Abstract
The firm arrest of leukocytes to the endothelium during inflammation is known to be mediated by endothelial intercellular adhesion molecules (ICAMs) binding to activated integrins displayed on leukocyte surface. Selectin-ligand interactions, which mediate rolling, are believed to be important for facilitating firm adhesion, either by activating integrins or by facilitating the transition to firm adhesion by making it easier for integrins to bind. Although leukocytes employ two distinct adhesion molecules that mediate different states of adhesion, the fundamental biophysical mechanisms by which two pairs of adhesion molecules facilitate cell adhesion is not well understood. In this work, we attempt to understand the interaction between two molecular systems using a cell-free system in which polystyrene microspheres functionalized with the selectin ligand, sialyl LewisX (sLeX), and an antibody against ICAM-1, aICAM-1, are perfused over P-selectin/ICAM-1 coated surfaces in a parallel plate flow chamber. Separately, sLeX/P-selectin interactions support rolling and aICAM-1/ICAM-1 interactions mediate firm adhesion. Our results show that sLeX/aICAM-1 microspheres will firmly adhere to P-selectin/ICAM-1 coated surfaces, and that the extent of firm adhesion of microspheres is dependent on wall shear stress within the flow chamber, sLeX/aICAM-1 microsphere site density, and P-selectin/ICAM-1 surface density ratio. We show that P-selectin's interaction with sLeX mechanistically facilitates firm adhesion mediated by antibody binding to ICAM-1: the extent of firm adhesion for the same concentration of aICAM-1/ICAM-1 interaction is greater when sLeX/P-selectin interactions are present. aICAM-1/ICAM-1 interactions also stabilize rolling by increasing pause times and decreasing average rolling velocities. Although aICAM-1 is a surrogate for β2-integrin, the kinetics of association between aICAM-1 and ICAM-1 is within a factor of 1.5 of activated integrin binding ICAM-1, suggesting the findings from this model system may be insightful to the mechanism of leukocyte firm adhesion. In particular, these experimental results show how two molecule systems can interact to produce an effect not achievable by either system alone, a fundamental mechanism that may pervade leukocyte adhesion biology.
INTRODUCTION
Adhesion of leukocytes to vascular wall in response to inflammation is an important, well-studied phenomenon that follows a multistep adhesion cascade, comprised of an initial tethering step, firm arrest, and transendothelial migration of leukocytes. Initial leukocyte (neutrophil) adhesion, referred to as rolling, is mediated by a class of adhesive molecules called selectins (L-, P-, and E-selectin). These molecules initiate rolling by binding to their carbohydrate counterreceptors on leukocyte surface (P- and E-selectin) or the endothelium (L-selectin) (Lawrence and Springer, 1991; Springer, 1994). After this initial tethering, leukocytes firmly adhere to the endothelium via the binding of activated β2-integrins to intercellular adhesion molecules (ICAMs) expressed on endothelial surface (Ebnet and Vestweber, 1999). To date, two independent mechanisms have been proposed for integrin activation. One mechanism suggests integrin activation results from chemotactic stimulation of leukocytes (Springer, 1994) whereas another suggests selectin ligation leads to integrin activation (Evangelista et al., 1999; Simon et al., 2000). Work done by Blanks and co-workers with Chinese hamster ovary cells expressing P-selectin suggests that P-selectin glycoprotein ligand-1 (PSGL-1) expressed on neutrophils binding to P-selectin results in the activation of β2-integrin, thereby facilitating neutrophil adhesion to ICAM-1 expressing cells (Blanks et al., 1998). Furthermore, Gopalan et al. showed that cross-linking L-selectin activates the integrin, LFA-1, that binds to ICAM-1 (Gopalan et al., 1997).
Regardless of the mechanism of integrin activation, what is clear is that rolling is essential for firm arrest and eventual transmigration of leukocytes to inflamed tissues. The question is, is there any role for selectin-mediated rolling in facilitating the transition to firm adhesion beyond the activation of integrins? More generally, the paradigm of two molecules, each with different physical chemistry and capable of supporting different dynamics states of adhesion, acting in concert, is ubiquitous in adhesion biology, but the precise interplay between two different molecule systems is not understood. Therefore, the goal of this article is to systematically construct an experimental system that will allow us to understand the fundamental biophysical interplay between two molecules, motivated by leukocyte adhesion biology, and thus suggest a mechanism by which selectins may facilitate the transition to firm adhesion via integrins.
To do this, we will use cell-free systems in which microspheres coated with two ligands, sialyl LewisX (sLex) and an antibody against ICAM-1 (BBIG-I1), are allowed to interact with selectin/ICAM-1 surfaces under flow, mimicking the weak ligand-strong ligand chemistry present in leukocyte adhesion. Specifically, sLeX/P-selectin interactions have been shown to support rolling adhesion (Rodgers et al., 2000), and here we show that binding between anti-ICAM-I (aICAM-1) and ICAM-1 mediates firm binding. Cell-free systems such as this have successfully been used to elucidate the physical chemistry of selectin-mediated rolling (Brunk and Hammer, 1997; Greenberg et al., 2000; Rodgers et al., 2000), but cell-free systems have not been used to understand the transition from rolling to firm adhesion. Fig. 1 shows a schematic illustration of the cell-free system used in this work. This system is ideal to understand the role of wall shear stress, the ratio of P-selectin/ICAM-1 surface density ratio, and the ratio of sLeX/aICAM-1 density in the transition from rolling to firm adhesion in a two-receptor system because in a cell-free system we can adjust the properties of the system systematically in ways that are not easily achieved in cell systems. Our results show that at several intermediate sLeX/aICAM-1 ratios, selectin-ligand interactions are critical for optimal ICAM-1-mediated firm adhesion. We find that the amount of firmly adhering microspheres is dependent on wall shear stress, P-selectin/ICAM-1 surface density ratio, and sLeX/aICAM-1 microsphere site density ratio. We show that removing sialyic acid from sLeX, which renders the carbohydrate inactive as a rolling ligand, greatly reduces the extent of firm adhesion. In addition, we show that ICAM-1 interactions stabilize rolling, by decreasing the average rolling velocity and increasing the pause times during microsphere rolling. The suggestion that the results of our model system are indicative of mechanisms that may be at play in leukocyte adhesion is enhanced by our finding that the on-rate for aICAM-1 binding to ICAM-1 is within a factor of 1.5 of that between activated β2-integrin and ICAM-1. Overall, we believe this article identifies a mechanistic role of selectins in facilitating the transition to firm adhesion in leukocytes.
FIGURE 1.
A schematic of a cell-free system used to understand the physical chemistry of ICAM-1 mediated leukocyte adhesion. Plastic surface with immobilized P-selectin and ICAM-1 represents the endothelial wall, and polystyrene microspheres coated with sLeX and anti-ICAM-1 mAb are used as model leukocytes.
