Abstract
Since the first occurrence of O139 Vibrio cholerae as a cause of cholera epidemics, this serogroup has been investigated intensively, and it has been found that its pathogenicity is comparable to that of O1 El Tor strains. O139 isolates express a thin capsule, composed of a polymer of repeating units structurally identical to the lipopolysaccharide (LPS) O side chain. In this study, we investigated the role of LPS O side chain and capsular polysaccharide (CPS) in intestinal colonization by with genetically engineered mutants. We constructed CPS-negative, CPS/LPS O side chain-negative, and CPS-positive/LPS O side chain-negative mutants. Furthermore, we constructed two mutants with defects in LPS core oligosaccharide (OS) assembly. Loss of LPS O side chain or CPS resulted in a ≈30-fold reduction in colonization of the infant mouse small intestine, indicating that the presence of both LPS O side chain and CPS is important during the colonization process. The strain lacking both CPS and LPS O side chain and a CPS-positive, LPS O side chain-negative core OS mutant were both essentially unable to colonize. To characterize the role of surface polysaccharides in survival in the host intestine, resistance to several antimicrobial substances was investigated in vitro. These investigations revealed that the presence of CPS protects the cell against attack of the complement system and that an intact core OS is necessary for survival in the presence of bile.
The causative agent of the intestinal disease cholera is Vibrio cholerae, a gram-negative motile bacterium (17). In general, the important steps of infection include ingestion of V. cholerae along with contaminated food or water, passage through the gastric acid barrier of the stomach, adherence to and penetration through the intestinal mucus lining, adherence to intestinal epithelial cells, multiplication, and cholera toxin production (12). While there are currently more than 200 recognized V. cholerae O serogroups, essentially only O1 and more recently O139 have been identified as the cause of large cholera epidemics.
The encapsulated O139 pathogen emerged in late 1992, causing cholera epidemics in Bangladesh and India (23). Molecular and epidemiological analysis as well as whole-genome comparison revealed that O139 strains are very similar to O1 El Tor strains, supporting the hypothesis that pathogenic O139 isolates evolved from the recent seventh pandemic El Tor strains (3, 7, 9, 10).
One genetic difference between O1 El Tor and O139 strains corresponds to the surface polysaccharide biosynthesis (wb∗) gene cluster. In O139 strains, the genes encoding the O1 antigen are deleted and replaced by the O139 antigen (wbf) locus, encoding the lipopolysaccharide (LPS) O side chain and the capsular polysaccharide (CPS) (34). The structures of the LPS O side chain and the CPS are identical, and it was found that one O139 side chain unit is linked to lipid A-core oligosaccharide (OS), whereas the LPS-unlinked CPS is formed by polymerized O139 side chain repeat units (15, 16, 41).
Consistent with the structural data, mutations in putative O139 side chain biosynthetic genes led to LPS O side chain- and CPS-negative mutants (5, 41). On the other hand strains with mutations in putative capsule translocation genes are still able to express the LPS O side chain but they are CPS-negative (4). No defined LPS O side chain-negative but CPS-positive mutants linked to the wbf cluster have been described; only spontaneous mutants with unknown mutation(s) have been isolated so far (2).
Other gram-negative bacteria are known that, like O139 strains, express side chain repeat units as a high-molecular-weight capsular and a low-molecular-weight lipid A-core-linked form. These strains are classified based on their biosynthetic gene clusters as expressing either group 1 or group 4 capsule (44). The current biosynthetic model for these groups proposes that individual repeat units are assembled on a lipid carrier in the cytoplasm and then transferred across the inner membrane by a polysaccharide exporter protein. The repeat units can serve as a substrate for the polymerization and capsule translocation machinery or for the WaaL enzyme, which ligates one repeating unit onto the lipid A-core OS (44).
Given the similarity, it is likely that V. cholerae O139 strains use a similar pathway. The structures of the LPS core OS of O1 and O139 strains were reported to be fairly similar (13), and we showed recently that they have identical core OS biosynthetic (wav) gene clusters (25). The putative lipid A core-surface polymer ligase WaaL is also encoded by the wav gene cluster, and mutations within this gene led to an O side chain-negative O1 El Tor mutant (25). Since the O1 and O139 waaL genes are identical (25), it is likely that ligation of the O139 side chain to the lipid A core is also catalyzed by this enzyme.
The role of the O139 surface polysaccharides in V. cholerae pathogenesis is still not completely understood. The CPS layer is hydrophobic and very thin, and it provides only partial protection against phagocytosis (1, 21). However, synthesis of the capsular material is required for efficient colonization of the small intestine in suckling mice (41). CPS is also required for serum resistance (41), and this phenotype might be relevant for intestinal colonization of humans by O139 strains, since complement proteins are detectable within the small intestine (29).
Expression of the LPS O side chain also seems to be important for colonization and complement resistance, as investigated with undefined spontaneous LPS O side chain-negative, CPS-positive mutants (2). The influence of the LPS core OS has not been investigated for O139 strains. Evidence for contribution of the core OS to V. cholerae virulence comes from our recent observation that strains of different serogroups associated with clinical cholera (O1, O139, and O37) have the same wav gene cluster, which differs markedly from those of environmental isolates (25). Indeed, preliminary data with V. cholerae O1 LPS core OS mutants suggested that an intact core OS contributes to resistance against bile salts, short-chain organic acids, and antimicrobial peptides, which should improve survival in the human small intestine (26). O139 strains can also produce another exopolysaccharide (EPS) which is important for biofilm formation but interferes with intestinal colonization (43).
