at large distances from the protein (dΔ/dz = 0 for z → ∞). The other boundary conditions are the natural ones (see, for example, Fox 1950), ensuring that the profile found indeed minimizes the system free energy. It should be emphasized that we focus here on symmetric copolymers and cylindrical inclusions, so that the contact angle, or curvature, at the interface between the inclusion and the bilayer are free to adjust to minimize the free energy (see, for example, Nielsen and Andersen 2000). Obviously, incorporation of noncylindrical proteins will result in a boundary condition fixed by the protein shape and lead to an increase in the system free energy. However, as shown by Dan and Safran (1998), the qualitative behavior of the system is unaffected by consideration of this added constraint.
RESULTS
Calculating the equilibrium perturbation profile yields
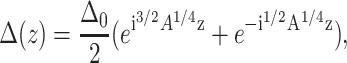 |
(4) |
where i = (−1)1/2 and A = B/K. Since both roots are complex numbers, the bilayer thickness does not decay as a simple exponential with distance from the protein, but has an oscillating component superimposed (Dan et al., 1993, 1994). The value of A defines the characteristic bilayer perturbation length. Thus, the perturbation length in this system is determined by the ratio between the bending modulus, K, and the compressibility, B. In general, when A is large, perturbations extend over large distances (compared to the bilayer thickness). When the bending rigidity is high, perturbations decay quickly with distance from the inclusion boundary. However, in the polymeric bilayer B and K are coupled to the chain length (see Eq. 3), so that A1/4 varies as N−2/3 (Milner and Witten, 1988; Dan et al., 1993; Halperin et al., 1992).
In Fig. 2 we plot the bilayer thickness profile for three membrane to protein thickness ratios. We see that, indeed, for all three cases the deformation profile does not simply decay as a function of distance from the protein boundary. The oscillations become more pronounced with increased thickness mismatch. The range of the profile perturbation scales roughly as four times the bilayer thickness. Thus, in lipid bilayers where Lm is small (of order 2 nm), the perturbation decays rapidly. However, in polymeric bilayers where the bilayer thickness may be much larger (Discher et al., 2000; Won et al., 2002; Bermudez et al., 2002), the perturbation may extend to distances of order 25–30 nm.
FIGURE 2.
Bilayer thickness profile, (Eq. 6) as a function of the distance from the inclusion boundary, z. All length scales are in units of a, the segment size. The flat monolayer thickness, Lm = 2 and the protein thickness, Lp = 8, 4 and 1.5 when Lp ≫ Lm, Lp = 2Lm and Lp < Lm respectively.
Substituting the perturbation profile, Eq. 4, into the expression for the deformation energy yields the energy penalty incurred through protein incorporation into the bilayer
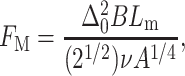 |
(5) |
Recall that for a symmetrical copolymer B, K, and A are given by Eq. 3, ν scales as N, and the thickness of the self-assembled layer Lm scales as a N2/3 (Halperin et al., 1992).
In Fig. 3 we plot the perturbation energy, FM, as a function of the polymer molecular weight N for several protein dimensions. As expected, the energy is minimal, for any given protein, at a finite chain length (N*) which corresponds to the case where Δ0 = 0, namely, when there is no thickness mismatch. It is interesting to note that although we use a linear perturbation model, the energy is asymmetrical, increasing more sharply for stretched bilayers (namely, when N < N*) than for compressed ones where N > N*.
FIGURE 3.
Protein-induced membrane perturbation energy, (Eq. 5) as a function of chain molecular weight.
DISCUSSION AND CONCLUSIONS
Using a mean-field model we derive here the perturbation energy of a symmetric polymeric bilayer due to the incorporation of a transmembrane protein. The protein perturbs the bilayer thickness, thereby inducing a deformation profile which extends over a distance roughly of order 3–4 times the bilayer thickness (Fig. 2). The perturbation range determines the range over which embedded proteins interact (Dan et al., 1994, 1993; Cantor, 1999). In lipid bilayers where Lm is of order 2 nm the interaction range is relatively short. However, in polymeric bilayers where Lm may be an order of magnitude larger (Won et al., 2002; Bermudez et al., 2002) the protein-protein interactions range becomes significant.
