Abstract
Citrobacter rodentium belongs to the attaching and effacing family of enteric bacterial pathogens that includes both enteropathogenic and enterohemorrhagic Escherichia coli. These bacteria infect their hosts by colonizing the intestinal mucosal surface and intimately attaching to underlying epithelial cells. The abilities of these pathogens to exploit the cytoskeleton and signaling pathways of host cells are well documented, but their interactions with the host's antimicrobial defenses, such as inducible nitric oxide synthase (iNOS), are poorly understood. To address this issue, we infected mice with C. rodentium and found that iNOS mRNA expression in the colon significantly increased during infection. Immunostaining identified epithelial cells as the major source for immunoreactive iNOS. Finding that nitric oxide (NO) donors were bacteriostatic for C. rodentium in vitro, we examined whether iNOS expression contributed to host defense by infecting iNOS-deficient mice. Loss of iNOS expression caused a small but significant delay in bacterial clearance without affecting tissue pathology. Finally, immunofluorescence staining was used to determine if iNOS expression was localized to infected cells by staining for the C. rodentium virulence factor, translocated intimin receptor (Tir), as well as iNOS. Interestingly, while more than 85% of uninfected epithelial cells expressed iNOS, fewer than 15% of infected (Tir-positive) cells expressed detectable iNOS. These results demonstrate that both iNOS and intestinal epithelial cells play an active role in host defense during C. rodentium infection. However, the selective expression of iNOS by uninfected but not infected cells suggests that this pathogen has developed mechanisms to locally limit its exposure to host-derived NO.
Enteropathogenic (EPEC) and enterohemorrhagic (EHEC) Escherichia coli are important causative agents of infectious diarrhea, belonging to a family of related pathogenic bacteria that together infect a wide range of animal species, including humans (16, 29, 43, 62). Unlike the enteroinvasive bacterial pathogens Salmonella and Shigella, EPEC and EHEC are noninvasive and infect their hosts by attaching to the surface of intestinal epithelial cells (4, 43). Following their initial adherence, these pathogens form attaching and effacing (A/E) lesions on infected epithelial cells, characterized by the effacement of microvilli and the production of pedestal-like structures beneath the adherent bacteria. All A/E pathogens share a homologous DNA region called the LEE (locus of enterocyte effacement), a pathogenicity island required for A/E lesion formation (9, 20, 43, 67). The LEE encodes a type III secretion apparatus and several virulence factors, including the secreted proteins EspA, EspB, and EspD, as well as the outer membrane adhesin intimin and the translocated intimin receptor (Tir) (30). These proteins are critical for the intimate attachment of A/E pathogens to host cells, as well as for their ability to cause disease (1, 45).
To successfully infect their hosts, A/E pathogens must not only subvert the cytoskeleton of intestinal epithelial cells but also encounter and survive the host's innate and immune defenses. Although few studies have examined these interactions, intestinal biopsy samples taken from children suffering from EPEC infection displayed significant intestinal inflammation, crypt hyperplasia, and tissue damage (12, 26, 28). Furthermore, testing of convalescent-phase sera as well as peripheral blood lymphocytes from volunteer studies indicates that EHEC and EPEC infections induce strong humoral and cellular immune responses (37, 59). Other studies indicate that the infected epithelial cells themselves may play an active role in the host inflammatory response, as EPEC attachment to cultured epithelial cells triggers NF-κB activation and the release of interleukin-8 (IL-8) (23, 50-52). In related studies, EPEC infection has been shown to activate mitogen-activated protein kinase pathways in host cells (7, 8, 53). In contrast, EPEC has been found to undermine the actions of professional immune cells, at least in vitro. A toxin secreted by EPEC can inhibit cytokine production by T lymphocytes (31, 32), while EPEC infection impairs the phagocytic ability of macrophages through a phosphatidylinositol 3-kinase-dependent mechanism (5, 21).