In this study, we constructed a set of defined CPS, LPS O side chain, and LPS core OS V. cholerae O139 mutants and investigated their behavior in intestinal colonization in vivo and their resistance to several antimicrobial compounds in vitro.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Escherichia coli strains SM10λpir (22) and XL-1 Blue (New England Biolabs, Schwalbach, Germany) were used for all genetic constructions. V. cholerae strains and plasmids used in this study are listed in Table 1. All strains were grown in Luria broth (LB; Difco) at 37°C, except as noted otherwise. For optimal cholera toxin and toxin-coregulated pilin (TCP) expression, the strains were incubated under inducing conditions. Cultures grown overnight in AKI medium (14) were diluted 1:100 in AKI without NaHCO3, grown for 4 h at 37°C under static growth conditions, and then transferred to vigorous shaking for 4 h at 37°C (20). Expression of waaL from the araBAD promoter was induced by supplementing the medium with 0.01% arabinose for in vitro assays; for the in vivo colonization assay, no induction was necessary. Antibiotics (Sigma, Deisenhofen, Germany) were used at the following concentrations: kanamycin, 50 μg/ml; ampicillin, 50 and 100 μg/ml for V. cholerae and E. coli, respectively; and chloramphenicol, 2 and 20 μg/ml for V. cholerae and E. coli, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotypea | Reference or source |
|---|---|---|
| Strains | ||
| MO10 | Clinical isolate of V. cholerae O139 | 42 |
| PW96 | MO10 lacZ::MCS | 43 |
| MO10 ΔwaaL | MO10 ΔwaaL | This study |
| MO10 ΔwaaLwbfF | MO10 ΔwaaL wbfF::pGP704 | This study |
| MO10 ΔgalEK | ΔgalE::cm ΔgalK | This study |
| MO10 wbfF | wbfF::pGP704 | This study |
| MO10 waaF | waaF::pGP704 | This study |
| MO10 wavB | wavB::pGP704 | This study |
| Plasmids | ||
| pTrcAkan | Cloning vector Kmr | 24 |
| pBAD28 | Cloning vector Apr Kanr | 8 |
| pGP704 | OriR6K mobRP4 Apr | 22 |
| pUC4K | Apr Kmr | 39 |
| pGPwaaF | ‘waaF’b Apr | 25 |
| pGPwavB | ‘wavB’ Apr | 25 |
| pKEK229 | OriR6K mobRP4 sacB Apr | 6 |
| pKEKΔgalE::cm | ΔgalE::cm Apr | 26 |
| pBADwaaL | waaL+ Kmr | 25 |
| pACYCgalE | galE+ Kmr | 26 |
| pACYCwaaF | waaF+ Kmr | 25 |
| pAKwavB | wavB tetR+ Cmr | 25 |
| pGPwbfF | ‘wbfF’ in pGP704 Apr | This study |
| pKEKΔgalK | ΔgalK in pKEK229 Apr | This study |
| pKEKΔwaaL | ΔwaaL in pKEK229 Apr | This study |
| pSSkanwavB1 | pAKwavB tetR+ Cmr Kmr | This study |
| pSSkanwavB2 | pSSkanwavB1 ΔtetR Cmr Kmr | This study |
MCS, multiple cloning site.
‘gene,’ internal gene fragment.
Plasmid constructions.
All DNA manipulations were carried out according to standard protocols (19). The synthetic oligonucleotides used for PCR amplification of an internal gene fragment of wbfF were otnA1 (5′-TTGAAGTTTTAATTGATGACCTGT-3′) and otnASalI (5′-TTCCATTCAATAAAACCTGGG-3′); subsequently this wbfF fragment was subcloned into pGP704 (4, 22). The construction of suicide plasmids to introduce chromosomal in-frame deletions of the genes galK and waaL were performed in the same manner. DNA fragments of about 500 bp encompassing the regions upstream of the corresponding genes were PCR amplified with primer pairs waaLupSmaI (5′-TAAACCCGGGGGCGTCTCTATCGAGCTCAA-3′) and waaLupSalI (5′-TAATAGTCGACGCACTCAGGGTTATAAGAG-3′), and galKupSmaI (5′-TCCCCCGGGTAAAAGATGGCGCGAGAACC-3′) and galKupSalI (5′-TACGCGTCGACAAGGTGCGTAGCATCATAGC-3′).
The downstream DNA regions of the corresponding genes were PCR amplified with primer pairs waaLdownSalI (5′-TACGCGTCGACAAACAGGCTCTCCAATCAGC-3′) and waaLdownApaI (5′-GTAAGGGCCCGCCAAAGCAACATACCTTTCC-3′), and galKdownSalI (5′-TACGCGTCGACCACCCGAAGCAAGTAGAAGC-3′) and galKdownApaI (5′-GTAAGGGCCCATTTGCCAGCGACGACGATC-3′). The upstream and downstream PCR products of waaL and galK were digested with SalI and ligated overnight. The ligation mix was digested with SmaI and ApaI, purified, and ligated into pKEK229 (6) that had been digested with SmaI and ApaI to give plasmids pKEKΔgalK and pKEKΔwaaL.
For the construction of complementing plasmid pSSwavB, we used plasmid pAKwavB, in which wavB was subcloned under the transcriptional control of the tet promoter (25). To make the plasmid usable for this study, we ligated a BamHI fragment containing a kanamycin resistance gene (kanR) (39) (Pharmacia, Biotech Europe, Freiburg, Germany) into the unique BamHI site of pAKwavB to obtain pSSkanwavB1. We deleted the tetracycline resistance gene (tetR) by first digesting pSSkanwavB1 with restriction enzymes SnaBI and NdeI, then treating with Klenow fragment (Sigma), and religating, yielding plasmid pSSwavB2, which had lost most of the tetR gene (341-bp internal fragment) and constitutively expressed wavB.