As may be expected, we find that the perturbation energy increases as a function of the thickness mismatch between the embedded protein and the hydrophobic bilayer core. The perturbation penalty for incorporation of a given protein into a polymer bilayer decreases and then increases with chain molecular weight, displaying a minimum at a finite molecular weight which corresponds to a bilayer thickness that is equal to the protein dimension (Fig. 3). Somewhat unexpectedly, the penalty for bilayer stretching is larger than for compression. For a given bilayer (namely, a fixed chain length), the penalty for stretching due to the incorporation of large proteins is much larger than the penalty for compression due to the incorporation of smaller proteins (Fig. 4). Considering any given protein, γLp is fixed by the protein conformation and the energetic gain due to protein incorporation in the bilayer decreases with increasing membrane perturbation energy (see Eq. 5 and Fig. 3). As a result, the energetic penalty is minimal (and the gain maximal) in bilayers where there is no thickness mismatch. Quite surprisingly, although the moduli of the bilayer increase significantly with the chain length (Eq. 3), the overall penalty for protein incorporation does not. As shown in Fig. 3, the perturbation energy increases relatively moderately with N.
FIGURE 4.
Perturbation energy of a given membrane, as a function of protein size, (Eq. 7). The membrane thickness is Lm = 2 and (γa2) is taken to be 0.1 kT. Note that higher values of γ will lead to an even more significant shift in the minimum toward larger proteins.
Why is the energetic penalty for protein incorporation relatively low, even for large thickness mismatch? In lipid bilayers the configurations of the core tails are relatively limited. As a result, the bilayers are relatively incompressible and cannot support perturbations in the thickness/surface density that are more than a few percent (see, for example, Gennis, 1989). Even a small thickness mismatch between the lipid bilayer and a transmembrane protein will therefore result in a large energetic penalty prohibiting protein incorporation. In self-assembled diblock copolymer bilayers, the surface density is set by an energetic balance between the surface tension at the hydrophobic/hydrophilic interface and the chain configurations. Although the preferred, equilibrium core dimensions of a given chain length N scales as N2/3, it can easily vary from a collapsed N1/3 to a stretched N1 (see, for example, Halperin et al., 1992). Bilayer compression, e.g., by matching to a small protein, results in an increase in the local surface tension energy and a decrease in the stretching energy. Thus, the energetic penalty due to the protein incorporation is mitigated by a gain in stretching energy.
The bilayer perturbation energy defines the membrane resistance to protein incorporation; however, transmembrane proteins are driven into the bilayer by the unfavorable interactions between their hydrophobic regions and the aqueous solution. Thus, the net energy gain due to protein incorporation in the bilayer (per unit width) is given by Eq. 1, namely, FM − γLp. It is interesting to examine the distribution of protein incorporation into a given bilayer, as a function of the protein size. In this case, both the perturbation energy and the energetic gain vary as a function of protein dimension. In Fig. 4 we plot, the different contributions and the difference between them as a function of the protein thickness (note that since Lm is fixed, Δ0 is proportional to Lp). We see that accounting for the protein-solution interactions shifts the minimum in the energy from Δ0 = 0 to a finite value, which depends on the magnitude of the surface tension γ. Thus, we expect that the distribution of proteins embedded in a given membrane will not favor proteins whose thickness is identical to the bilayer thickness, but somewhat larger proteins. The degree of shift (from Δ0 = 0 to a finite value) increases with increasing γ. The analysis presented here pertains to the incorporation of transmembrane proteins across the bilayer, a category that includes a variety of proteins (e.g., ion channel forming ones like gramicidin alamethicin).
It is interesting to note that the overall system free energy is reduced by protein incorporation over a significantly large range of thickness mismatches (for the example plotted ion Fig. 4, it encompasses mismatches ranging from Δ0 ≈ −0.8 to Δ0 ≈ 5, or from a protein to bilayer thickness ratio of order 0.2 to 6). Obviously, above a certain thickness mismatch the membrane perturbation energy would become prohibitive for this type of incorporation. Proteins may then either continue to circulate in solution, or be partially embedded in the bilayer (see Fig. 5). Both scenarios would result in some hydrophilic/hydrophobic interfacial penalty, so that determining under which conditions transmembrane proteins would be partially incorporated is sensitive to the specific protein characteristics.
FIGURE 5.
A schematic of bilayer perturbation by nonincorporated transmembrane proteins. As is shown, partial embedding of the protein, although reducing the membrane perturbation, involves an energetic penalty due to the surface tension between hydrophobic and hydrophilic regions.
