Abstract
Gramicidin is a membrane pentadecapeptide that acts as a channel, allowing the passage of monovalent metal ions and assisting in bacterial cell death. The active form is a noncovalently bound dimer. One means to study the self-assembly of this peptide has been to compare the state of the peptide in various solvents ranging from hydrophilic (e.g., trifluoroethanol) to hydrophobic (e.g., n-propanol). In this article, we report the use of electrospray mass spectrometry to study the self-association of gramicidin in various organic and mixed solvents that are introduced directly into the mass spectrometer. The dimer (both homo and hetero) can survive the introduction into the gas phase, and the amount in the gas phase increases with the decreasing dielectric constant of the solvent, reflecting solution-phase behavior. Tandem mass spectrometry data reveal that the stability of dimer in the gas phase decreases with increasing metal ion size, strongly suggesting that the metal ion binds inside the dimer between the monomers.
INTRODUCTION
Small linear peptides are generally characterized by large conformational flexibility because the small number of amino acid residues cannot promote the formation of a stable secondary structure. An early exception is S-peptide, the N-terminal 20 residues of ribonuclease, which was characterized by Brown and Klee (1971). Since then, a number of examples in which some secondary structure exist for small linear peptides have been uncovered (Blanco et al., 1994; de Alba et al., 1996; Krstenansky et al., 1989; Motta et al., 1989; Schaefer et al., 1998; Temussi et al., 1989). One means to promote the formation of the stable secondary structure is to dissolve the peptide in an appropriate solvent to reduce the dielectric constant (Breeze et al., 1991; Luo and Baldwin, 1997; Lynch and Kaiser, 1988; Pascal and Cross, 1993). These solvent-induced changes are more pronounced when the polypeptide has a high surface-to-volume ratio (Luo and Baldwin, 1997; Rizo et al., 1993; Schiffer and Dotsch, 1996; Xu and Cross, 1998).
Gramicidin is another, striking exception to the rule because it exists as various stable helical structures and self assembles to give stable dimers that have different stagger and handedness of the polypeptide chains. Gramicidin is a 15-residue, membrane-spanning peptide with alternating L- and D-amino acids and serves as an antibiotic. It is produced by Bacillus brevis during transition from the vegetative phase to sporulation. The peptide's activity against gram-positive bacteria stems from its ability to form cation-specific channels. Its amino-acid sequence is HCO-L-Val1-Gly2-L-Ala3-D-Leu4-L-Ala5-D-Val6-L-Val7-DVal8-L-Trp9-D-Leu10-L-Xxx11-D-Leu12-L-Trp13-D-Leu14-L-Trp15-NHCH2CH2OH, where Xxx is Trp in gramicidin A (GA), Phe in gramicidin B (GB), and Tyr in gramicidin C (GC). For a small fraction of the gramicidins (∼5%), Val1 is replaced with Ile (e.g., when Val1 is replaced with Ile in gramicidin A, referred to as IGA in this article). Gramicidin D is a mixture of A:B:C in the ratio 80:5:15 (Sarges and Witkop, 1965).
A peptide of this amino-acid sequence is hydrophobic. No chargeable or hydrophilic side chains are present and both N- and C-termini are blocked. As a result, gramicidin does not form a zwitterion or become charged at any pH, explaining its poor solubility in water. Gramicidin, however, is soluble in a number of organic solvents and prefers the hydrophobic environment of the membranes. The Bull and Breese indices (Bull and Breese, 1974) for all the variants are highly negative, in accord with its poor solubility in water.
When gramicidin dimerizes, it exists in β-sheet-like secondary structures that are folded to form a helix (for a review, see Wallace, 1998). With its alternating L- and D-amino acids, the structural motifs have all the side chains on one side of the surface, and when folded to form a helix, produce structures with hydrophilic polypeptide in the interior of the helices and hydrophobic side chains coating the surface. The resulting structures function as ion channels with their hydrophobic surfaces embedded in a membrane and their hydrophilic interiors binding and transporting metal ions. This ability to conduct monovalent metal ions across cell membranes explains its antibiotic nature.
The dimer is biologically active (Urry, 1971; Veatch et al., 1974), and it binds selectively Na+, K+, and other monovalent cations. Its structure is either a head-to-head single-stranded dimer (Urry, 1971) or a double-stranded helical dimer (Veatch et al., 1974). This interesting property of self assembly into helical structures depends on the solvent. Conformations that have low dipole moments are favored in solvents with low polarity, whereas those with high dipole moments exist preferentially in polar solvents.
These interesting properties have made gramicidin an appropriate model for studying solvent effects on the structural changes of peptides and proteins. Its small size and stable structure have made it suitable for study by amino acid mutations, two-dimensional and three-dimensional NMR, x-ray crystallography, and circular dichroism (for reviews, see Busath, 1993; Doyle and Wallace, 1996; Greathouse et al., 1999; Ivanov and Sychev, 1982; Killian, 1992; Koeppe and Andersen, 1996; and Wallace, 1988, 1990, 1998).