Despite these observations, it remains to be determined if epithelial cells actually play an active role during infection in vivo and to what degree innate and immune responses contribute to host defense or instead become subverted by A/E pathogens. Furthering our understanding in this area has been limited by the inability of EPEC and EHEC to infect and cause A/E lesions in laboratory animal species. Fortunately, there are several veterinary A/E pathogens that infect their hosts in a manner similar to EPEC and EHEC (3, 43, 48). Our laboratory (8, 9, 61) and others (3, 24, 40) have used Citrobacter rodentium, the causative agent of transmissible murine colonic hyperplasia, to model these infections. So far, studies have identified the elevated expression of several Th1 cytokines, including gamma interferon (IFN-γ), that contribute to both host defense and tissue damage in the inflamed colons of infected mice (24, 25, 57). Another element of host defense, nitric oxide (NO), is a central component of innate immunity and an effective antimicrobial agent. Unlike the constitutive NO synthases, the inducible form of nitric oxide synthase (iNOS) can produce large quantities of reactive nitrogen intermediates (RNI) such as nitrite and S-nitrosoglutathione (GSNO) (39, 44), as well as NO at concentrations that can exert antimicrobial effects. Increased expression of iNOS is frequently observed during colonic inflammation (35, 38, 46) and is induced in response to both IFN-γ (34, 49) and bacterial lipopolysaccharide (15, 33). Therefore, iNOS expression and the subsequent release of NO could contribute not only to host defense but also to the tissue damage seen during A/E pathogen infections.
To assess the potential role of iNOS in A/E pathogen infections, we infected wild-type mice with C. rodentium and examined iNOS expression. Finding that epithelial cells strongly expressed iNOS during infection, we also studied the effects of chemical sources of NO on C. rodentium growth in vitro and monitored the course of C. rodentium infections in mice lacking iNOS. Our findings demonstrate that iNOS expression contributes to C. rodentium clearance but, more importantly, that epithelial cells play an active role in host defense during infection by an A/E lesion-causing bacterial pathogen. Nonetheless, when we examined the proximity of iNOS staining to bacterial attachment, iNOS was selectively expressed by uninfected but not infected epithelial cells. These results suggest that, although the host response to C. rodentium infection leads to upregulation of iNOS expression, this pathogen has developed mechanisms to locally limit its exposure to host-derived NO.
MATERIALS AND METHODS
Mice.
Three- to four-week-old iNOS-deficient mice (65) (on a C57BL/6 background) and wild-type C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Mice were kept in sterilized cages with filter tops, handled in tissue culture hoods, and fed autoclaved food and water under specific-pathogen-free conditions at our animal facilities. Sentinel animals were routinely tested for common pathogens. The protocols employed were in direct accordance with guidelines drafted by the University of British Columbia's Animal Care Committee and the Canadian Council on the Use of Laboratory Animals.
Bacterial strains and infection of mice.
Mice were orally inoculated with either the wild-type C. rodentium (formerly Citrobacter freundii biotype 4280) strain DBS100 (55) or the type III secretion mutant (ΔescD). For inoculations, bacteria were grown overnight with shaking in Luria broth (LB) at 37°C. Mice were infected by oral gavage with 0.1 ml of LB containing approximately 2.5 × 108 CFU of C. rodentium. To minimize any differences between infections, both wild-type and iNOS-deficient mice were infected with the same bacterial preparation. Mice were sacrificed at various time points postinfection (p.i.), and tissues were prepared for histological analysis, viable bacterial counts, or RNA isolation as described below.
C. rodentium ΔescD possesses a phenotype similar to that of the corresponding EPEC mutant in that it does not secrete effector proteins and is unable to cause pedestals on host cells in tissue culture. ΔescD was generated as follows. A PCR fragment (3.75 kb) containing the escD gene as well as flanking regions from 2.2 kb upstream and 0.4 kb downstream of the escD gene was cloned into pCR2.1-TOPO to create pTOPO-ISDL. The primers used for the PCR were Seq-1 and Seq-6. The template pTOPO-ISDL was used for inverse PCR (Elongase) with deletion primers ΔescD+ and ΔescD− to create an internal deletion and truncation in the escD gene. About 471 bp of the escD gene (from codons 26 to 149) was deleted. A BamHI site and a stop codon were introduced into the site of deletion. The inverse PCR product was treated with Klenow enzyme and T4 polynucleotide kinase and then gel purified before self-ligation and transformation into DH10B. The escD gene with the internal deletion was subcloned as an XbaI-SacI fragment into the suicide vector pRE118 (XbaI-SacI) along with its flanking regions and used for allelic exchange in wild-type C. rodentium.