Mutant strain constructions.
The chromosomal mutations in V. cholerae O139 strain MO10 were constructed with different suicide plasmids (Table 1) as described previously (25, 26). The correct chromosomal insertion for all mutants was confirmed by Southern blot analysis (data not shown).
Analysis of cell surface polysaccharides.
Cell surface polysaccharides from proteinase K-digested whole-cell lysates were isolated as described by Hitchcock and Brown (11) and analyzed by electrophoresis on sodium dodecyl sulfate-15% polyacrylamide gels. LPS was visualized by silver staining (38). CPS and LPS O side chain expression was assessed by Western blot analysis. Samples were electrophoretically transferred to nitrocellulose membranes (37) and probed with a polyclonal rabbit anti-O139 serum (25).
In vitro resistance assays.
Serum resistance was determined under laboratory conditions. Briefly, cells were grown to mid-logarithmic phase in LB broth, washed, and mixed to a final concentration of 20% with normal human serum, obtained and pooled from four donors, or 20% heat-inactivated normal human serum in phosphate-buffered saline (PBS) with 0.1% peptone. Approximately 108 CFU were used in each assay. After incubation at 37°C for 1 h, the cells were harvested, washed, and resuspended in PBS-0.1% peptone. The viable cells were determined by plating serial dilutions onto LB agar. The minimal bactericidal concentration (MBC) of protamine (Sigma), ranging from 600 μg/ml to 9.3 μg/ml, was determined as described by Steinberg et al. (33). The MBC was defined as the lowest concentration of drug that eliminated viable cells after 18 h of incubation at 37°C without shaking. The sensitivity to bile salts was tested on thiosulfate-citrate-bile-sucrose (TCBS) agar plates, prepared as instructed for the commercially available TCBS (Difco), containing no or 0.8% bile.
In vivo colonization assay.
The infant mouse colonization assay for O139 strains has been described previously (43). Briefly, mutant strains (lacZ+) were mixed with strain PW96 (MO10 wild type lacZ) and given as an oral inoculum at a ratio of approximately 105 CFU of mutant to 105 CFU of wild type to 5- to 6-day-old CD-1 suckling mice. After a 22-h period of colonization, intestinal homogenates were collected, and the ratio of mutant to wild-type cells was determined by plating appropriate dilutions on LB agar containing streptomycin and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal).
We also determined the in vitro competition index. The same inoculum which was used for the mice was added to 5 ml of LB and allowed to grow at 37°C overnight, and then cells were plated on X-Gal-LB agar medium to determine the cell counts.
CTX-Kmφ transduction and GM1-ELISA.
CTX-Kmφ utilizes TCP as its receptor, and therefore TCP expression was investigated by determining CTX-Kmφ phage transduction frequency (40). The level of cholera toxin in culture supernatants was measured by the GM1-ganglioside enzyme-linked immunosorbent assay (GM1-ELISA) (35) with purified cholera toxin to generate a standard curve.
TCP Western blot analysis.
Whole-cell lysates were matched by optical density at 600 nm, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a nitrocellulose membrane (37), and probed with TcpA polyclonal antiserum (kindly provided by J. Mekalanos).
RESULTS
Construction of V. cholerae O139 LPS and capsule mutants.
Two core OS biosynthesis genes, the putative heptosyl II transferase (waaF, VC0236) and the putative 1-4-β-glycosyl transferase (wavB, VC0224), as well as the putative O antigen ligase gene waaL (VC0237) have recently been characterized in O1 El Tor strains (25). In this study, we generated mutations in the corresponding genes in the V. cholerae O139 isolate MO10.
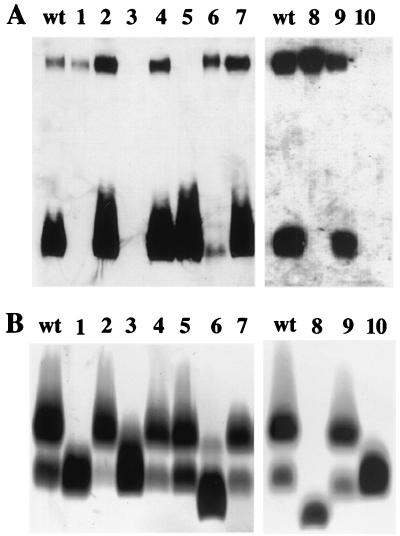
LPS extracted from the waaF mutant displayed in SDS-PAGE analysis, as expected, a faster-migrating core OS and no LPS O side chain (Fig. 1B, lane 8). The presence of the CPS was confirmed in Western blot analysis (Fig. 1A, lane 8). Inactivation of wavB resulted in a truncated LPS core OS with attached O side chain, but the amount of O side chain was significantly less compared to the wild type (Fig. 1B, lane 6). This was in contrast to the O1 wavB mutant (25), in which no significant reduction of O side chain was observed. The synthesis of the CPS was not affected in the O139 wavB strain (Fig. 1A, lane 6). Deletion of waaL led to an LPS O side chain-negative strain (Fig. 1A and B, lane 1), which was consistent with the proposed function of WaaL as the lipid A core-surface polymer ligase. Furthermore, the waaL deletion did not affect capsule biogenesis (Fig. 1A, lane 1).
FIG. 1.