Mass spectrometry is emerging as a viable biophysical technique for studying self-association. The “soft” ionization property of electrospray ionization (ESI) (Fenn et al., 1989), for example, allows noncovalent complexes of proteins to be admitted to the gas phase for detection and investigation (for review, see Loo, 1997, 2000). ESI produces multiply charged, gas-phase protein ions that sometimes reflect solution structure. In the only investigation of self-association of gramicidin by ESI-mass spectrometry, the authors used hydrogen/deuterium (H/D) exchange to reveal that gramicidin in trifluoroethanol (TFE) is more ordered than the tripeptide Ala-Ala-Ala (Bouchard et al., 2000). The insufficient mass-resolving power of the instrument used in that study did not permit a homodimer to be observed in any of the solvents.
Here we report a study of the solvent-dependent dimerization of gramicidin, using a higher mass-resolving power mass spectrometer than was used previously. Although the results do not permit us to assign an exact structure of the dimer (i.e., to distinguish head-to-head or intertwined double-helix dimers), we are able to determine 1), the extent of dimerization in solutions containing solvents of varying dielectric constants, 2), the effect of dielectric constant on the dimerization, and 3), the relative strengths of various metal-ion-bound dimers in the gas phase. The approach may be useful for the study of other self-associated and membrane-bound peptides.
METHODS AND MATERIALS
Gramicidin D was purchased from ICN Biochemicals (Costa Mesa, CA) and used without further purification. The solvents methanol, ethanol, and n-propanol were purchased from Sigma-Aldrich (Milwaukee, WI).
Mass spectra of the dimer in various solvents were acquired with a Micromass Q-Tof II (Micromass, Manchester, UK), which is a tandem mass spectrometer consisting of a quadrupole (Q) mass analyzer, a quadrupole collision cell, and a second-stage time-of-flight (Tof) analyzer. The experiments that examined the extent of dimerization as a function of dielectric constant and the strength of the various metal ion-bound dimers using tandem mass spectrometry (MS/MS) were conducted on a Micromass Q-Tof Ultima GLOBAL. Both instruments were operated in the positive-ion mode. The solvent for ESI was that in which gramicidin had been incubated before the mass spectrometry experiment. The solutions of gramicidin were made by dissolving gramicidin in the appropriate solvent (i.e., methanol, ethanol, n-propanol, trifluoroethanol) to a concentration of 2 mM. The solutions were then diluted to 50 μM before injection into the mass spectrometer. The ESI conditions were optimized for the highest sensitivity detection of the dimer in the gas phase. The needle voltage was 1.8 and 3 kV, and the cone voltage was 60 and 90 V, for the Q-Tof II and the Ultima GLOBAL, respectively. The temperatures of the source block and for desolvation were 90°C. A flow rate of 10 μL/min was used. All parameters (e.g., aperture to the Tof, transport voltage, offset voltages) were optimized to achieve maximum sensitivity and a mass resolving power of 10,000 (full width at half-maximum).
For the study of the dependence of the dimer on dielectric constant, solvents were made on a volume basis. A “master” gramicidin solution in n-propanol was diluted to 50 μM in the mixed solvent that was adjusted such that the addition of the n-propanol solution gave the desired volume ratio. The solution was admitted to the ESI source after saturating it with NaCl by adding 0.1 mg of the salt to the solvent, stirring with a vortex mixer, and centrifuging before using the supernatant. The percent dimer found in these experiments was calculated by integrating over the whole spectrum using the integrate function of the MassLynx software (Waters, Milford, MA) of the spectrometer and then computing the ratio of the intensity of the ion signal of interest to that of the total ion current (the sum of the monomer and dimer intensities).
Metal-ion-bound gramicidin dimer ions were obtained by spraying from n-propanol that was saturated with the corresponding metal chloride salt. Proton-bound dimers were obtained by adding 0.1% trifluoroacetic acid to the gramicidin solution in propanol. MS/MS experiments were performed by introducing argon gas into the collision cell to a pressure of ∼6 × 10−5 torr, which was measured indirectly by a gauge situated on the housing of the collision cell. A precursor ion corresponding to the mass of the mass of the (M2 + 2 metal)2+ was selected in the Q1. The width of the selection was set wide enough for the whole isotopic distribution to pass through. The collision energy, which is the translational energy of ions entering the collision cell, was changed by varying the voltage applied to the collision cell. The spectra were a sum of 2-s scans over 2 min, and the spectra were smoothed twice using a three-point Savitzky-Golay method.