Many types of proteins do not adopt the transmembrane configuration, but adsorb at the hydrophobic/hydrophilic bilayer interface (Gennis, 1989). Although the thickness mismatch scenario does not fit these cases, protein incorporation at the interface between the hydrophilic/hydrophobic polymer blocks perturbs the bilayer structure in a manner quite similar to that of transmembrane ones (see, for example, Dan and Safran 1998). However, in these cases the perturbation energy is much more sensitive to the specific protein characteristics (e.g., shape, contact angle). Thus, we expect that the analysis presented here applies for all types of membrane proteins, once the degree of perturbation is accurately accounted for.
The model presented here is based on several simplifying assumptions. The first one regards the (a)symmetry of the copolymer. Most diblock copolymers are not symmetric, so that the membrane perturbation energy (Eq. 2) includes a spontaneous curvature term. Previous studies (Dan et al., 1993, 1994; Dan and Safran, 1995) have shown that accounting for the bilayer spontaneous curvature plays a significant role when examining transmembrane proteins whose thickness matches that of the bilayer, but is overwhelmed by the thickness mismatch in cases where that applies. Therefore, accounting for the copolymer asymmetry (in either molecular weight or segment size) and bilayer spontaneous curvature would not affect the qualitative findings presented here. Our second simplifying assumption regards polymer polydispersity; although the large majority of models analyzing polymeric self assembly neglect the effect of polydispersity (see, for example, Milner and Witten, 1988; Halperin et al., 1992; Wang and Safran, 1991), the only synthetic polymers that are truly monodisperse are those synthesized using biological methods (see, for example, Dougherty et al., 1992). In diblock copolymers polydispersity may be manifested through a molecular weight distribution and/or a composition distribution. How would polydispersity affect our results? In general, entropy should drive different chains to mix uniformly. However, the perturbation induced by embedded protein is likely to lead to local segregation, where shorter chains that match the protein dimensions more closely would concentrate in the region adjacent to the protein boundary. This segregation should not affect the basis of our analysis, but the difference between the real thickness mismatch in polydisperse systems and the nominal one is expected to lead to even easier protein incorporation than what we predict here.
What do our results indicate regarding the use of polymersomes as drug carriers? One of the most significant issues regarding the use of any type of nanoparticle for drug delivery is their relatively rapid clearance by the immune system, triggered by immunoprotein adsorption. In liposomes, the incorporation of hydrophilic polymer chains has been shown to slow the kinetics of protein adsorption, thereby increasing the circulation time in vivo (Needham et al., 1992; Woodle et al., 1994; Storm et al., 1995; Szleifer, 1997a,b,c; Satulovsky et al., 2000; Efremova et al., 2000; Storm and Crommelin, 1998; Allen et al., 1991; Blume and Cevc, 1990; Klibanov et al., 1990). While such kinetic effects are expected to occur in polymer-based bilayers, we find that the equilibrium concentration of proteins incorporated into a bilayer depends on the bilayer thickness. Assuming that most natural proteins are designed to match lipid bilayers, this indicates that increasing the bilayer thickness (or the molecular weight of the diblock copolymer chains) will suppress, to some degree, protein incorporation. This indicates that the polymersome tagging for clearance by immunoprotein incorporation will be moderately suppressed, but not extinguished, for high molecular weight copolymers. Recently, Photos et al. (2003) have shown that the circulation time, in vivo of polymersomes increases nearly linearly with the chain molecular weight, implying thereby that immunoprotein adsorption/incorporation decreases with increasing N. It is hard to determine whether this suppression is due to a slowdown in the adsorption kinetics (a process dominated by the hydrophilic block), or to the membrane perturbation mechanism proposed here (which is dominated by the hydrophobic block). More revealing is the observation of Photos et al. (2003), that for a given hydrophilic chain length, the circulation half-time in vivo increases from order 15 h in stealth liposomes carrying a moderate density of polymer chains to order 20 h in polymersomes. This difference, which is relatively small, is likely to be dominated by the hydrophobic core, thereby supporting our conclusion that equilibrium protein incorporation is diminished, but not overly suppressed, as the chain length increases.
Acknowledgments
We thank Peter Photos and Dennis Discher for sharing their data, and for helpful discussions.
This research has been supported by the National Science Foundation CAREER grant 0096004.