Surface polysaccharides expressed by LPS and CPS mutants. (A) Western blot analysis with O139-specific antiserum (see Materials and Methods). Reactive bands are indicated as follows: the upper band represents the capsular form of O139 side chain, and the lower band corresponds to the O side chain/core OS/lipid A portion. (B) Silver-stained polyacrylamide gel after SDS-PAGE. Stained bands are indicated as follows: the upper bands represent the O side chain/core OS/lipid A, and the lower bands are the core OS/lipid A portion. Lanes: wt, MO10 wild type; 1, MO10 ΔwaaL; 2, MO10 ΔwaaL pBADwaaL; 3, MO10 ΔgalEK; 4, MO10 ΔgalEK pACYCgalE; 5, MO10 wbfF; 6, MO10 wavB; 7, MO10 wavB pSSkanwavB2; 8, MO10 waaF; 9, MO10 waaF pACYCwaaF; 10, MO10 ΔwaaL ΔwbfF.
In order to be able to compare any effects of these mutants with CPS-negative and LPS O side chain/CPS-negative mutants, we inactivated wbfF and galEK. The putative outer membrane protein WbfF (formerly OtnA) was described earlier to be involved in CPS synthesis, probably in the translocation process (4). Inactivation of wbfF in MO10 caused a CPS-negative but LPS O side chain-positive phenotype (Fig. 1A and B, lane 5), which is in accordance with the otnA characterization reported previously (4).
Recently, we showed that mutations in galE (VCA0774) did not interfere with LPS biosynthesis and intestinal colonization in O1 El Tor strains (26). In contrast, O139 strains do contain Gal residues in the O139 side chain (15, 16), and hence we concluded that the inability to produce UDP-Gal should prevent the biosynthesis of both LPS O side chain and CPS. A strain mutated in galE could still synthesize LPS O side chain and CPS (data not shown), presumably because it can utilize exogenous galactose present in the LB medium. To prevent the utilization of exogenous galactose, we additionally inactivated galK. The galEK double mutant was unable to synthesize CPS and LPS O side chain (Fig. 1A and B, lane 3) and was also unable to form a biofilm (data not shown).
Finally, we constructed a wbfF waaL double mutant, which, like the galEK mutant, was deficient in CPS and LPS O side chain synthesis (Fig. 1A and B, lane 10). Both mutant combinations allowed us to determine whether there was any other UDP-Gal-dependent complex carbohydrate structure not associated with O139 side chain synthesis that might affect intestinal colonization of the galEK mutant. In the presence of plasmids encoding galE, waaL, wavB, or waaF, the phenotypes of the corresponding mutants could be restored (Fig. 1A and B).
In vivo colonization phenotypes of LPS and CPS mutants.
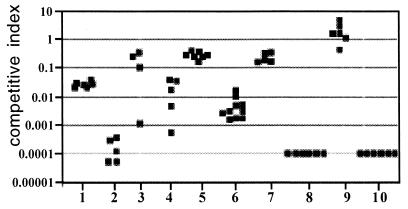
The ability to colonize the small intestine was investigated in the infant mouse model. In a direct comparison of wild-type versus mutant strains, the colonization phenotype for all mutants was determined. In these competition assays, all mutants investigated were attenuated in their ability to successfully colonize the suckling mouse small intestine (Fig. 2). When CPS (wbfF, Fig. 2, data set no. 1) or LPS O side chain synthesis (waaL, Fig. 2, data set no. 4) was prevented, similar decreases in colonization were observed for each of these mutants (≈30-fold). Mutants unable to express O side chain and capsule (galEK, no. 2; waaL wbfF, no. 10) were severely defective for colonization, as indicated by a competitive index of less than 0.0001. No mutant bacteria were recovered for the waaL wbfF strain (competitive index <0.0001), and very few were recovered for the galEK strain (competitive index ≈0.0001), which was inoculated at a higher mutant/wild-type ratio. We believe that these two mutant strains are equally defective in colonization, because when they were coinoculated, they were recovered at extremely low but equal frequencies (data not shown).
FIG. 2.
Colonization deficiency of LPS and CPS mutants. Each point represents the value from a single mouse competition assay. The competitive index is defined as the output ratio of mutant to wild-type bacteria divided by the input ratio of mutant to wild-type bacteria. Strains with the wild-type ability to colonize have a competitive index of approximately 1.0. Data sets: 1, MO10 wbfF; 2, MO10 ΔgalEK; 3, MO10 ΔgalEK pACYCgalE; 4, MO10 ΔwaaL; 5, MO10 ΔwaaL pBADwaaL; 6, MO10 wavB; 7, MO10 wavB, pSSkanwavB2; 8, MO10 waaF; 9, MO10 waaF, pACYCwaaF; 10, MO10 ΔwaaL ΔwbfF. The defect in colonization of all mutant strains was statistically significant (P < 0.01) compared with the wild-type strain MO10 by Student's two-tailed t test.
A similar strong effect on colonization was observed for the CPS-positive, LPS O side chain-negative, core OS-defective waaF mutant (Fig. 2, no. 8). The CPS-expressing wavB mutant, which synthesizes an incomplete core and less LPS O side chain, was slightly more attenuated compared to the waaL and wbfF mutants. The colonization defects of the waaL, galE, wavB, and waaF mutants were essentially restored in the presence of the respective complementation plasmids (Fig. 2, no. 3, 5, 7, and 9). The small divergences in the complementation strains compared to the wild-type strain were most probably due to differences in the level of gene expression.
Next, we examined whether the alteration of the surface polysaccharides caused other deleterious effects which could explain the attenuation in intestinal colonization. None of the mutants demonstrated any general growth defects in in vitro competition assays, with the exception of the waaF mutant, which had an approximately 100-fold attenuation (competitive index of 0.014 for this strain). We also tested the abilities of these strains to induce virulence factors known to be important for intestinal colonization. The formation of TCP is critical for colonization (36), and all strains were able to induce TCP expression under in vitro inducing conditions.