RESULTS AND DISCUSSION
ESI-mass spectra of gramicidin D in organic solvents
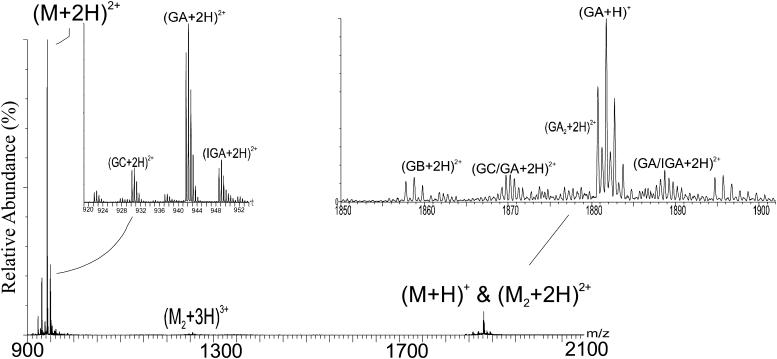
The ESI-mass spectra of gramicidin were taken in a variety of solvents. A mass spectrum of gramicidin in methanol (Fig. 1) shows three sets of peaks, corresponding to the different gramicidin species that are present in the sample. Peaks in the range of m/z 1880 represent both doubly charged, (M + 2H)2+, homo and heterodimers and singly charged, (M + H)+, monomers of the various gramicidin species (e.g., GA, GB, and GC). Heterodimer species (e.g., GA-GC) are identifiable by their distinctive m/z values. Unambiguous evidence for homodimers, on the other hand, is the pattern of C-13 isotope peaks, which are separated by 0.5 u (Fig. 1, inset on right). This establishes that the charge on the species is +2 and that the species is a dimer. The distribution of peaks is overlapped with peaks from the singly charged monomer, but the (M + H)+ is easily distinguishable as its isotope peaks are separated by 1 u. A lower cone voltage and source temperature make the ESI process sufficiently “soft” to introduce the dimers into the gas phase and permit their detection. In the earlier mass spectrometric study of gramicidin, Bouchard and co-workers (Bouchard et al., 2000) did not observe the homodimer in the gas phase, although it did exist in an ethanol solution. They suggested that ESI caused dissociation upon introduction to the gas phase.
FIGURE 1.
Mass spectrum, obtained with a Q-Tof II, of Gramicidin in methanol. The experimental details are described in the methods section. The cluster at ∼m/z 1880 contains both homo and heterodimers that are doubly charged and singly charged monomers. The ions of ∼m/z 1253 are triply charged dimers and the ions of ∼m/z 942 are doubly charged monomers. The abbreviations used to label the peaks are GA for gramicidin A, IGA for isoleucine-gramicidin A, GB for gramicidin B, and GC for gramicidin C.
More evidence for the dimer in the gas phase comes from the peaks at ∼m/z 1254, which represent the triply charged gramicidin dimers (both homo and hetero). These peaks, corresponding to the homodimers of gramicidin A/B/C, unambiguously demonstrate the existence of the dimer as there can be no overlap from peaks representing the monomer. No dimers with four charges could be detected as the C-13 isotope peaks at ∼m/z 942 are cleanly separated by 0.5 u, suggesting they represent the doubly charged monomer (Fig. 1, left inset). All the major variants of gramicidin (see Table 1) are also represented by the peaks around m/z 942, the intensities roughly corresponding to their relative abundances in the mixture.
TABLE 1.
Various mono (+1) and di (+2) Na-bound species of gramicidin and their expected monoisotopic nominal masses
| Species | Charge | Monoisotopic nominal m/z | Charge | Monoisotopic nominal m/z |
|---|---|---|---|---|
| Gramicidin A (GA) | 1+ | 1904 | 2+ | 963.5 |
| Gramicidin B (GB) | 1+ | 1865 | 2+ | 944 |
| Gramicidin C (GC) | 1+ | 1881 | 2+ | 952 |
| Isoleucine-gramicidin A (IGA) | 1+ | 1918 | 2+ | 970.5 |
| Homodimer GA | 1+ | 3785 | 2+ | 1904 |
| Heterodimer, GA/IGA | 1+ | 3799 | 2+ | 1911 |
| Heterodimer GA/GC | 1+ | 3762 | 2+ | 1892.5 |
ESI of gramicidin from various solvents
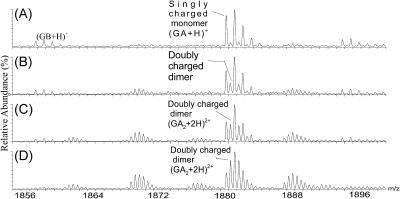
The amount of dimer that exists in organic solvents often increases with increasing solvent hydrophobicity (decreasing dielectric) (Arseniev et al., 1986, 1985; Chen et al., 1996; Glickson et al., 1972; Killian, 1992; Pascal and Cross, 1992; Veatch et al., 1974). The capability of mass spectrometry to provide evidence for the solvent-dependent dimerization or self association is revealed by the ESI of gramicidin from various organic solvents (Fig. 2). The amount of dimer in the solvent increases in the order: TFE < methanol < ethanol < n-propanol. In TFE, the peaks represent mainly a singly charged monomer, whereas in n-propanol, the peaks represent nearly exclusively the doubly charged dimer. The distribution of species in the various solvents is consistent with known solution-phase behavior, demonstrating that gramicidin in the gas phase retains a “memory” of its state in solution.