Survival and body weight measurement.
The systemic effects of C. rodentium infection on the host, the survival of infected mice, as well as changes in their body weight were measured over the course of infection. Mice were weighed just prior to infection, as well as at 4-day intervals until day 24 p.i. Survival data are presented as the percentages of the initial 12 mice still surviving at the end of the experiment.
Tissue collection.
Over the course of the infection, mice were euthanized and, following careful dissection, the first 4 cm of the colon beginning at the anal verge was collected. Fecal pellets were removed before the tissue was weighed. Tissues were then placed in 10% neutral-buffered formalin (Sigma) for histological analysis, or the colon plus fecal pellets were collected in phosphate-buffered saline, pH 7.4, and kept on ice before being processed for viable bacterial counts.
Histology.
Full-thickness colonic tissues were fixed in 10% neutral-buffered formalin. Sections (3 μm) were cut and stained with hematoxylin and eosin stain. Photomicrographs were taken using a Nikon Eclipse E400 microscope. Crypt heights were measured by micrometry by an observer blinded to the experimental condition, with 10 measurements being taken in the distal colon of each mouse. Only well-oriented crypts were measured.
Bacterial counts.
Colonic tissues plus any fecal pellets were homogenized at a low speed with a Kinematica tissue homogenizer (Brinkmann). Homogenates were serially diluted and plated onto MacConkey agar plates (selective for gram-negative organisms). Bacterial colonies were enumerated the following day. C. rodentium colonies were easily distinguished from colonies derived from commensal flora by their size and appearance (pink center with white rim), as previously described (54, 55). The validity of this approach was verified by PCR analysis for LEE genes.
C. rodentium response to GSNO and SNP.
To test the in vitro effects of NO on the growth of C. rodentium, wild-type cultures were grown in LB overnight at 37°C in a shaking incubator. The cultures were divided in half, with those cultures receiving the NO donor sodium nitroprusside (SNP; Sigma) diluted to 106 CFU/ml in LB at pH 7.0, while those receiving the NO releaser GSNO (Alexis Biochemicals) were diluted in LB at pH 5.0 (the optimal pH for GSNO) (58). Different doses of the compounds were then added to the cultures, which were incubated with shaking at 37°C for 4 h. Cultures were then serially diluted and plated onto LB plates. Bacterial colonies were enumerated the following day.
Modified Griess reaction for levels of NO.
NO has an extremely short half-life and rapidly degrades to nitrate and nitrite. Thus, NO levels were determined indirectly by measuring the total levels of these stable NO end products in plasma. In brief, blood samples were centrifuged and plasma was collected. Nitrate was reduced to nitrite by incubating the samples at room temperature with 0.05 U of nitrate reductase (Oxford Biomedical Research)/ml following the manufacturer's instructions. Samples were then mixed in 96-well microtiter plates with Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, 25% H3PO4) to measure nitrite levels. After 10 min, the optical density was measured at 560 nm.
Immunostaining.
Immunoperoxidase staining was performed using standard techniques. In brief, tissues were rinsed with ice-cold phosphate-buffered saline, embedded in OCT (optimal cutting template) compound (Sakura Finetech), frozen with isopentane (Sigma) and liquid N2, and stored at −70°C. Serial sections were cut at a thickness of 6 μm and fixed in ice-cold acetone for 10 min. Endogenous peroxidase activity was blocked by treatment with 1% H2O2 in Tris-buffered saline, pH 7.4, for 30 min. Tissues were then blocked with 1% bovine serum albumin. For iNOS detection, a rabbit polyclonal immunoglobulin G (IgG) antibody targeted against the carboxyl terminus of mouse iNOS (SC 650; Santa Cruz Biotechnology, Santa Cruz, Calif.) was used. Detection was performed with goat anti-rabbit polyclonal IgG conjugated to horseradish peroxidase by using 3-amino-9-ethyl carbazole as the chromogen source. Specificity was confirmed by performing the staining with the omission of the primary antibody. Photomicrographs were taken using Nikon Eclipse E400 microscope.