MATERIALS AND METHODS
Adhesion molecules and antibodies
Soluble, recombinant P-selectin (sP) containing the entire extracellular domain of the molecule was a generous gift from Dr. Raymond T. Camphausen, Wyeth (Cambridge, MA) (Rodgers et al., 2000). Biotinylated, multivalent sialyl LewisX and LewisX (LeX) carbohydrate were purchased from Glycotech (Rockville, MD). Recombinant human ICAM-1/Fc chimera (human IgG1), biotinylated anti-ICAM-1 monoclonal antibody (mAb) BBIG-I1, FITC-labeled anti-P-selectin mAb 9E1, and anti-ICAM-1 mAb BBIG-I1 were purchased from R & D systems (Minneapolis, MN). Anti-sLeX mAb HECA-452, FITC-labeled anti-rat IgM, and FITC-labeled anti-mouse IgG1 were purchased from Pharmingen (San Diego, CA).
Substrate preparation
P-selectin/ICAM-1 surfaces were prepared by a method similar to that described in previous publication (Rodgers et al., 2000). In this method, double well, rectangular, flexiperm gaskets (Sigma, St. Louis, MO) were placed on microscope slides cut from bacteriological polystyrene dishes. The surface enclosed by one of the two wells was incubated overnight with 374 μL of 2.5, 5, or 10 μg/mL ICAM-1 in binding buffer (0.1 M NaHCO3) and the other well with binding buffer. After ICAM-1 incubation, ICAM-1 coated surfaces were washed twice with 2 mL binding buffer, after which the double well gaskets were replaced with single well, rectangular gaskets. The surfaces enclosed by the single well gaskets were coated with 800 μL of 12, 10, or 4 μg/mL P-selectin in binding buffer for a period of 2 h. After coating with P-selectin, the surfaces were incubated with 2 mL of a heat-denatured solution of 2% bovine serum albumin (BSA) in Dulbecco's phosphate buffered saline (DPBS) (pH = 7.4) for a minimum of 30 min, and before use in laminar flow assays, slides were incubated for 2 min with 1% Tween 20 in DPBS. Control surfaces were prepared using the BSA blocking step only.
P-selectin/ICAM-1 site density determination
For site density determination, eight-well, rectangular, flexiperm gaskets were placed on polystyrene slides. ICAM-1 was incubated overnight at one concentration, followed by P-selectin incubation for 2 h as described in the previous section. After protein incubation, wells were coated with 2% denatured BSA solution. BSA-blocked, P-selectin/ICAM-1 surfaces were then incubated with a saturating concentration of FITC-labeled aICAM-1 mAb (BBIG-I1) or anti-P-selectin mAb (9E1). Fluorescence intensity was obtained using FeliX software (Photon Technologies, Severna Park, MD) and a Nikon Diaphot inverted microscope (Melville, NY) equipped with a FITC cube and connected to a photomultiplier tube (Photoscan, Nikon, Tokyo, Japan). P-selectin/ICAM-1 site density (molecules/μm2) was obtained using calibration curves generated by measuring the fluorescence of known concentrations of anti-P-selectin and aICAM-1 mAbs as previously described (Brunk and Hammer, 1997).
Microsphere preparation
Biotinylated sLeX, a 30-kD carbohydrate probe that has been described in previous publications (Brunk and Hammer, 1997; Greenberg et al., 2000; Rodgers et al., 2000), and biotinylated aICAM-1 mAb were diluted to 5 μg/mL in DPBS+, (DPBS, 1% BSA, pH = 7.4). These two solutions were then mixed at ratios between 0% and 100% sLeX. A total of 106 SuperAvidin-coated beads (∼10 μm) were simultaneously coated with sLeX and anti-ICAM-1 by incubating the beads with 100 μL solution containing different ratios of biotinylated sLeX and aICAM-1 mAb for 1 h at room temperature, with continuous vortexing. Beads were then washed three times in DPBS+ and resuspended in 2 mL DPBS+ until ready for use in adhesion experiments.
Carbohydrate and antibody site density determination
For sLeX site density determination, 105 aICAM-1/sLeX-coated beads were incubated with 100 μL of 30 μg/ml rat-IgM anti-human Cutaneous Lymphocyte Antigen (CLA) reactive to sLeX (HECA-452) for 1 h. Beads were then washed two times with DPBS+ and incubated for 1 h with 100 μL of 30 μg/ml FITC-labeled anti-rat IgM mAb. Fluorescence intensities were measured using flow cytometry, and sLeX site densities were estimated as described previously (Brunk and Hammer, 1997). Fluorescence shifts were converted to site densities using a calibration curve relating mean peak fluorescence of Quantum 26 calibration beads (Bangs Laboratories, Fishers, IN) to their molecules of equivalent soluble fluorochrome (MESF). Using the molecules of equivalent soluble fluorochrome/protein ratio of the FITC-conjugated secondary antibody and assuming a 1:1 relationship between sLeX, the primary and secondary antibody, the number of surface-bound molecules was determined. For anti-ICAM-1 density determination, 105 aICAM-1/sLeX-coated beads were incubated with 100 μL of 30 μg/ml FITC-conjugated anti-mouse IgG1 monoclonal antibody reactive to the mouse IgG1 residue on the biotinylated aICAM-1 mAb. Assuming a 1:1 binding ratio between aICAM-1 and the fluorescence antibody, aICAM-1 site density was calculated similar to that of sLeX.
Determination of binding constant of anti-ICAM-1 mAb (BBIG-I1) to ICAM-1
Binding kinetics of anti-ICAM-1 mAb was measured on a BIAcore 1000 instrument (BIAcore, Piscataway, NJ). StreptAvidin (SA)-chips were purchased from BIAcore and stored at 4°C before use. To begin measurement, a SA-chip was loaded into the BIAcore 1000, after which the surface was exposed to three 1-min injections of conditioning buffer (10 mM NaCl in 50 mM NaOH) at 30 μl/min. After conditioning, the chip was washed with HBS-EP buffer (10 mM HEPES, pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% Tween 20) for 5 min at 20 μl/min to block nonspecific adhesion. Biotinylated anti-ICAM-1, BBIG-I1, was immobilized onto the surface by flowing 16 μg/ml antibody in HBS-EP buffer over the chip at 5 μl/min until saturation (∼1700 response units). After the adsorption of aICAM-1, the surface was washed with HBS-EP for ∼15 min at 20 μl/min to remove excess antibody. Binding constants for the interactions between aICAM-1 and ICAM-1 were obtained by injecting various concentrations of ICAM-1 in HBS-EP buffer over the anti-ICAM-1 surface at 20 μl/min for 3 min, after which HBS-EP buffer is allowed to flow over the surface for 3 min to access the rate of ICAM-1 detachment from the surface. Fig. 2 shows how the response units (RU) vary as a function of time for the association and dissociation of ICAM-1 to antibody-immobilized surface. ka and kd, the on-rate and off-rate of aICAM-1 binding to ICAM-1, were determined by fitting a kinetic expression given by Eq. 1 to this binding data using the BIAevaluation software (BIAcore) as previously described by Labadia and co-workers (Labadia et al., 1998).