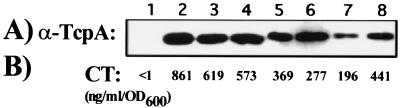
TcpA-directed Western blot analysis revealed that only the waaF mutant expressed slightly less TcpA (Fig. 3A, lane 7), which could be restored in the presence of a waaF expressing plasmid (Fig. 3A, lane 8). However, the waaF mutant assembled functional TCP on the surface, since it could be infected with phage CTX-Kmφ, which is known to use TCP as its receptor (40) (data not shown). All mutants also expressed and secreted relatively normal amounts of cholera toxin, indicating that the mutations affected neither cholera toxin induction nor the type II protein secretion pathway (Fig. 3B). In summary, these investigations indicated that the alterations of the surface polysaccharides caused no detectable cell damage or alteration in virulence factor induction that might explain the colonization defects, with the possible exception of the waaF mutation.
FIG. 3.
Expression of TcpA and cholera toxin in LPS and CPS mutants. Strains were grown under inducing conditions (Materials and Methods). (A) Whole-cell lysates were subjected to Western blot with TcpA antiserum. (B) Cell-free supernatants were measured for cholera toxin (CT) by ELISA and given in per optical density unit at 600 nm. Lanes: 1, MO10 (noninduced); 2, MO10 (induced); 3, MO10 wbfF (induced); 4 MO10 ΔwaaL (induced); 5, MO10 ΔgalEK (induced); 6, MO10 wavB (induced); 7, MO10 waaF (induced); 8, MO10 waaF pACYCwaaF (induced).
Resistance against antimicrobial compounds in vitro.
To investigate the survival of the mutants in the host intestine in more detail, we performed in vitro assays in the presence of compounds known to be present in the human small intestine (26). Resistance against the complement system was investigated in survival assays in the presence of 20% human blood serum (Table 2). All strains synthesizing CPS, including the LPS O side chain-negative mutant (waaL) and both LPS core OS mutants (wavB and waaF), were fully protected against attack by the complement system. In mutants in which the CPS was not expressed, LPS O side chain-positive strains (wbfF) were clearly more resistant to complement than LPS O side chain-negative mutants (galEK and waaL wbfF). These results indicated that the CPS prevents lysis by serum components, whereas protection by the LPS O side chain alone is significantly less effective.
TABLE 2.
Resistance to antimicrobial peptides and the complement system
| Strain | MBC of protamine (μg/ml) | Serum resistancea (% survival) |
|---|---|---|
| MO10 | 150 | 96.75 ± 6.50 |
| MO10 ΔwaaL | 150 | 99.15 ± 1.69 |
| MO10 wbfF | 150 | 3.68 ± 5.58 |
| MO10 ΔwaaL ΔwbfF | 150 | 0.03 ± 0.03 |
| MO10 ΔgalEK | 150 | 0.05 ± 0.06 |
| MO10 ΔgalEK pACYCgalE | ND | 90.25 ± 11.84 |
| MO10 waaFb | 75 | 100 ± 0 |
| MO10 waaF pACYCwaaF | 150 | ND |
| MO10 wavB | 150 | 92.48 ± 8.68 |
Serum resistance was calculated as the ratio of the number of CFU found after cells were incubated for 45 min in 20% normal human serum divided by the number of CFU found after cells were incubated for 45 min in 20% heat-inactivated normal human serum. Indicated is the mean ± standard deviation. All assays were performed at least four times. ND, not determined.
This mutant was also tested in the presence of control plasmid pTrcAkan.
Next, we investigated survival in the presence of the antimicrobial peptide protamine. The only mutant that exhibited any reduction in MBC was the waaF mutant, with a deep core OS defect, and this sensitivity could be complemented in trans by the waaF-expressing plasmid pACYCwaaF (Table 2).
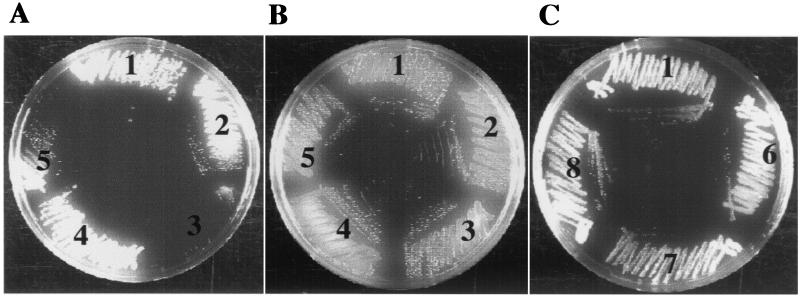
Survival in the presence of bile was tested on TCBS plates containing 0.8% bile. Growth defects were only observed for mutants with defects in core OS assembly (Fig. 4A) and not for the ΔwaaL, wbfF, ΔgalEK (Fig. 4C, sets 6 to 8), and ΔwaaL ΔwbfF (data not shown) mutants. The waaF mutant could not grow (Fig. 4A, set 3), while the wavB mutant grew poorly on these plates (Fig. 4A, 5). The growth defects could be complemented in the presence of waaF- and wavB-expressing plasmids (Fig. 4A, sets 2 and 4).
FIG. 4.
Growth phenotypes of LPS and core OS mutants on TCBS plates. (A) TCBS plus ampicillin (100 μg/ml) containing 0.8% bile. (B) TCS plus ampicillin (100 μg/ml) without bile. (C) TCBS containing 0.8% bile. Sets: 1, MO10 pBAD28; 2, MO10 waaF pACYCwaaF; 3, MO10 waaF; 4, MO10 wavB pSSkanwavB2; 5, MO10 wavB; 6, MO10 ΔgalEK; 7, MO10 ΔwaaL; 8, MO10 wbfF.