FIGURE 2.
Partial mass spectra taken with a Q-Tof II by electrospray from various solvents and showing the m/z region for singly charged monomers and doubly charged dimers. The experimental details are described in the methods section. The amount of dimer depends on the dielectric constant of the solvent and increases in the order: TFE (A) < methanol (B) < ethanol (C) < n-propanol (D).
The evidence presented here, when taken together, shows that mass spectrometry is sufficient to show that gramicidin dimerizes in some solvents. If the dimer were formed in the ESI process, the amount of dimer would be comparable for the various solvents under the same instrument conditions. This was not the case.
Predicting dielectric constant from the extent of dimerization
We decided to study the dimerization of gramicidin in solvent mixtures of different dielectric constant to understand more fully the properties of peptide dimerization. An NMR study (Xu and Cross, 1998) showed that antiparallel conformations are preferred as the solvent polarity decreases, but that study did not address the extent of dimerization as a function of solvent dielectric constant. Others have investigated the effect of dielectric constant on ESI (Cole and Harrata, 1993; Labowsky, 1998). The goal of these reports, however, is to understand the mechanism of ESI. One study sharing our objective to understand solvent-dependent association was directed at the clustering of [KBrO3]nKxx+ (Charles et al., 2001).
A secondary goal is to determine whether the propensity of a small peptide to dimerize varies in a systematic way such that a peptide can serve as an “indicator” for solution dielectric much like a colored organic acid can be an indicator for pH. Is there a correlation between the amount of dimer in the gas phase and the dielectric constant of the solvent from which the peptide is introduced into the gas phase? Although there are numerous methods for measuring the dielectric constant of liquids (e.g., microwave spectroscopy (Yao and Hiejima, 2002), NMR (Mallnowski and Garg, 1977), thickness shear mode bulk acoustic wave sensor, (Kinart and Kinart, 2000; Murthy, 1994; Zhang and Vetelino, 2001)), the addition of a complementary method that could be carried out as needed in a central research facility would be a useful.
The dielectric constant of a mixed solvent can be approximately written as
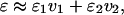 |
(1) |
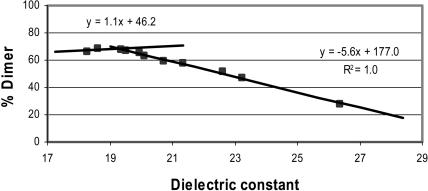
where ɛ1 and ɛ2 are the dielectric constants of the pure solvents and v1 and v2 represent the corresponding volume fractions (Akerlof, 1932; Xu and Cross, 1998). Although dielectric constants are not a quantitative measure of the polarity of mixed solvents, we will make use of Eq. 1 as a starting point because no other quantitative treatment of mass spectral abundances and dielectric constant currently exists. We chose n-propanol as the reference because ESI from this solvent produces an appreciable amount of dimer in the gas phase. We then added methanol or n-butanol to increase or decrease, respectively, the dielectric constant. (Solvents compatible with ESI were chosen. The dielectric constants of the solvents used are: water, 78.53; methanol, 32.63; ethanol, 24.3; n-propanol, 20.1; and n-butanol, 17.1.) For example, the dielectric constant of n-butanol is lower than that of n-propanol, leading to the expectation that the amount of dimer in n-butanol would be higher than that in n-propanol. The mass spectral abundance data fit two intersecting lines (Fig. 3). The percent dimer increases with decreasing dielectric constant and then reaches a maximum after which the percent dimer no longer increases even as the dielectric constant decreases. We interpret that this leveling of dimer abundance (e.g., at ∼70%) is due to dissociation of the dimer in the ESI process even though the dimer is completely formed in solution. Although the ESI conditions are “soft”, they are not sufficiently soft to ward off a small amount (∼30%) of dimer dissociation that accompanies the ESI process.
FIGURE 3.
Plot of percent dimer in the gas phase versus the dielectric constant of a solvent mixture. The data were fit with a linear regression using Microsoft Excel. The data were acquired on a Q-Tof Ultima GLOBAL, as described in the Methods and Materials section.
When water was added to n-propanol, the amount of dimer introduced by ESI from those solvents decreases very rapidly. At 4% water, only the doubly charged monomer remains. The data do not fit a single linear plot, supporting the conclusion that Eq. 1 applies when ɛ1 and ɛ2 are similar to each other. For this case, the dielectric constant for n-propanol is 20.1 (Maryott and Edgar, 1951), whereas for water it is 78.5 (Maryott and Edgar, 1951). Reasonable agreement, however, is found for the percent dimer in TFE, which has a dielectric constant of ∼26.1 and a predicted percent dimer of ∼25% (observed percent dimer ∼15%).