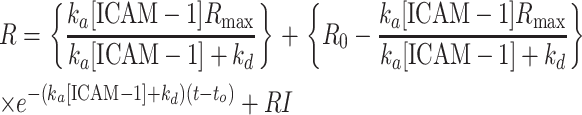 |
(1) |
This expression is applied to all kinetic data, estimating the parameters ka, kd, and Rmax. In this expression, R0 is the refractive index before the injection of ICAM-1, R is the refractive index as a function of time after injection of ICAM-1, RI is the bulk refractive index contribution, and Rmax is the maximal value of R observed when all the immobilized aICAM-1 is saturated with ICAM-1. Surface regeneration (removal of bound ICAM-1) was achieved by exposing the surface to a 1-min injection of 10 mM HCl at 20 μl/min. Control experiments where different concentrations of ICAM-1 were perfused over a blank SA-chip registered no response above the base level (data not shown).
FIGURE 2.
Plot of response unit (RU) versus time showing the association and dissociation of ICAM-1 to immobilized anti-ICAM-1 at different concentrations of ICAM-1. Arrows indicated points of injection of specific reagents.
Laminar flow assay
A straight-channel, parallel-plate flow chamber was used for laminar flow assays. The straight-channel template was cut from 0.01-inch-thick Duralastic sheeting (Allied Biomedical, Goose Creek, SC). For each flow experiment, the template was placed over P-selectin/ICAM-1 coated polystyrene slide. The template and slide were placed in the bottom well of the flow chamber and the top secured with nuts and bolts. Flow chamber experimental setup was as previously described, where the assembled chamber was mounted on the stage of a Nikon Diaphot inverted microscope with phase-contrast optics (Rodgers et al., 2000; Eniola et al., 2002). sLeX/aICAM-1 beads were placed in a test tube and connected to the flow chamber using rubber tubing. Flow of bead solution over P-selectin/ICAM-1 surfaces was initiated with an infusion/withdrawal syringe pump (Harvard Apparatus, South Natick, MA). Experiments were recorded using a Cohu black and white CCD camera (Cohu, San Diego, CA) and Sony SVO-9500MD S-VHS recorder (Sony Medical Systems, Montvale, NJ). Recognizing that the rate of transport of microspheres to substrate surface is likely a function of flow rate as described by Munn and co-workers (Munn et al., 1994), the start time for data collection was adjusted according to the time it takes microspheres to enter the chamber at each flow rate studied. Rolling and firm binding data were collected 3 min after microspheres enter the chamber for all flow rates explored. Furthermore, to account for the variation in fluxes along the chamber length, data were collected at a constant position along the chamber for all experiments. For each experiment, wall shear stress (τw) was calculated according to
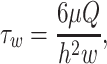 |
(2) |
where μ is the fluid viscosity, Q is the volumetric flow rate, h is the channel height, and w is the channel width.
Data analysis
Rolling and bound flux of interacting beads were obtained manually by counting the number of rolling and bound beads in the window of view (0.32 mm2) over a period of 1 min. Instantaneous and average rolling velocities were obtained through digital image analysis of video recordings of adhesion experiments, using LabView software (National Instrument, Austin, TX). Instantaneous rolling velocity data were obtained every 1/10 of a second, and the average rolling velocities were obtained by taking the mean velocity of at least 10 particles continuously rolling for 3 s or longer (Rodgers et al., 2000). The average fraction of time paused by beads was obtained by taking the mean fraction of pause time of at least five particles over a period of at least 10 s. Error bars were plotted using SE calculation unless otherwise noted. Differences in the level of firm adhesion between microspheres with different ratios of sLeX/aICAM-1 were examined using the two-tailed Student's t-test. A value of p < 0.05 was considered statistically significant.
RESULTS
Characterization of microspheres and polystyrene surfaces
Microsphere sLeX/aICAM-1 site densities were determined by flow cytometry. Fig. 3 shows the fluorescence histograms obtained for sLeX and aICAM-1 labeling on the bead surface, and the resulting plot of sLeX microsphere site density as a function of the coating ratio of sLeX used in solution. Rightward shift in fluorescence intensity of sample beads from the control shown in Fig. 3, A and B, confirms the presence of both sLeX and anti-ICAM-1 on microsphere surface, and Fig. 3 C suggests a linear relationship between the coating ratio and site density ratio of sLeX/aICAM-1. The fluorescence intensities were related to molecular density using fluorescent calibration beads. The site densities of sLeX and aICAM-1 on microsphere surface are listed in Table 1. The total amount of aICAM-1 on microspheres coated with solutions containing 50:50 and 25:75 sLeX/aICAM-1, 500 and 1057 sites/μm2 (based on a microsphere surface area of 314 μm2), respectively, is within the range of site densities of β2-integrins on activated neutrophils, 515–1400 sites/μm2 (based on an apparent surface area of 227 μm2; Shao et al., 1998), that has been reported in literature (Thiel et al., 1997). Similarly, the amount of aICAM-1 on beads coated with 75:25 sLeX/aICAM-1, 168 sites/μm2, is slightly lower than, but of the same order as, the amount of β2-integrins, ∼300 sites/μm2, on resting neutrophils (Thiel et al., 1997; Edwards et al., 2000).
FIGURE 3.

Flow cytometry data for sLeX/anti-ICAM-1 microspheres. (A and B) Fluorescence histograms for SuperAvidin microspheres incubated with 5 μg/ml solutions containing 0%, 25%, 50%, 75%, and 100% sLeX. (A) Microspheres with HECA-452 binding to sLeX epitopes and FITC labeled anti-rat IgM mAb binding to the rat IgM residue on HECA-452. Microspheres incubated with solution containing 0% sLeX (100% anti-ICAM-1) were used as control. (B) Microspheres with FITC-labeled anti-mouse IgG1 mAb binding to the mouse IgG1 residue on anti-ICAM-1 mAb. (C) sLeX microsphere site density ratio as a function of sLeX coating ratio. Error bars are 95% confidence intervals.
TABLE 1.
Molecular site densities of sLeX and anti-ICAM-1 on microsphere surface
| Molecule site density (sites/μm2) | ||
|---|---|---|
| Coating concentration (% aICAM-1) | sLeX | Anti-ICAM-1 |
| 0 | 1167 | 0 |
| 25 | 1019 | 168 |
| 50 | 796 | 500 |
| 75 | 444 | 1047 |
| 100 | 0 | 2282 |
Microsphere surface area = 314 μm2.