In the absence of bile, no growth defect for the waaF and wavB strains was observed (Fig. 4B, sets 3 and 5), indicating that bile was inhibitory to growth. We found no evidence that the bile sensitivity was due to lower levels of OmpU, an outer membrane porin that has been shown to increase resistance against bile (27), since both mutants showed normal levels of OmpU expression (data not shown). These results revealed that an intact LPS core OS was essential for resistance against bile.
DISCUSSION
Initial reports during the outbreak in 1993 suggested that the pathogenic V. cholerae O139 strains originated in the Bay of Bengal (28, 30, 32). Since then, O139 outbreaks have occurred in 11 countries in southeast Asia. However, beginning in 1994, O1 EI Tor strains have reemerged as the predominant cholera-causing organisms on the Indian subcontinent, although O139 strains continue to cause cholera there also.
Pathogenic V. cholerae O139 strains have a complex life cycle, including survival and growth in the estuarine environment and the human intestine. We were interested in the contribution of surface polysaccharides to these processes. The exopolysaccharide VPS seems to be mainly important for survival in aquatic environments by stabilization of biofilms (43), while the role of the other surface polysaccharides, LPS core OS, LPS O side chain, and CPS, during the life cycle is less clear. The behavior of LPS O side chain and CPS mutants in intestinal colonization of O139 isolates was addressed previously, but these investigations were performed with spontaneous mutants (2, 41). With the intention of establishing a comprehensive analysis, we investigated the role of surface polysaccharides in intestinal colonization with a set of genetically defined LPS O side chain-CPS mutants and two LPS core OS mutants.
What is the role of LPS O side chain and CPS during O139 intestinal colonization? To investigate this further, we constructed mutants defective in ligation of the O139 side chain to lipid A core (waaL; LPS O side chain-negative, CPS-positive), capsule translocation (wbfF; LPS O side chain-positive, CPS-negative), biosynthesis of UDP-galactose (galEK; LPS O side chain-negative, CPS-negative), and lipid A core ligation and capsule translocation (waaLwbfF; LPS O side chain-negative, CPS-negative) mutants; the last two mutants behaved similarly under all conditions tested, indicating that in both mutants the loss of CPS and O side chain is responsible for the observed effects.
The galEK and waaL wbfF mutants were essentially unable to colonize the small intestine of infant mice (i.e., no mutant bacteria were recovered in competition experiments with a wild-type strain), which is in accordance with earlier reports (2, 41). The observed ≈30-fold decrease in colonization of strains lacking either the LPS O side chain or the CPS alone (waaL and wbfF mutants, respectively) is significant and, in the case of the CPS-negative mutant, similar to results reported previously (2, 41). Our defined waaL mutant colonized better than a spontaneous LPS O side chain-negative but CPS-positive mutant described previously (2), for which a three- to fourfold decrease in intestinal colonization was reported. We believe the discrepancy may be due to the nature of the mutation in the two studies. In the present study, a strain with a nonpolar deletion in waaL was used, which should have no effect on the expression of other genes and, as shown here, have no effect on capsule translocation, representing a true LPS O side chain-negative but CPS-positive mutant.
What is the mechanism of the attenuation? We excluded the possibility that the observed colonization deficiency was caused simply by a loss of functional pili, since Western blot and CTX phage transduction experiments (data not shown) demonstrated the presence of TCP. It has been proposed that the colonization defect of CPS- and/or LPS O side chain-negative V. cholerae mutants is mainly due to increased complement sensitivity (2, 41). However, we found that the presence of CPS alone protects against the complement system, independent of the LPS defect. When the CPS was absent, the LPS O side chain could partially compensate for this loss. Increased complement sensitivity would therefore explain only the attenuated colonization of the CPS (wbfF) and the CPS-LPS O side chain double mutants (ΔgalEK and ΔwaaL ΔwbfF) but not of the CPS-positive LPS O side chain-negative mutant (ΔwaaL).
It is not clear how much the complement system contributes to defense within the intestine, e.g., by bacteriolysis of gram-negative bacteria, since there is only evidence for activation of initial factors, such as C3, but not the terminal components of the complement cascade (29). Intestinal colonization likely constitutes a multifactorial process, and the mucus lining of the small intestine probably serves as the initial matrix to which V. cholerae cells adhere. During this stage of colonization, the ToxR regulatory cascade (including TCP expression) is probably not fully operative, as suggested by several lines of research (18, 31). In this scenario, O139 CPS or O139 side chain molecules might be involved in initial adhesion (45). However, there is still a lack of experiments addressing the function of surface polysaccharides as adhesins, e.g., measurement of mucus binding by defined surface polysaccharide mutants or conjugated LPS or CPS material.
The contribution of the V. cholerae LPS core OS to V. cholerae virulence was neglected for a long time. In a genetic study, we recently found evidence that clinical V. cholerae strains of different serogroups have a common core OS structure that was distinguishable from those of environmental isolates (25). This finding prompted us to investigate O139 LPS core OS mutants in intestinal colonization. Two defined knockout mutants were addressed in this study. The waaF mutant, which lacked core material and LPS O side chain but was still encapsulated, could not be recovered from the mouse intestine, indicating a severe defect in colonization. Notably, alteration of the core OS of the waaF mutant resulted in a 1.000-fold reduction in colonization compared to the O side chain-negative, CPS-positive ΔwaaL mutant.