The results show that the amount of dimer in the gas phase correlates inversely with the dielectric constant of the solvent over a range of 19–28. This suggests that self-associating molecules such as gramicidin can be “indicators” of the solvent dielectric constant. For example, increasing the range of solvent to those having dielectric constants >28 requires a dimer that is more strongly bound than that of gramicidin. For solvents with dielectric constants <19, dimers that are more weakly bound than that of gramicidin would be required. The high sensitivity of mass spectrometry, its ease of use, and the simple data analysis recommend its use.
Binding strength of the metal-ion-bound dimers in gas phase
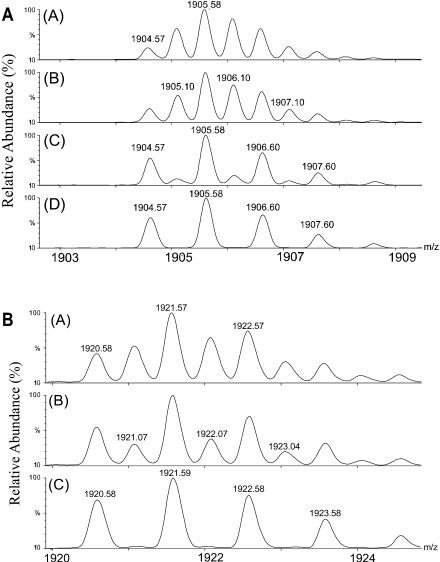
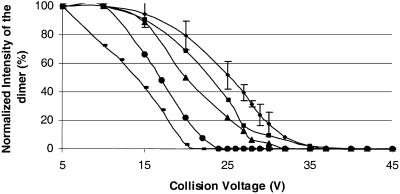
The solvent-dependent dimerization of gramicidin presents the opportunity to assess the binding strength of the dimer in the gas phase. We chose n-propanol as the solvent from which the dimer was put in the gas phase for studying the relative strengths of the metal-bound dimers because the (M2 + 2 metal)2+ region from n-propanol consists of just the dimer peaks with no apparent “contamination” from the monomer peaks at the low collision voltages. We found that the percent dimer decreases with increasing collision energy (varied by changing the collision voltage from 5 to 40 V) until a voltage is reached where the distribution represents all monomer (Fig. 4, A and B). A plot of the data for proton and various metal-ion-bound dimers (Fig. 5) shows, for example, that the collision voltage for 50% dissociation of a Na-bound dimer is ∼28 eV (laboratory frame), suggesting that the monomers are relatively strongly bound in the gas phase. Although we cannot convert the collision voltage into the internal energy that is introduced in the dimer, we suggest that a requirement of 28 eV is a characteristic of relatively strong binding. Small linear peptides that are doubly charged generally fragment in a Q-Tof instrument, such as the one we are using, at ∼30 eV to give b and y ions. The only detectable fragmentation of the gramicidin dimer at 28 eV, however, is partial dissociation to monomer.
FIGURE 4.
(A) Magnified view of the mass spectrum for the region of (GA2 + 2 Na)2+ as a function of collision energy. Spectrum A was obtained at a collision voltage of 10 V, B at 20 V, C at 30 V, and D at 40 V. (B) Magnified view of the mass spectrum in the region of (GA2 + 2 K)2+ as a function of collision energy. Collision voltages were (A) at 10 V, (B) at 20 V, and (C) at 30 V applied to collision cell. The data in panels A and B were acquired on a Q-Tof Ultima GLOBAL, as described in the Methods and Materials section.
FIGURE 5.
Normalized intensity of the dimer versus collision voltage for various metal-ion-bound homodimers of gramicidin A. The stability of the metal-ion-bound dimer decreases as Na (♦) > H (▪) > K (▴) > Rb (•) > Cs (-).
The extent of dimerization as a function of collision energy decreases in the order Na > H > K > Rb > Cs for metal-ion and proton binding (data not shown for Rb and Cs). This dependence on collision energy (Fig. 5) indicates that the strength of the dimer decreases with increasing metal-ion size; the proton-bound dimer is an exception. One explanation is the metal ions bind inside the dimer between the monomers of a double-helix. As the metal-ion becomes larger, the monomers comprising the dimer are pushed apart, weakening the complex. There would be little metal-ion dependence on the strength of the dimer were the metal binding on the outside. The proton-bound dimer is weaker than the sodium-bound dimer, despite their relative sizes, suggesting that the proton-bound dimer has a different structure than the metal-ion bound dimers in the gas phase. No Li-bound dimers could be detected.