P-selectin/ICAM-1 density ratios on polystyrene surfaces were determined by surface fluorescence imaging. A 50:50 P-selectin/ICAM-1 ratio was obtained when slides were coated with 5 μg/ml ICAM-1 followed by 10 μg/ml P-selectin. Similarly, slides coated with 2.5 μg/ml ICAM-1 and 12.5 μg/ml P-selectin yielded an 80:20 P-selectin/ICAM-1 surface ratio, and a 30:70 ratio was obtained when slides were coated with 10 μg/ml and 4 μg/ml ICAM-1 and P-selectin, respectively. The average total substrate site density of surfaces used in flow experiments was 408 ± 35.5 sites/μm2.
Binding kinetics of aICAM-1 binding to ICAM-1
The binding kinetics of anti-ICAM-1 mAb, BBIG-I1, to ICAM-1 was measured on BIACORE 1000. Fig. 4 shows the data (symbols) and global analysis fitted (line) curves of the association and dissociation interactions between antibody and ICAM-1. The on-rate (kon) and off-rate (koff) for the antibody/ICAM-1 interactions obtained using the BIAevaluation software as described in the Materials and Methods section, were 156,000 (±46,000) M−1s−1 and 1.13 (±0.11) × 10−4 s−1 respectively (where the numbers in parentheses represent SE for n = 5).
FIGURE 4.
Determination of the kinetic rate constant for the interactions between immobilized anti-ICAM-1 mAb and ICAM-1. Symbols are experimental data obtain from the (A) association and (B) dissociation of ICAM-1 to immobilized anti-ICAM-1, and the lines represents fitted values predicted by Eq. 1. Nonlinear regression global analysis converged to ka and kd values of 156,000 (±46,000) M−1s−1 and 1.13 (±0.11) × 10−4 s−1, respectively (numbers in parentheses represent SE for n = 5).
Rolling and firm adhesion of microspheres
Flow adhesion assays with polystyrene microspheres coated with different ratios of sLeX/aICAM-1 show these beads can roll and firmly adhere to P-selectin/ICAM-1-coated surfaces. Fig. 5 shows the binding flux of 50:50 sLeX/aICAM-1 beads over 50:50 P-selectin/ICAM-1 and P-selectin surfaces at 131 s−1. Under these conditions, beads bind firmly to P-selectin/ICAM-1 surfaces; however, these same beads showed an insignificant level of firm adhesion on surfaces coated with P-selectin only. Furthermore, no significant firm adhesion was recorded when sLeX-coated (no aICAM-1) beads were perfused over the 50:50 P-selectin/ICAM-1 surfaces (Fig. 4). Thus, firm adhesion requires the presence of anti-ICAM-1 on the bead surface, and ICAM-1 on the substrate.
FIGURE 5.
Firm attachment flux of sLeX/anti-ICAM-1 9.95-μm microspheres with 50% (▪) and 100% (□) sLeX site density ratio interacting with 50:50 P-selectin/ICAM-1 (204 ± 17.8 sites/μm2 P-selectin) and P-selectin surface (363 ± 5.5 sites/μm2) at 131 s−1. Abscissa labels describe surface coating.
Dynamics of microsphere adhesion depends on densities of two molecules
Fig. 6, A and B, illustrates how binding flux for two-molecule microspheres depends on the amount of anti-ICAM-1 on the bead surface and wall shear stress on both P-selectin and 50:50 P-selectin/ICAM-1 surfaces, respectively. Fig. 6 A shows that the rolling flux of sLeX/aICAM-1 microspheres on P-selectin decreases with increasing wall shear stress. This figure also shows that rolling flux on P-selectin decreases as the ratio of aICAM-1 to sLeX on the bead surface increases.
FIGURE 6.

Rolling and binding flux of 10.2-μm sLeX/anti-ICAM-1 microspheres. (A) Rolling flux on a P-selectin surface and (B) binding flux on 50:50 selectin/ICAM-1 surface as a function of anti-ICAM-1 microsphere coating ratio at shear rates of 125 (⧫), 152 (▪), and 208 s−1(•). (C) Comparison of binding (▪) and rolling (▴) flux on 50:50 selectin/ICAM-1 surface as a function of anti-ICAM-1 microsphere coating ratio at a shear rate of 152 s−1. Circles represent the average of the total accumulation of microspheres at 100%–25% sLeX/aICAM-1 coating ratios. Data averaged over three experiments and four different positions in the flow chamber.
Fig. 6 B shows how the level of firm binding (left ordinate) and the amount of aICAM-1 (right ordinate) vary with the ratio of sLeX/aICAM-1 on the bead. This figure shows that firm binding of microspheres on an P-selectin/ICAM-1 surface decreases with increasing wall shear stress. When there is little or no aICAM-1 on the bead, there is no firm adhesion. Furthermore, at any wall shear stress, the number of beads firmly bound to P-selectin/ICAM-1 surface increases as the relative amount of aICAM-1 to sLeX on the bead surface increases up to a maximum, and then plateaus or decreases (Fig. 6 B). Interestingly, there is about a 5-fold increase in the number of firmly adherent beads as the ratio of aICAM-1 to sLeX on microsphere increases from 25% to 50% aICAM-1. This observation suggests that sLeX/P-selectin interactions might be augmenting microsphere firm binding to P-selectin/ICAM-1 since there is only a ∼3-fold increase in the amount of aICAM-1 on microsphere surface as the aICAM-1 to sLeX ratio increases from 25% to 50% (see Table 1 and right ordinate on Fig. 6 B). Furthermore, at most shear rates studied, there seems to be no statistical difference in the firm adhesion (p-values were between 0.25 and 0.27) on P-selectin/ICAM-1 surfaces as the ratio of aICAM-1 to sLeX on microsphere increases from 50% to 100% aICAM-1, although there is a significant increase in the amount of aICAM-1 on microsphere surface (from 500 to 2282 sites/μm2; see Table 1 and right abscissa in Fig. 6 B). In other words, similar levels of firm adhesion are observed with microspheres with significantly different amounts of aICAM-1 present on their surface. This strongly suggests that at 50% and 75% aICAM-1 to sLeX microsphere site density ratios, the presence of rolling interactions enhances the firm adhesion of microspheres, since the same level of firm adhesion is seen with fewer aICAM-1 molecules. Perfusion of sLeX/aICAM-1 beads over P-selectin surfaces shows an insignificant level of firm adhesion at all microsphere site density ratios, which suggests there is no interaction between aICAM-1 mAb and P-selectin, and that P-selectin interactions do not support firm binding (Fig. 6 A).