The waaF mutant was sensitive to both bile and polycationic peptides, a phenotype which was observed exclusively in this core OS mutant. In addition, the waaF mutation caused pleiotropic effects, such as a noticeable growth defect and decreased TcpA expression, which could explain the deleterious colonization defect. These findings indicate that the LPS core OS structure synthesized by the waaF mutant was not able to maintain the integrity of the outer membrane. The other core OS mutant (wavB) was constructed with the intention of investigating the role of the LPS core OS in a strain that expresses the LPS O side chain and CPS. This mutation had no pleiotropic effects on growth rate or expression of TCP and OmpU but, unfortunately, affected O side chain attachment, leading to a decreased amount of LPS O side chain in this mutant compared to the wild-type strain. For this reason, we cannot exclude the decrease in LPS O side chain as the cause of the attenuated colonization behavior of this mutant. However, we also observed that the wavB strain grew poorly in the presence of bile, a phenotype which was not associated with the LPS O side chain-negative (ΔwaaL) mutant, and thus identifying at least one exclusive phenotype associated with the LPS core OS that may contribute to colonization.
In summary, our results presented here and our previous results with O1 El Tor LPS core OS mutants (26) provide evidence that the LPS core OS structure might be critical for survival of pathogenic V. cholerae strains in the mammalian intestine, but the nature of its role and whether a specific core OS structure is important for colonization will be addressed in more detail in future investigations. A detailed understanding of core OS distribution among pathogenic strains and its contribution to virulence could be beneficial for the development of a vaccine providing protection against pathogenic V. cholerae of different serogroups.
Acknowledgments
For the gift of O139-specific antiserum, we thank J. Bockemühl.
This work was funded by BMBF grant 01KI8906 and Nachwuchsgruppenförderprogramm Bayern to J.R. and NIH AI43486 to K.E.K.
Jutta Nesper, Stefan Schild, and Crystal M. Lauriano contributed equally to this work.
Editor: J. T. Barbieri
REFERENCES
- 1.Albert, M. J., F. Qadri, N. A. Bhuiyan, S. M. Ahmad, M. Ansaruzzaman, and A. Weintraub. 1999. Phagocytosis of Vibrio cholerae O139 Bengal by human polymorphonuclear leukocytes. Clin. Diagn. Lab. Immunol. 6:276-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attridge, S. R., A. Fazeli, P. A. Manning, and U. H. Stroeher. 2001. Isolation and characterization of bacteriophage-resistant mutants of Vibrio cholerae O139. Microb. Pathog. 30:237-246. [DOI] [PubMed] [Google Scholar]
- 3.Berche, P., C. Poyart, E. Abachin, H. Lelievre, J. Vandepitte, A. Dodin, and J. M. Fournier. 1994. The novel epidemic strain O139 is closely related to the pandemic strain O1 of Vibrio cholerae. J. Infect. Dis. 170:701-704. [DOI] [PubMed] [Google Scholar]
- 4.Bik, E. M., A. E. Bunschoten, R. J. Willems, A. C. Chang, and F. R. Mooi. 1996. Genetic organization and functional analysis of the otn DNA essential for cell-wall polysaccharide synthesis in Vibrio cholerae O139. Mol. Microbiol. 20:799-811. [DOI] [PubMed] [Google Scholar]
- 5.Comstock, L. E., J. A. Johnson, J. M. Michalski, J. G. Morris, and J. B. Kaper. 1996. Cloning and sequence of a region encoding a surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol. Microbiol. 19:815-826. [DOI] [PubMed] [Google Scholar]
- 6.Correa, N. E., C. M. Lauriano, R. McGee, and K. E. Klose. 2000. Phosphorylation of the flagellar regulatory protein FlrC is necessary for Vibrio cholerae motility and enhanced colonization. Mol. Microbiol. 35:743-755. [DOI] [PubMed] [Google Scholar]
- 7.Dziejman, M., E. Balon, D. Boyd, C. M. Fraser, J. F. Heidelberg, and J. J. Mekalanos. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc. Natl. Acad. Sci. USA 99:1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall, R. H., F. M. Khambaty, M. Kothary, and S. P. Keasler. 1993. Non-O1 Vibrio cholerae. Lancet i:342-430. [DOI] [PubMed]
- 10.Higa, N., Y. Honma, J. M. Albert, and M. Iwanaga. 1993. Characterization of Vibrio cholerae O139 synonym Bengal isolated from patiens with cholera-like disease in Bangladesh. Microbiol. Immunol. 37:971-974. [DOI] [PubMed] [Google Scholar]
- 11.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmgren, J., and A. M. Svennerholm. 1977. Mechanisms of disease and immunity in cholera: a review. J. Infect. Dis. 136:105-112. [DOI] [PubMed] [Google Scholar]
- 13.Holst, O. 1999. Chemical structure of the core region of lipopolysaccharides, p. 115-154. In H. Brade, S. M. Opal, S. N. Vogel, and D. C. Morrison (ed.), Endotoxin in health and disease. Marcel Dekker Inc., New York, N.Y.