Gramicidin is known to transport metal-ions with diameters as large as 0.5 nm (Urry 1971; Veatch et al., 1974), but Cs+ ion (radius <0.5 nm) weakens it considerably in the gas phase. These trends also suggest that the dimer is intrinsically stronger in the gas phase than in solution. In the absence of any solvent interactions with gramicidin, the monomers are pulled even closer and interact more strongly than in solution. In fact there is a body of evidence indicating that electrostatic noncovalent interactions are strengthened in the absence of solvent shielding, whereas hydrophobic interactions are weakened (for a review, see Daniel et al., 2002).
CONCLUSION
Homodimers of gramicidin can be introduced by ESI into the gas phase. Their existence clearly indicates self association in organic solvents of low dielectric constant. Mass spectrometry also shows that the extent of self association in organic solvents and solvent mixtures varies linearly with the solvent's dielectric constant over a certain range, becoming constant at a value of <100% when solvents of low dielectric constant are used. The incomplete formation of dimer in the gas phase when solvents of low dielectric constant are used is likely caused by dissociation of the dimer in the process of electrospray. These solvent effects on dimerization are consistent with those determined by NMR and CD, which previously revealed that the amount of dimer in solution increases with decreasing polarity of the solvent. Tandem mass spectrometry of various metal-ion-bound dimers strongly suggests that the metal ions bind inside the dimer, causing the dimer to weaken with increasing metal-ion size.
The mass spectrometric information reported here cannot be interpreted in terms of the structural details of the gramicidin dimer. Efforts are under way in our laboratory to probe the structure and dynamics of the dimer in organic solvents and model membranes, using H/D exchange and mathematical modeling. H/D exchange (Demmers et al., 2000; Engen and Smith, 2000; Marshall et al., 2000; Miranker et al., 1996; Smith, 1998) combined with mass spectrometry does reveal information about the secondary structure, hydrogen bonding, and dynamics of peptides and proteins in solution (Engen and Smith, 2000; Kaltashov and Eyles, 2002; Smith, 1998).
Acknowledgments
We thank the National Center for Research Resources of the National Institutes of Health for financial support (grant 2P41RR000954).
References
- Akerlof, G. 1932. Dielectric constant of some organic solvent-water mixtures at various temperatures. J. Am. Chem. Soc. 54:4125–4139. [Google Scholar]
- Arseniev, A. S., I. L. Barsukov, and V. F. Bystrov. 1986. Conformation of gramicidin A in solution and micelles: two-dimensional proton NMR study. Chem. Pept. Proteins. 3:127–158. [Google Scholar]
- Arseniev, A. S., I. L. Barsukov, V. F. Bystrov, A. L. Lomize, and YuA Ovchinnikov. 1985. 1H-NMR study of gramicidin A transmembrane ion channel. Head-to-head right-handed, single-stranded helices. FEBS Lett. 186:168–174. [DOI] [PubMed] [Google Scholar]
- Blanco, F. J., G. Rivas, and L. Serrano. 1994. A short linear peptide that folds into a native stable β-hairpin in aqueous solution. Nat. Struct. Biol. 1:584–590. [DOI] [PubMed] [Google Scholar]
- Bouchard, M., D. R. Benjamin, P. Tito, C. V. Robinson, and C. M. Dobson. 2000. Solvent effects on the conformation of the transmembrane peptide gramicidin A: insights from electrospray ionization mass spectrometry. Biophys. J. 78:1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeze, A. L., T. S. Harvey, R. Bazzo, and I. D. Campbell. 1991. Solution structure of human calcitonin gene-related peptide by 1H NMR and distance geometry with restrained molecular dynamics. Biochemistry. 30:575–582. [DOI] [PubMed] [Google Scholar]
- Brown, J. E., and W. A. Klee. 1971. Helix-coil transition of the isolated amino terminus of ribonuclease. Biochemistry. 10:470–476. [DOI] [PubMed] [Google Scholar]
- Bull, H. B., and K. Breese. 1974. Surface tension of amino acid solutions. Hydrophobicity scale of the amino acid residues. Arch. Biochem. Biophys. 161:665–670. [DOI] [PubMed] [Google Scholar]
- Busath, D. D. 1993. The use of physical methods in determining gramicidin channel structure and function. Annu. Rev. Physiol. 55:473–501. [DOI] [PubMed] [Google Scholar]
- Charles, L., D. Pepin, F. Gonnet, and J. C. Tabet. 2001. Effects of liquid phase composition on salt cluster formation in positive ion mode electrospray mass spectrometry: implications for clustering mechanism in electrospray. J. Am. Soc. Mass Spectrom. 12:1077–1084. [DOI] [PubMed] [Google Scholar]
- Chen, Y., A. Tucker, and B. A. Wallace. 1996. Solution structure of a parallel left-handed double-helical gramicidin A determined by 2D 1H NMR. J. Mol. Biol. 264:757–769. [DOI] [PubMed] [Google Scholar]