Correspondingly, one can examine the rolling flux under the same conditions. Fig. 6 C compares the rolling and binding flux of sLeX/aICAM-1 beads on a 50:50 P-selectin/ICAM-1 surface. This plot shows that rolling flux progressively decreases as the ratio of sLeX to aICAM-1 decreases. Clearly, the absence of sLeX (for beads with 100% aICAM-1) prevents rolling, i.e., anti-ICAM-1 does not support rolling interactions by itself as suggested by its high affinity binding to ICAM-1. Furthermore, the large drop (80% decrease) in the rolling flux of beads over P-selectin/ICAM-1 surfaces seen as aICAM-1 ratio increases from 25% to 50% reflects the transition from rolling to firm adhesion of sLeX/aICAM-1 coated microspheres, since such a sharp decrease is not observed with beads interacting with P-selectin only (17% decrease) (Fig. 6 A). Fig. 6 C also illustrates that the total accumulation of beads on a surface is constant or decreases slightly for all aICAM-1 sLeX combinations, except for beads without any sLeX (100% aICAM-1). This data suggest some minimal presence of sLeX is necessary for maximal accumulation, but that aICAM-1 is capable of some extent of accumulation in the absence of sLeX.
We also examined the influence of the P-selectin/ICAM-1 surface ratio on the extent of firm adhesion. Microspheres with different ratios of sLeX/aICAM-1 were perfused over surfaces with 30:70, 50:50, 80:20, and 100:0 P-selectin/ICAM-1 site density ratios. Fig. 7 A illustrates how the firm binding of microspheres changes on these P-selectin/ICAM-1 surfaces. When the beads with lower sLeX/aICAM-1 ratios (aICAM-1 >50%) interact with P-selectin/ICAM-1 surfaces, firm adhesion increased as the amount of ICAM-1 on the surface increased. However, binding of beads with aICAM-1 ratio <50% (higher sLeX/aICAM-1 ratios) was limited; increasing ICAM-1 concentration did not always lead to an increase in adhesion, suggesting that the extent of adhesion is not solely due to ICAM-1 interactions. Fig. 7 B compares the rolling and binding flux of 50% sLeX/aICAM-1 coated beads on surfaces with different ratios of P-selectin/ICAM-1. As the molecular surface density ratio increases from 0% to 50% ICAM-1, we see a correlation between the decrease in rolling flux and the increase in firm binding flux, suggesting beads that had been rolling on P-selectin were now firmly bound via ICAM-1. Also, the maximum in firm binding occurs not at the highest ICAM-1 surface density, but rather when there is a 70:30 ratio of ICAM-1 to P-selectin on the surface. Therefore, firm adhesion is most likely when there is a rolling ligand in combination with a firm adhesion ligand.
FIGURE 7.
(A) Binding flux of 9.95-μm sLeX/anti-ICAM-1 microspheres at 131 s−1 as a function of anti-ICAM-1 microsphere coating ratio on 100% (•), 80% (*), 50% (◊), and 30% (▵) P-selectin surfaces. Average total P-selectin/ICAM-1 site density was 408 ± 35.5 sites/μm2. (B) Comparison of binding (▪) and rolling (⧫) flux of 9.95 μm, 50:50 sLeX/aICAM-1 beads as a function of substrate molecular site density ratio of ICAM-1 to P-selectinat a shear rate of 131 s−1.
P-selectin-ligand (sLeX) interactions (rolling) facilitate ICAM-1-mediated firm binding
Data displayed in Figs. 6 and 7 suggest that firm binding of two-molecule beads can be facilitated by rolling. To demonstrate this biophysical principle yet another way, microspheres were coated with anti-ICAM-1 and LeX, a carbohydrate structure similar to sLeX but devoid of sialic acid, and not capable of mediating rolling on selectins (Rodgers et al., 2000). Lex/aICAM-1 microspheres at any concentration of molecules do not roll when perfused over P-selectin or P-selectin/ICAM-1 surfaces (data not shown). Fig. 8 compares binding of Lex/aICAM-1 beads on P-selectin/ICAM-1 surfaces to that of sLeX/aICAM-1 beads on the same surface. Since sLeX can support rolling and LeX cannot, this comparison clearly indicates the contribution of rolling interactions to the extent of firm adhesion. Beads coated with greater than 50:50 LeX/aICAM-1 did not bind to P-selectin/ICAM-1 surfaces because they do not have enough aICAM-1 to support firm adhesion themselves, and LeX does not support rolling. When beads are coated with 50% aICAM-1, replacing sLeX with LeX decreases firm binding at all P-selectin concentrations (Fig. 8). Specifically, replacing LeX with sLeX at 50:50 ratios of carbohydrate to aICAM-1 leads to a 12-, 9-, and 4-fold increase in the extent of firm adhesion on 80:20, 50:50, and 30:70 P-selectin/ICAM-1 surfaces, respectively. Examination of the results from 25% to 75% aICAM-1 on all P-selectin/ICAM-1 densities indicate rolling greatly facilitates the transition to firm adhesion of dually coated microspheres. As the density of ICAM-1 decreases on the substrate surface (higher values of P-selectin/ICAM-1), the critical need for sLeX in facilitating firm adhesion increases, and higher aICAM-1 densities are required to compensate for the decrease in ICAM-1 density. For example, for 80:20 P-selectin/ICAM-1 surfaces, the maximal increase in firm binding between sLeX/aICAM-1 and LeX/aICAM-1 beads is at 75% aICAM-1. Overall, selectin interactions aid the transition to firm adhesion at intermediate aICAM-1/sLeX densities, suggesting the two molecules work synergistically to increase adhesion.
FIGURE 8.

Comparison of binding fluxes of 9.95-μm LeX/anti-ICAM-1 and sLeX/anti-ICAM-1 beads on (top) 30:70, (middle) 50:50, and (bottom) 80:20 P-selectin/ICAM-1 surfaces at a shear rate of 131 s−1.
ICAM-1-antibody interactions slow rolling
In addition to measuring the binding fluxes of firmly bound sLeX/aICAM-1 beads, rolling velocities of microspheres on P-selectin and P-selectin/ICAM-1 surfaces were measured. Fig. 9, top compares the average rolling velocity of sLeX/aICAM-1 coated beads on P-selectin and P-selectin/ICAM-1 surfaces. Average microsphere rolling velocity on P-selectin/ICAM-1 surfaces decreases with decreasing sLeX/aICAM-1 bead density ratio (increasing %aICAM-1). This observation is in contrast to what is seen with the same microspheres rolling on P-selectin surfaces (see Fig. 9, top) where, because sLeX density is decreasing, rolling velocity increases. This shows that microsphere rolling on P-selectin/ICAM-1 surfaces is stabilized by ICAM-1-antibody bonds between microsphere and surface, and that some degree of ICAM-1 binding can be tolerated and support rolling adhesion. In addition, Fig. 9, bottom, which compares microsphere rolling velocity on surfaces with different ratios of P-selectin/ICAM-1 (30:70, 50:50, and 80:20), shows an interesting transition between P-selectin-controlled and ICAM-controlled regimes. When the microspheres are predominately coated with sLeX (low aICAM-1), average rolling velocity decreases when P-selectin is added to the surface (i.e., increasing P-selectin surface ratio). In this regime, the dynamics of adhesion are controlled by P-selectin interactions. However, when the beads are predominately (but not exclusively) coated with a-ICAM-1, increasing P-selectin on the surface can lead to an increase in the rolling velocity. At one surface ratio of P-selectin/ICAM-1 (80:20), the net effect of these counteropposing forces is that rolling velocity is independent of the relative amount of ligands on the bead surface. These results suggest a two-molecule system can illustrate interesting dynamic control mechanisms to maintain constant levels of rolling adhesion over wide range of surface densities.