References
- Allen, T. M., C. Hansen, F. Martin, C. Redemann, and A. Yauyoung. 1991. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim. Biophys. Acta. 1066:29–36. [DOI] [PubMed] [Google Scholar]
- Aranda-Espinoza, H., A. Berman, N. Dan, P. Pincus, and S. Safran. 1996. Interaction between inclusions embedded in membranes. Biophys. J. 71:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez, H., A. K. Brannan, D. A. Hammer, F. S. Bates, and D. E. Discher. 2002. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules. 35:8203–8208. [Google Scholar]
- Bezrukov, S. M. 2000. Functional consequences of lipid packing stress. Current Opinion in Colloid & Interface Science. 5:237–243. [Google Scholar]
- Blume, G., and G. Cevc. 1990. Liposomes for the sustained drug release in vivo. Biochim. Biophys. Acta. 1029:91–97. [DOI] [PubMed] [Google Scholar]
- Bradley, A. J., D. V. Devine, S. M. Ansell, J. Janzenand, and D. E. Brooks. 1998. Inhibition of liposome-induced complement activation by incorporated poly(ethylene glycol) lipids. Arch. Biochem. Biophys. 357:185–194. [DOI] [PubMed] [Google Scholar]
- Brown, M. D., A. Schätzlein, A. Brownlie, V. Jack, W. Wang, L. Tetley, A. I. Gray, and I. F. Uchegbu. 2000. Preliminary characterization of novel amino acid based polymeric vesicles as gene and drug delivery agents. Bioconjug. Chem. 11:880–891. [DOI] [PubMed] [Google Scholar]
- Cantor, R. S. 1997. Lateral pressures in cell membranes: a mechanism for modulation of protein function. J. Phys. Chem. B. 101:1723–1725. [Google Scholar]
- Cantor, R. S. 1999. The influence of membrane lateral pressures on simple geometric models of protein conformational equilibria. Chem. Phys. Lipids. 101:45–56. [DOI] [PubMed] [Google Scholar]
- Cantor, R. S. 2002. Size distribution of barrel-stave aggregates of membrane peptides. Biophys. J. 82:2520–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F. Y., M. T. Lee, and H. W. Huang. 2002. Sigmoidal concentration dependence of antimicrobial peptide activities: a case study of alamethicin. Biophys. J. 82:908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, I. H., and Y. D. Kim. 1998. Formation of stable polymeric vesicles by tocopherol-containing amphiphiles. Macromolecular Rapid Communications. 19:27–30. [Google Scholar]
- Cladera, J., J. L. Rigaud, J. Villaverde, and M. Dunach. 1997. Liposome solubilization and membrane protein reconstitution using Chaps and Chapso. Eur. J. Biochem. 243:798–804. [DOI] [PubMed] [Google Scholar]
- Dan, N., A. Berman, P. Pincus, and S. A. Safran. 1994. Membrane-induced interactions between inclusions. J. Phys. II France. 4:1713–1725. [Google Scholar]
- Dan, N., P. Pincus, and S. A. Safran. 1993. Membrane deformation and induced interactions. Langmuir. 9:2768–2771. [Google Scholar]
- Dan, N., and S. A. Safran. 1995. Solubilization of proteins in membranes. Isr. J. Chem. 35:37–40. [Google Scholar]
- Dan, N., and S. A. Safran. 1998. Effect of lipid characteristics on the structure of transmembrane proteins. Biophys. J. 74:1410–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine, D. V., and J. M. J. Marjan. 1997. The role of immunoproteins in the survival of liposomes in the circulation. Crit. Rev. Ther. Drug Carrier Syst. 14:105–131. [PubMed] [Google Scholar]
- Dimova, R., U. Seifert, B. Pouligny, S. Forster, and H. G. Dobereiner. 2002. Hyperviscous diblock copolymer vesicles. Eur. Phys. J. 7:241–250. [Google Scholar]
- Discher, B. M., D. A. Hammer, F. S. Bates, and D. E. Discher. 2000. Polymer vesicles in various media. Current Opinion in Colloid & Interface Science. 5:125–131. [Google Scholar]
- Discher, B. M., Y.-Y. Won, D. S. Ege, J. C. M. Lee, D. E. Discher, and D. A. Hammer. 1999. Polymersomes: tough vesicles made from diblock copolymers. Science. 284:1143–1146. [DOI] [PubMed] [Google Scholar]