- Cole, R. B., and A. K. Harrata. 1993. Solvent effect on analyte charge state, signal intensity, and stability in negative ion electrospray mass spectrometry; implications for the mechanism of negative ion formation. J. Am. Soc. Mass Spectrom. 4:546–556. [DOI] [PubMed] [Google Scholar]
- Daniel, J. M., S. D. Friess, S. Rajagopalan, S. Wendt, and R. Zenobi. 2002. Quantitative determination of noncovalent binding interactions using soft ionization mass spectrometry. Int. J. Mass Spectrom. 216:1–27. [Google Scholar]
- de Alba, E., M. A. Jimenez, M. Rico, and J. L. Nieto. 1996. Conformational investigation of designed short linear peptides able to fold into β-hairpin structures in aqueous solution. Fold. Des. 1:133–144. [DOI] [PubMed] [Google Scholar]
- Demmers, J. A., J. Haverkamp, A. J. Heck, R. E. Koeppe 2nd, and J. A. Killian. 2000. Electrospray ionization mass spectrometry as a tool to analyze hydrogen/deuterium exchange kinetics of transmembrane peptides in lipid bilayers. Proc. Natl. Acad. Sci. USA. 97:3189–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, D. A., and B. A. Wallace. 1996. The dynamic nature of gramicidin. Biomembranes. 6:327–359. [Google Scholar]
- Engen, J. R., and D. L. Smith. 2000. Investigating the higher order structure of proteins. Hydrogen exchange, proteolytic fragmentation, and mass spectrometry. Methods Mol. Biol. 146:95–112. [DOI] [PubMed] [Google Scholar]
- Fenn, J. B., M. Mann, C. K. Meng, S. F. Wong, and C. M. Whitehouse. 1989. Electrospray ionization for mass spectrometry of large biomolecules. Science. 246:64–71. [DOI] [PubMed] [Google Scholar]
- Glickson, J. D., D. F. Mayers, J. M. Settine, and D. W. Urry. 1972. Spectroscopic studies on the conformation of gramicidin A′. Proton magnetic resonance assignments, coupling constants, and H-D exchange. Biochemistry. 11:477–486. [DOI] [PubMed] [Google Scholar]
- Greathouse, D. V., R. E. Koeppe 2nd, L. L. Providence, S. Shobana, and O. S. Andersen. 1999. Design and characterization of gramicidin channels. Methods Enzymol. 294:525–550. [DOI] [PubMed] [Google Scholar]
- Ivanov, V. T., and S. V. Sychev. 1982. The gramicidin A story. Struct. Complexes Biopolym. Low Mol. Weight Mol. Proc. Workshop Conf. Hoechst. 11:107–125. [Google Scholar]
- Kaltashov, I. A., and S. J. Eyles. 2002. Crossing the phase boundary to study protein dynamics and function: combination of amide hydrogen exchange in solution and ion fragmentation in the gas phase. J. Mass Spectrom. 37:557–565. [DOI] [PubMed] [Google Scholar]
- Killian, J. A. 1992. Gramicidin and gramicidin-lipid interactions. Biochim. Biophys. Acta. 1113:391–425. [DOI] [PubMed] [Google Scholar]
- Kinart, C. M., and W. J. Kinart. 2000. Physicochemical methods used to study internal structures of liquid binary mixtures. Phys. Chem. Liq. 38:155–180. [Google Scholar]
- Koeppe 2nd, R. E., and O. S. Andersen. 1996. Engineering the gramicidin channel. Annu. Rev. Biophys. Biomol. Struct. 25:231–258. [DOI] [PubMed] [Google Scholar]
- Krstenansky, J. L., T. J. Owen, K. A. Hagaman, and L. R. McLean. 1989. Short model peptides having a high α-helical tendency: design and solution properties. FEBS Lett. 242:409–413. [DOI] [PubMed] [Google Scholar]
- Labowsky, M. 1998. Discrete charge distributions in dielectric droplets. J. Colloid Interface Sci. 206:19–28. [DOI] [PubMed] [Google Scholar]
- Loo, J. A. 1997. Studying noncovalent protein complexes by electrospray ionization mass spectrometry. Mass Spectrom. Rev. 16:1–23. [DOI] [PubMed] [Google Scholar]
- Loo, J. A. 2000. Electrospray ionization mass spectrometry: a technology for studying noncovalent macromolecular complexes. Int. J. Mass Spectrom. 200:175–186. [Google Scholar]
- Luo, P., and R. L. Baldwin. 1997. Mechanism of helix induction by trifluoroethanol: a framework for extrapolating the helix-forming properties of peptides from trifluoroethanol/water mixtures back to water. Biochemistry. 36:8413–8421. [DOI] [PubMed] [Google Scholar]
- Lynch, B., and E. T. Kaiser. 1988. Biological properties of two models of calcitonin gene related peptide with idealized amphiphilic α-helices of different lengths. Biochemistry. 27:7600–7607. [DOI] [PubMed] [Google Scholar]
- Mallnowski, E. R., and S. K. Garg. 1977. Dielectric constants of liquids from nuclear magnetic resonance phase control studies. J. Phys. Chem. 81:685–686. [Google Scholar]
- Marshall, A. G., M. R. Emmett, M. A. Freitas, C. L. Hendrickson, and Z. Zhang. 2000. Isotopic amplification, H/D exchange, and other mass spectrometric strategies for characterization of biomacromolecular topology and binding sites. In Mass Spectrometry in Biology & Medicine. A. L. Burlingame, S. A. Carr, and M. A. Baldwin, editors. Humana Press, Totowa, NJ. 31–52.