FIGURE 9.
Rolling velocity of 9.95-μm sLeX/anti-ICAM-1 microspheres on P-selectin and P-selectin/ICAM-1 surfaces. (Top) Comparison of rolling velocity on P-selectin (⧫) and 30:70 P-selectin/ICAM-1 (◊) surfaces as function of anti-ICAM-1 microsphere coating ratio at a shear rate of 131 s−1. (Bottom) Comparison of rolling velocity on 30:70 (▵), 50:50 (⋄), and 80:20 (*) P-selectin/anti-ICAM-1 surfaces as function of anti-ICAM-1 microsphere coating ratio at a shear rate of 131 s−1.
To further understand the rolling interactions of the two-molecule microspheres on P-selectin/ICAM-1 surfaces, instantaneous rolling velocities of microspheres on P-selectin and P-selectin/ICAM-1 surfaces were examined. Fig. 10, A and B, shows the instantaneous velocity traces as a function of time for 50% aICAM-1 beads on P-selectin (363 ± 5.5 sites/μm2) and 50:50 P-selectin/ICAM-1 (∼204 ± 17.8 sites/μm2 P-selectin). As shown in these traces, sLeX/aICAM-1 beads interacting with P-selectin/ICAM-1 surfaces displayed slower rolling velocity when compared to beads rolling on pure P-selectin surface, though the selectin site density was lower on the P-selectin/ICAM-1 surface. Furthermore, these 50% aICAM-1 beads displayed long duration of pauses (periods of zero instantaneous velocity) when interacting with P-selectin/ICAM-1 surface, suggesting a gradual transition from rolling to firm adhesion. These periods of pausing are due to ICAM-1/aICAM-1 interactions, since similar beads showed short pauses or no pauses when rolling on surfaces without ICAM-1, as shown in Fig. 10 A. Fig. 10 C, showing a plot of average fraction of time paused by sLeX/aICAM-1 beads as a function of aICAM-1 bead coating ratio, shows that the fraction of time during which beads are paused increases as the ratio of aICAM-1 to sLeX increases from 0% to 100%; where at a 100% aICAM-1 ratio, no beads are rolling. This plot illustrates that the periods of pausing displayed by sLeX/aICAM-1 beads on P-selectin/ICAM-1 surfaces are indeed mediated by ICAM-1 interacting with the antibody present on microsphere surface.
FIGURE 10.

Instantaneous velocity traces of 10.2-μm microspheres coated with 50% sLeX beads on a (A) P-selectin (363 ± 5.5 sites/μm2) surface and (B) 50:50 P-selectin/ICAM-1 at 125 s−1. Instantaneous velocities were taken every 1/10 s. (C) Average fraction of time paused by sLeX/anti-ICAM-1 beads on a 50:50 P-selectin/ICAM-1 surface as a function of anti-ICAM-1 coating ratio (aICAM-1 bead site density) over a period of 10 s at 125 s−1.
DISCUSSION
The role of endothelial selectin in ICAM-mediated firm arrest and eventual transmigration of leukocytes in response to inflammation is a major focus of research. The mechanism of initial leukocyte tethering or rolling is well characterized, and is believed to be important in bringing leukocyte within the proximity necessary for integrin activation by chemoattractants such as platelet-activating factor (PAF) (Butcher, 1991; Lawrence and Springer, 1991; Springer, 1994). In addition, selectins (molecules known to mediate rolling) have been implicated in the direct activation of cell arrest, independent of chemotactic signaling (Gopalan et al., 1997; Evangelista et al., 1999; Simon et al., 2000). Unlike lymphocytes, in which chemokines directly involved in arrest have been found that target lymphocytes to specific sites (Campbell and Butcher, 2000), no chemokines has been found in granulocytes, which has led to a focus on the novel signal transduction mechanisms involved in the process of granulocyte firm adhesion. Overlooked, however, is the role that selectin physical chemistry plays in facilitating firm adhesion, outside of its role as a direct activator of β2-integrins. In this work, we attempted to begin to understand the dynamics of a two-receptor system comprised of a weak receptor, sLeX, and a stronger receptor, anti-ICAM-1, interacting with their corresponding ligands, P-selectin and ICAM-1, respectively. Our goal was to develop a two-receptor system that allows us to elucidate basic scientific principles in adhesion. Understanding these interactions will afford us the fundamental understanding necessary to explore the role of selectin-ligand interactions (rolling) in facilitating the transition from rolling to firm adhesion in leukocytes. Our work utilizes a cell-free system that is comprised of a parallel plate flow chamber with a P-selectin/ICAM-1 coated surface making the bottom part of the chamber. We perfused over this P-selectin/ICAM-1 coated surface polystyrene microspheres whose surfaces have been modified with P-selectin ligand, sLeX, and a monoclonal antibody against ICAM-1 via avidin-biotin chemistry. Similar cell-free systems with microspheres coated with selectin ligand, such as sLeX and PSGL-1, interacting with selectin surfaces in parallel flow chambers have been successfully used to identify the role of selectin-ligand physical chemistry in supporting rolling interactions (Brunk and Hammer, 1997; Greenberg et al., 2000; Rodgers et al., 2000).
Using our cell-free assay, we show that microspheres coated with sLeX and aICAM-1 will roll and bind firmly to P-selectin/ICAM-1 surfaces under flow. We show that microsphere rolling and firm adhesion is dependent on the wall shear stress within the flow chamber. As wall shear stress increases, the number of microspheres able to bind firmly with P-selectin/ICAM-1 surfaces diminishes, resulting in a decrease in the number of firmly adherent beads. Most interestingly, we find that firm adhesion of sLeX/aICAM-1 beads on P-selectin/ICAM-1 surfaces is dependent on sLeX/aICAM-1 bead site density ratio as well as the substrate surface density ratio of P-selectin/ICAM-1.
There are several distinct lines of evidence that suggest selectin-mediated rolling can facilitate the transition to firm adhesion presented in this article. They are:
As illustrated in Fig. 6 B, beads bearing combinations of aICAM-1 and sLeX, at certain ratios, display equivalent levels of firm adhesion to beads with only aICAM-1, although beads with only aICAM-1 have a higher density of aICAM-1 as shown in Table 1 and plotted in Fig. 6 B.
As illustrated in Fig. 7, the maximal level of firm binding of 50:50 sLeX/aICAM-1 coated beads occurs on substrates with 80% ICAM-1 and 20% P-selectin, not when the surfaces have purely ICAM-1.