- 14.Iwanaga, M., K. Yamamoto, N. Higa, Y. Ichinose, N. Nakasone, and M. Tanabe. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 30:1075-1083. [DOI] [PubMed] [Google Scholar]
- 15.Knirel, Y. A., L. Paredes, P.-E. Jansson, A. Weintraub, G. Widmalm, and M. J. Albert. 1995. Structure of the capsular polysaccharide of Vibrio cholerae O139 synonym Bengal containing d-galactose-4,6-cyclophosphate. Eur. J. Biochem. 232:391-396. [DOI] [PubMed] [Google Scholar]
- 16.Knirel, Y. A., G. Widmalm, S. N. Senchenkova, P.-E. Jansson, and A. Weintraub. 1997. Structural studies on the short-chain lipopolysaccharide of Vibrio cholerae O139 Bengal. Eur. J. Biochem. 247:402-410. [DOI] [PubMed] [Google Scholar]
- 17.Koch, R. 1884. An address on cholera and its bacillus. Br. Med. J. 2:403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, S. H., D. L. Hava, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 19.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 20.Medrano, A. I., V. J. DiRita, G. Castillo, and J. Sanchez. 1999. Transient transcriptional activation of the Vibrio cholerae El Tor virulence regulator ToxT in response to culture conditions. Infect. Immun. 67:2178-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meno, Y., M. K. Waldor, J. J. Mekalanos, and K. Amako. 1998. Morphological and physical characterization of the capsular layer of Vibrio cholerae O139. Arch Microbiol. 170:339-344. [DOI] [PubMed] [Google Scholar]
- 22.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris, J. G. 1994. Vibrio cholerae O139 Bengal, p. 95-102. In I. K. Wachsmuth, P. A. Blake, and O. Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, D.C.
- 24.Nesper, J., J. Blaβ, M. Fountouloakis, and J. Reidl. 1999. Characterization of the major control region of Vibrio cholerae bacteriophage K139: immunity, exclusion and integration. J. Bacteriol. 181:2902-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nesper, J., A. Kraiss, S. Schild, J. Blass, K. E. Klose, J. Bockemühl, and J. Reidl. 2002. Comparative and genetic analysis of the putative Vibrio cholerae lipopolysaccharide core oligosaccharide biosynthesis (wav) gene cluster. Infect. Immun. 70:2419-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nesper, J., C. M. Lauriano, K. E. Klose, D. Kapfhammer, A. Kraiss, and J. Reidl. 2001. Characterization of Vibrio cholerae O1 El Tor galU and galE mutants: influence on lipopolysaccharide structure, colonization, and biofilm formation. Infect. Immun. 69:435-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Provenzano, D., and K. E. Klose. 2000. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc. Natl. Acad. Sci. USA 97:10220-10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramamurthy, T., S. Grag, and R. Sharma. 1993. Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet 341:703-704. [DOI] [PubMed] [Google Scholar]
- 29.Riordan, S. M., C. J. McIver, D. Wakefield, P. C. Andreopoulos, V. M. Duncombe, T. D. Bolin, and M. C. Thomas. 1997. Local and systemic complement activity in small intestinal bacterial overgrowth. Dig. Dis. Sci. 42:1128-1136. [DOI] [PubMed] [Google Scholar]
- 30.Rivas, M., C. Toma, E. Miliwebsky, M. I. Caffer, M. Galas, P. Varela, M. Tous, A. M. Bru, and N. Binsztein. 1993. Cholera isolates in relation to the eight pandemic. Lancet 342:926-927. [PubMed] [Google Scholar]
- 31.Schumacher, D. A., and K. E. Klose. 1999. Environmental signals modulate ToxT-dependent virulence factor expression in V. cholerae. J. Bacteriol. 181:1508-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimada, T., G. B. Nair, B. C. Deb, M. J. Albert, R. B. Sack, and Y. Takeda. 1993. Outbreak of Vibrio cholerae non-O1 in India and Bangladesh. Lancet 341:1347.. [DOI] [PubMed] [Google Scholar]
- 33.Steinberg, D. A., M. A. Hurst, C. A. Fujii, A. H. Kung, J. F. Ho, F.-C. Cheng, D. J. Loury, and J. C. Fiddes. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stroeher, U. H., K. E. Jedani, and P. A. Manning. 1998. Genetic organization of the regions associated with surface polysaccharide synthesis in Vibrio cholerae O1, O139 and Vibrio anguillarum O1 and O2: a review. Gene 223:269-282. [DOI] [PubMed] [Google Scholar]
- 35.Svennerholm, A. M., and J. Holmgren. 1978. Identification of Escherichia coli heat-labile enterotoxin by means of a ganglioside immunosorbant assay (GM1-ELISA) procedure. Curr. Microbiol. 1:19-23. [Google Scholar]
- 36.Tacket, C. O., R. K. Taylor, G. Losonsky, Y. Lim, J. P. Nataro, J. B. Kaper, and M. M. Levine. 1998. Investigation of the roles of toxin-coregulated pili and mannose-sensitive hemagglutinin pili in the pathogenesis of Vibrio cholerae O139 infection. Infect. Immun. 66:692-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Towbin, H., T. Staehlin, and J. Gordon. 1979. Electrophoretic transfer of proteins. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai, C. M., and C. E. Frasch. 1982. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119:115-119. [DOI] [PubMed] [Google Scholar]
- 39.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 40.Waldor, K. W., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 41.Waldor, M. K., R. Colwell, and J. J. Mekalanos. 1994. The Vibrio cholerae O139 serogroup antigen includes an O antigen capsule and lipopolysaccharide virulence determinants. Proc. Natl. Acad. Sci. USA 91:11388-11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waldor, M. K., and J. J. Mekalanos. 1994. ToxR regulates virulence gene expression in non-O1 strains of Vibrio cholerae that cause epidemic cholera. Infect. Immun. 62:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watnick, P. I., C. M. Lauriano, K. E. Klose, L. Croal, and R. Kolter. 2001. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 39:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto, T., M. J. Albert, and R. B. Sack. 1994. Adherence to human small intestines of capsulated Vibrio cholerae O139. FEMS Microbiol. Lett. 119:229-235. [DOI] [PubMed] [Google Scholar]