- Maryott, A. A. S., and R. Edgar. 1951. Table of Dielectric Constants of Pure Liquids. National Bureau of Standards, Gaithersburg, MD.
- Miranker, A., C. V. Robinson, S. E. Radford, and C. M. Dobson. 1996. Investigation of protein folding by mass spectrometry. FASEB J. 10:93–101. [DOI] [PubMed] [Google Scholar]
- Motta, A., D. Picone, P. A. Temussi, M. Marastoni, and R. Tomatis. 1989. Conformational analysis of peptide T and of its C-pentapeptide fragment. Biopolymers. 28:479–486. [DOI] [PubMed] [Google Scholar]
- Murthy, V. R. K. 1994. Methods of measurement of dielectric constant and loss in the microwave frequency region. Microwave Materials. 100–111.
- Pascal, S. M., and T. A. Cross. 1992. Structure of an isolated gramicidin A double helical species by high-resolution nuclear magnetic resonance. J. Mol. Biol. 226:1101–1109. [DOI] [PubMed] [Google Scholar]
- Pascal, S. M., and T. A. Cross. 1993. High-resolution structure and dynamic implications for a double-helical gramicidin A conformer. J. Biomol. NMR. 3:495–513. [DOI] [PubMed] [Google Scholar]
- Rizo, J., F. J. Blanco, B. Kobe, M. D. Bruch, and L. M. Gierasch. 1993. Conformational behavior of Escherichia coli OmpA signal peptides in membrane mimetic environments. Biochemistry. 32:4881–4894. [DOI] [PubMed] [Google Scholar]
- Sarges, R., and B. Witkop. 1965. Gramicidin A. V. The structure of valine- and isoleucine-gramicidin A. J. Am. Chem. Soc. 87:2011–2020. [DOI] [PubMed] [Google Scholar]
- Schaefer, M., C. Bartels, and M. Karplus. 1998. Solution conformations and thermodynamics of structured peptides: molecular dynamics simulation with an implicit solvation model. J. Mol. Biol. 284:835–848. [DOI] [PubMed] [Google Scholar]
- Schiffer, C. A., and V. Dotsch. 1996. The role of protein-solvent interactions in protein unfolding. Curr. Opin. Biotechnol. 7:428–432. [DOI] [PubMed] [Google Scholar]
- Smith, D. L. 1998. Local structure and dynamics in proteins characterized by hydrogen exchange and mass spectrometry. Biochemistry. 63:285–293. [PubMed] [Google Scholar]
- Temussi, P. A., D. Picone, M. A. Castiglione-Morelli, A. Motta, and T. Tancredi. 1989. Bioactive conformation of linear peptides in solution: an elusive goal? Biopolymers. 28:91–107. [DOI] [PubMed] [Google Scholar]
- Urry, D. W. 1971. The gramicidin A transmembrane channel: a proposed pi(L,D) helix. Proc. Natl. Acad. Sci. USA. 68:672–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch, W. R., E. T. Fossel, and E. R. Blout. 1974. The conformation of gramicidin A. Biochemistry. 13:5249–5256. [DOI] [PubMed] [Google Scholar]
- Wallace, B. A. 1988. Gramicidin, a “simple” ion channel. Curr. Top. Membr. 33:35–50. [Google Scholar]
- Wallace, B. A. 1990. Gramicidin channels and pores. Annu. Rev. Biophys. Biophys. Chem. 19:127–157. [DOI] [PubMed] [Google Scholar]
- Wallace, B. A. 1998. Recent advances in the high resolution structures of bacterial channels: gramicidin A. J. Struct. Biol. 121:123–141. [DOI] [PubMed] [Google Scholar]
- Xu, F., and T. A. Cross. 1998. Changes in polypeptide conformer populations induced by the solvent environment. Magn. Reson. Chem. 36:651–655. [Google Scholar]
- Yao, M., and Y. Hiejima. 2002. Dielectric relaxation of supercritical water and methanol. J. Mol. Liq. 96–97:207–220. [Google Scholar]
- Zhang, C., and J. F. Vetelino. 2001. Liquid dielectric constant measurement based on thickness shear mode quartz resonators. Proc. Electrochem. Soc. 18:125–129. [Google Scholar]