As illustrated in Fig. 8, there is a significant increase in firm binding of beads bearing the functional selectin ligand, sLeX, in combination with aICAM-1, compared to the beads bearing the nonfunctional ligand, LeX, with aICAM-1 at the same surface densities of aICAM-1. If rolling had no effect on firm adhesion, the extent of firm adhesion should be the same.
Furthermore, the results of this article suggest there are regimes in which each of the molecules can control binding. In the P-selectin controlled regime, there is little firm adhesion; increasing P-selectin density or P-selectin-ligand interactions decreases the rolling velocity. In the ICAM-1 controlled regime, antibody/ICAM-1 interactions dominates, more firm adhesion is seen, and increasing P-selectin densities have little effect on the extent of firm adhesion and can actually increase rolling velocity. In between these regimes, P-selectin density plays a critical role in switching adhesion from a rolling phenotype to a firm adhesion phenotype, and greatly facilities the transition to firm adhesion. We also showed that under the right conditions, combinations of the two molecules can maintain stable rolling at a constant velocity across a wide range of concentrations.
The results of this article correspond well to the theoretical results calculated and published recently by our laboratory (Bhatia et al., 2003). In that article, we used Adhesive Dynamics to calculate the dynamics of adhesion of leukocytes to surfaces mediated by two adhesion molecules—a selectin and an integrin. The validity of this model was verified several ways; the most compelling was a systematic comparison to experimental data on rolling in E-selectin or β2-integrin knockout mice, in which the progressive change in rolling velocity could be accurately recreated (Fig. 11 in Bhatia et al., 2003). This article anticipated many of the principal effects elucidate here experimentally. For instance, the state diagram for adhesion indicates that adding selectin interactions for existing concentration of activated integrin can lead to firm adhesion (Fig. 3 in Bhatia et al., 2003). Also, progressive addition of a firm adhesion ligand can lead to the progressive slowing of rolling, with increasing fraction of time paused as shown experimentally in Fig. 10 here (Figs. 4 and 5 in Bhatia et al., 2003). Furthermore, subsequent theoretical results show that a constant rolling velocity may be achieved through a combination of different integrin and selectin densities, as shown in Fig. 9 here (our unpublished results). Thus the basic principles elucidated by experiment were anticipated by theory, and the results of theory and experiment correspond well.
The question is, to what extent does our cell-free system, in which we explore the effect of P-selectin/ligand interactions on the firm adhesion mediated by ICAM-1 interacting with an antibody, in lieu of activated integrin or I-domain, illustrate principles that apply to leukocytes? We trust readers will recognize that reconstitution of an active integrin in a well-designed orientation, density, and activation state is not trivial given current technology, and a comprehensive biophysical study would not be easily achieved given the likely struggles such a molecule would engender. We do not claim that our system is a mimetic of a leukocyte. Rather, we believe our results suggest mechanisms that leukocytes may use in securing firm binding.
However, we did measure the kinetics of binding of aICAM-1, to compare to the kinetics of activated β2-integrin binding to ICAM-1. The kinetics of the antibody to ICAM-1 used in our system is similar to that of activated LFA-1. The measured on-rate (kon) for anti-ICAM-1 mAb, BBIG-I1, of 156,000 M−1s−1 is on the same order of magnitude as that of purified I-domain in the locked open position (107,000 M−1s−1) and that of activated αLβ2 integrin (224,000 M−1s−1) (Shimaoka et al., 2002). The off-rate of the antibody (1.13 × 10−4 s−1) from ICAM-1 is two orders of magnitude lower than that of LFA-1 (2.98 × 10−2 s−1) in its active state. The surprising close correspondence of the on-rate of the BBIG-I1 antibody with ICAM-1, and much lower off-rate, leads to the following interpretations of the results. First a lower off-rate should mean aICAM-1/ICAM-1 interactions are stronger, and better able to support firm adhesion than activated integrin (Chang et al., 2000). However, the value of having a lower off-rate than integrin is questionable, since the activated β2-integrin's off-rate is already two orders of magnitude lower than that of P-selectin/PSGL-1 interactions (Alon et al., 1995). Therefore, aside from some details in the dynamics of rolling conveyed by the lower off-rate, the ability to secure firm adhesion of the antibody should be similar to that of an activated integrin. Also, the ability of aICAM-1/ICAM-1 interactions to support (stabilize) some extent of rolling is consistent with that seen with the integrin I-domain or purified LFA-1 (Salas et al., 2002), which have also been shown to support rolling. Thus, the antibody functions like integrin—it can support firm adhesion at high densities, yet at lower densities, it stabilizes rolling just like β2-integrins. Second, the on-rate of the antibody is similar to that of β2-integrins, and therefore the ability of the antibody to capture beads (i.e., the extent of firm adhesion) should also be similar. Thus, we expect the synergy between selectin and antibody to ultimately be similar to that between selectin and integrin, although we fully recognize and look forward to testing these principles in leukocytes or with beads bearing physiological ligands.
A comment on the use of sLeX as a ligand for P-selectin is warranted. Rodgers and co-workers showed that sLeX is a fully functional P-selectin ligand (Rodgers et al., 2000). Similarly, Brunk and co-workers showed that sLeX and isomer, sLea, are functional ligands for E-selectin (Brunk and Hammer, 1997). Also, Somers and co-workers showed that a desulfated form of PSGL-1, which bears sLeX, is functional as a P-selectin ligand and structural studies show that an sLeX residue ligates with P-selectin in the desulfated form (Somers et al., 2000). Rodgers et al. also showed in a cell-free system that desulfated PSGL-1 bearing sLeX supported rolling in a cell-free system (Rodgers et al., 2000). Thus, with the goal of creating a two-molecule system to identify the interplay between two molecules on beads, the use of sLeX as a selectin ligand seems reasonable. Using this carbohydrate also allowed for a clean comparison with LeX that would not be possible with PSGL-1.
Given the results of this article, and the possibility that the principle illustrated herein may be at play in the transition of leukocyte rolling to firm adhesion, we suggest the following similarities to the adhesion of leukocytes. For one, the dependency of the firm adhesion of our two-receptor microspheres on the wall shear stress within the flow chamber is similar to what has been previously measured for leukocyte adhesion by Simon and co-workers, where they found that the efficiency of integrin dependent adhesion of leukocytes on stimulated, cultured endothelial cells decreases from a maximum as applied shear increases (Simon et al., 2000). Furthermore, although it has been shown that ICAMs can facilitate some firm adhesion of leukocytes in the absence of selectin-ligand interactions (Gopalan et al., 1997; Simon et al., 2000), our results clearly suggest the role selectin-ligand can have in facilitating firm adhesion mediated by a high affinity ligand to ICAM-1 as highlighted in Fig. 6. Finally, rolling velocity data obtained for sLeX/aICAM-1 microspheres interacting with P-selectin/ICAM-1 surfaces show that ICAM-1/antibody interactions slow rolling as shown in Figs. 9 and 10. This observation is similar to what has previously been reported with β2-integrin (CD18) deficient mice, where the velocities at which leukocytes roll in these mice were observed to be higher than the wild-type suggesting synergistic roles of selectin and ICAMs in leukocyte rolling adhesion (Kunkel et al., 1996; Jung et al., 1998). This observation also suggests that the transition from rolling to firm adhesion is a gradual process, where the initial interaction of intercellular adhesion molecules with their ligands serves to stabilize selectin-mediated adhesion to achieve optimum cell interaction with the endothelium. These interactions then eventually lead to firm arrest followed by cell transmigration. Also, the pause times are suspected of having critical effects on downstream signaling, so the ratio of selectin/ICAMs likely has a major effect on the ability of the leukocyte to see and harvest information from endothelium during rolling adhesion. Thus, the biophysical principles elucidated here may very well be involved in leukocyte adhesion.