- Discher, B. M., H. Bermudez, D. A. Hammer, and D. E. Discher. 2002. Cross-linked polymersome membranes: vesicles with broadly adjustable properties. J. Phys. Chem. B. 106:2848–2854. [Google Scholar]
- Discher, D. E., and A. Eisenberg. 2002. Polymer vesicles. Science. 297:967–973. [DOI] [PubMed] [Google Scholar]
- Dougherty, M. J., S. Kothakota, M. T. Krejchi, G. H. Zhang, T. L. Mason, D. A. Tirrell, and M. J. Fournier. 1992. Biosynthesis of new polymers of controlled molecular structure. Makromol Chemie- Macromol Symp. 62:225–229. [Google Scholar]
- Dufes, C., A. G. Schatzlein, L. Tetley, A. I. Gray, D. G. Watson, J. C. Olivier, W. Couet, and I. F. Uchegbu. 2000. Niosomes and polymeric chitosan based vesicles bearing transferrin and glucose ligands for drug targeting. Pharm. Res. 17:1250–1258. [DOI] [PubMed] [Google Scholar]
- Efremova, N. V., B. Bondurant, D. O'Brien, and D. E. Leckband. 2000. Measurements of interbilayer forces and protein adsorption on uncharged lipid bilayers displaying polyethylene glycol chains. Biochemistry. 39:3441–3451. [DOI] [PubMed] [Google Scholar]
- Fattal, D. R., and A. Ben-Shaul. 1995. Lipid chain packing and lipid-protein interaction in membranes. Physica A. 220:192–216. [Google Scholar]
- Fox, C. 1950. An Introduction to the Calculus of Variations. Oxford Press.
- Gennis, R. B. 1989. Biomembranes: Molecular Structure and Function. Springer-Verlag, New York.
- Halperin, A., M. Tirell, and T. P. Lodge. 1992. Tethered chains in polymer microstructures. Advances in Polymer Science. 100:31–71. [Google Scholar]
- Kahya, N., E.-I. Pecheur, W. P. De Boeij, D. A. Wiersma, and D. Hoekstra. 2001. Reconstitution of membrane proteins into giant unilamellar vesicles via peptide-induced fusion. Biophys. J. 81:1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, S. L., S. M. Bezrukov, S. M. Gruner, M. W. Tate, I. Vodyanoy, and V. A. Parsegian. 1993. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer. Biophys. J. 65:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanov, A. L., K. Maruyama, V. P. Torchilin, and L. Huang. 1990. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 268:235–237. [DOI] [PubMed] [Google Scholar]
- Lee, J. C. M., H. Bermudez, B. M. Discher, M. A. Sheehan, Y.-Y. Won, F. S. Bates, and D. E. Discher. 2001. Preparation, stability, and in vitro performance of vesicles made with diblock copolymers. Biotechnol. Bioeng. 73:135–145. [DOI] [PubMed] [Google Scholar]
- Maddox, M. W., and M. L. Longo. 2002. A Monte Carlo study of peptide insertion into lipid bilayer. Biophys. J. 82:244–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama, K., T. Yuda, A. Okamoto, C. Ishikura, S. Kojima, and M. Iwatsuru. 1991. Effect of molecular-weight in amphipathic polyethyleneglycol on prolonging the circulation time of large unilamellar liposomes. Chem. Pharm. Bull. 39:1620–1622. [DOI] [PubMed] [Google Scholar]
- May, S. 2000. Theories on structural perturbations of lipid bilayers. Current Opinion in Colloid & Interface Science. 5:244–249. [Google Scholar]
- Milner, S. T., and T. A. Witten. 1988. Bending moduli of polymeric surfactant interfaces. J. Phys. (Fr.). 49:1951–1962. [Google Scholar]
- Montesano, G., R. Bartucci, S. Belsito, D. Marsh, and L. Sportelli. 2001. Lipid membrane expansion and micelle formation by polymer-grafted lipids: Scaling with polymer length studied by spin-label electron spin resonance. Biophys. J. 80:1372–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, A., A. L. Klibanov, V. P. Torchilin, and L. Huang. 1991. Influence of the steric barrier activity of amphipathic poly(ethyleneglycol) and ganglioside Gm1 on the circulation time of liposomes and on the target binding of immunoliposomes in vivo. FEBS Lett. 284:263–266. [DOI] [PubMed] [Google Scholar]
- Mouritsen, O. G. 1998. Self assembly and organization of lipid-protein membranes. Current Opinion in Colloid & Interface Science. 3:78–87. [Google Scholar]
- Needham, D., T. J. McIntosh, and D. D. Lasic. 1992. Repulsive interactions and mechanical stability of polymer-grafted lipid membranes. Biochim. Biophys. Acta. 108:40–48. [DOI] [PubMed] [Google Scholar]
- Nielsen, C., and O. S. Andersen. 2000. Inclusion-induced bilayer deformations: effects of monolayer equilibrium curvature. Biophys. J. 79:2583–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar, M. M., K. Edwards, and T. D. Madden. 1999. Incorporation of bacterial membrane proteins into liposomes: factors influencing protein reconstitution. Biochim. Biophys. Acta. 1421:77–90. [DOI] [PubMed] [Google Scholar]
- Photos, P. J., L. Bacakova, B. Discher, F. S. Bates, and D. E. Discher. 2003. Polymer vesicles in vivo: correlations with PEG molecular weight. J. Control. Release. 90:323–324. [DOI] [PubMed] [Google Scholar]
- Rigaud, J. L., M. T. Paternostre, and A. Bluzat. 1988. Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents 2. Incorporation of the light driven proton pump bacteriorhodopsin. Biochemistry. 27:2677–2688. [DOI] [PubMed] [Google Scholar]
- Satulovsky, J., M. A. Carignano, and I. Szleifer. 2000. Kinetic and thermodynamic control of protein adsorption. Proc. Natl. Acad. Sci. USA. 97:9037–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple, S. C., A. Chonn, and P. R. Cullis. 1998. Interactions of liposomes and lipid-based carrier systems with blood proteins: Relation to clearance behavior in vivo. Adv. Drug Deliv. Rev. 32:3–17. [DOI] [PubMed] [Google Scholar]
- Shimada, K., S. Matsuo, Y. Sadzuka, A. Miyagishima, Y. Nozawa, S. Hirota, and T. Sonobe. 2000. Determination of incorporated amounts of poly(ethylene glycol)-derivatized lipids in liposomes for the physicochemical characterization of stealth liposomes. Int. J. Pharm. 203:255–263. [DOI] [PubMed] [Google Scholar]
- Storm, G., S. O. Belliot, T. Daemen, and D. D. Lasic. 1995. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv. Drug Deliv. Rev. 17:31–48. [Google Scholar]
- Storm, G., and D. J. A. Crommelin. 1998. Liposomes: quo vadis? Pharm. Sci. Technol. Today. 1:19–31. [Google Scholar]
- Szleifer, I. 1997a. Polymers and proteins: interactions at interfaces. Current Opinion in Solid State & Materials Science. 2:337–344. [Google Scholar]
- Szleifer, I. 1997b. Protein adsorption on surfaces with grafted polymers: a theoretical approach. Biophys. J. 72:595–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szleifer, I. 1997c. Protein adsorption on tethered polymer layers: effect of polymer chain architecture and composition. Physica A. 244:370–388. [Google Scholar]
- Wang, W., L. Tetley, and I. F. Uchegbu. 2001. The level of hydrophobic substitution and the molecular weight of amphiphilic poly-L-lysine-based polymers strongly affects their assembly into polymeric bilayer vesicles. J. Colloid Interface Sci. 237:200–207. [DOI] [PubMed] [Google Scholar]
- Wang, Z. G., and S. A. Safran. 1991. Curvature elasticity of diblock copolymer monolayers. J. Chem. Phys. 94:679–687. [Google Scholar]
- Won, Y.-Y., A. K. Brannan, H. T. Davis, and F. S. Bates. 2002. Cryogenic transmission electron microscopy (Cryo-TEM) of micelles and vesicles formed in water by poly(ethylene oxide)-based block copolymers. J. Phys. Chem. B. 106:3354–3364. [Google Scholar]
- Woodle, M. C., M. S. Newman, and J. A. Cohen. 1994. Sterically stabilized liposomes- physical and biological properties. J. Drug Target. 2:397–403. [DOI] [PubMed] [Google Scholar]
- Zhelev, D. V., N. Stoicheva, P. Scherrer, and D. Needham. 2001. Interactions of synthetic HA2 influenza fusion peptide analog with model membranes. Biophys. J. 81:285–304. [DOI] [PMC free article] [PubMed] [Google Scholar]