Acknowledgments
We thank Dr. M. King, Dr. W. Qie, Mr. E. Johnston, Mr. J. Lin, and Mr. D. Ege at the University of Pennsylvania for their valuable suggestions in this work.
We gratefully acknowledge support from National Institutes of Health grants GM59100 and HL18208.
References
- Alon, R., D. A. Hammer, and T. A. Springer. 1995. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 374:539–542. [DOI] [PubMed] [Google Scholar]
- Bhatia, S. K., M. R. King, and D. A. Hammer. 2003. The state diagram for cell adhesion mediated by two receptors. Biophys. J. 84:2671–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks, J. E., T. Moll, R. Eytner, and D. Vestweber. 1998. Stimulation of P-selectin glycoprotein ligand-1 on mouse neutrophils activates beta 2-integrin mediated cell attachment to ICAM-1. Eur. J. Immunol. 28:433–443. [DOI] [PubMed] [Google Scholar]
- Brunk, D. K., and D. A. Hammer. 1997. Quantifying rolling adhesion with a cell-free assay: E-selectin and its carbohydrate ligands. Biophys. J. 72:2820–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher, E. C. 1991. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 67:1033–1036. [DOI] [PubMed] [Google Scholar]
- Campbell, J. J., and E. C. Butcher. 2000. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr. Opin. Immunol. 12:336–341. [DOI] [PubMed] [Google Scholar]
- Chang, K. C., D. F. Tees, and D. A. Hammer. 2000. The state diagram for cell adhesion under flow: leukocyte rolling and firm adhesion. Proc. Natl. Acad. Sci. USA. 97:11262–11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet, K., and D. Vestweber. 1999. Molecular mechanisms that control leukocyte extravasation: the selectins and the chemokines. Histochem. Cell Biol. 112:1–23. [DOI] [PubMed] [Google Scholar]
- Edwards, B. S., M. S. Curry, H. Tsuji, D. Brown, R. S. Larson, and L. A. Sklar. 2000. Expression of P-selectin at low site density promotes selective attachment of eosinophils over neutrophils. J. Immunol. 165:404–410. [DOI] [PubMed] [Google Scholar]
- Eniola, A. O., S. D. Rodgers, and D. A. Hammer. 2002. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials. 23:2167–2177. [DOI] [PubMed] [Google Scholar]
- Evangelista, V., S. Manarini, R. Sideri, S. Rotondo, N. Martelli, A. Piccoli, L. Totani, P. Piccardoni, D. Vestweber, G. de Gaetano, and C. Cerletti. 1999. Platelet/polymorphonuclear leukocyte interaction: P-selectin triggers protein-tyrosine phosphorylation-dependent CD11b/CD18 adhesion: role of PSGL-1 as a signaling molecule. Blood. 93:876–885. [PubMed] [Google Scholar]
- Gopalan, P. K., C. W. Smith, H. Lu, E. L. Berg, L. V. McIntire, and S. I. Simon. 1997. Neutrophil CD18-dependent arrest on intercellular adhesion molecule 1 (ICAM-1) in shear flow can be activated through L-selectin. J. Immunol. 158:367–375. [PubMed] [Google Scholar]
- Greenberg, A. W., D. K. Brunk, and D. A. Hammer. 2000. Cell-free rolling mediated by L-selectin and sialyl Lewis(x) reveals the shear threshold effect. Biophys. J. 79:2391–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, U., K. E. Norman, K. Scharffetter-Kochanek, A. L. Beaudet, and K. Ley. 1998. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J. Clin. Invest. 102:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel, E. J., U. Jung, D. C. Bullard, K. E. Norman, B. A. Wolitzky, D. Vestweber, A. L. Beaudet, and K. Ley. 1996. Absence of trauma-induced leukocyte rolling in mice deficient in both P-selectin and intercellular adhesion molecule 1. J. Exp. Med. 183:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labadia, M. E., D. D. Jeanfavre, G. O. Caviness, and M. M. Morelock. 1998. Molecular regulation of the interaction between leukocyte function-associated antigen-1 and soluble ICAM-1 by divalent metal cations. J. Immunol. 161:836–842. [PubMed] [Google Scholar]
- Lawrence, M. B., and T. A. Springer. 1991. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 65:859–873. [DOI] [PubMed] [Google Scholar]
- Munn, L. L., R. J. Melder, and R. K. Jain. 1994. Analysis of cell flux in the parallel plate flow chamber: implications for cell capture studies. Biophys. J. 67:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers, S. D., R. T. Camphausen, and D. A. Hammer. 2000. Sialyl Lewis(x)-mediated, PSGL-1-independent rolling adhesion on P- selectin. Biophys. J. 79:694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas, A., M. Shimaoka, S. Chen, C. V. Carman, and T. A. Springer. 2002. Transition from rolling to firm adhesion is regulated by the conformation of the I domain of the integrin LFA-1. J. Biol. Chem. 277:50255–50262. [DOI] [PubMed]
- Shao, J. Y., H. P. Ting-Beall, and R. M. Hochmuth. 1998. Static and dynamic lengths of neutrophil microvilli. Proc. Natl. Acad. Sci. USA. 95:6797–6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka, M., J. Takagi, and T. A. Springer. 2002. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 31:485–516. [DOI] [PubMed] [Google Scholar]
- Simon, S. I., Y. Hu, D. Vestweber, and C. W. Smith. 2000. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J. Immunol. 164:4348–4358. [DOI] [PubMed] [Google Scholar]
- Somers, W. S., J. Tang, G. D. Shaw, and R. T. Camphausen. 2000. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selection bound to sLe(x) and PSGL-1. Cell. 103:467–479. [DOI] [PubMed] [Google Scholar]
- Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76:301–314. [DOI] [PubMed] [Google Scholar]
- Thiel, M., C. Zourelidis, J. D. Chambers, U. H. von Andrian, K. E. Arfors, K. Messmer, and K. Peter. 1997. Expression of beta 2-integrins and L-selectin on polymorphonuclear leukocytes in septic patients. Eur. Surg. Res. 29:160–175. [DOI] [PubMed] [Google Scholar]