Immunofluorescence staining was performed in a fashion similar to that used for immunoperoxidase staining. Following acetone fixation, tissue sections were directly blocked with 1% bovine serum albumin, followed by the addition of the rabbit anti-iNOS polyclonal antibody or rabbit anti-E. coli serum no. 8 (Biotech Labs, Ipswich, England) as well as rat anti-C. rodentium His-Tir serum generated in the lab. Following extensive washing with Tris-buffered saline, Alexa488-conjugated goat anti-rabbit as well as biotinylated goat anti-rat polyclonal IgG antibodies were added. Following incubation, the tissues were again washed, followed by the addition of streptavidin labeled with Alexa568. Finally, 1 μg of 4′,6′-diamidino-2-phenylindole (DAPI; Sigma)/ml was used to stain the host cell DNA. Coverslips were mounted in Mowiol mounting medium (Aldrich) and viewed at 350, 488, and 594 nm on a Zeiss Axiophot epifluorescence microscope.
For studies examining whether iNOS and Tir expression colocalized to the same cells, we examined at least 10 randomly chosen high-power fields in the distal colon of each mouse, with four mice tested per group. Using DAPI staining to estimate epithelial cell numbers, we counted the number of epithelial cells that stained positively for one or both markers. Infected cells were characterized as mildly or heavily infected based on the number of focal sites of Tir expression, presumably equivalent to the number of attaching bacteria. Ten or fewer Tir foci designated a cell as mildly infected; more than 10 designated it as heavily infected.
RNA extraction and semiquantitative PCR.
Immediately following dissection, colonic tissues were transferred to 1 ml of TRIzol reagent (Gibco Life Technologies), frozen in liquid N2, and stored at −70°C. RNA was purified according to the manufacturer's instructions. Total cellular RNA was isolated by homogenization of the tissue in 2 ml of TRIzol reagent (Gibco Life Technologies). Following incubation at room temperature for 5 min, RNA was extracted with chloroform (Sigma) and then centrifuged for 15 min at 12,000 × g and 4°C. The aqueous phase was precipitated with an equal volume of isopropanol (Sigma) and subsequently centrifuged for 15 min at 12,000 × g and 4°C. The pellet was washed with 70% ethanol and resuspended in 50 μl of water. Total RNA was determined by spectrometric analysis. RNA was treated with DNase I (Clontech, Palo Alto, Calif.) to remove contaminant genomic DNA for 1.5 h in the presence of the RNase inhibitor (Ambion, Austin, Tex.). A 10× termination mixture (0.1 M EDTA [pH 8.0], 1 mg of glycogen/ml) was added to stop the reaction. The enzyme was removed with phenol-chloroform extraction, and RNA was precipitated with 2 volumes of ethanol and 1/10 volume of 3 M sodium acetate, pH 5.2. RNA was resuspended in 15 μl of diethyl pyrocarbonate-treated H2O containing the RNase inhibitor and stored at −70°C. RNA was tested for the presence of remaining DNA contamination by 35 cycles of PCR amplification with the glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific primers CR127 (AGAACATCATCCCTGCATCC) and CR128 (CTGGGATGGAAATTGTGAGG).
Reverse transcription (RT) was performed with Superscript (Gibco) according to the manufacturer's instructions by using 3 μg of total RNA in each reaction mixture. cDNA was synthesized in 20-μl reaction mixtures with oligo(dT). PCR amplification was performed by using 0.5 μl of cDNA as template with the specific primers for mouse iNOS CR104 (CAGAGGACCCAGAGACAAGA) and CR105 (ACCTGATGTTGCCATTGTTG). The following PCR conditions were used: a 10-min denaturing step at 94°C and 35 cycles of 40 s at 94°C, 40 s at 61°C, and 50 s at 72°C. PCR products were analyzed by 1.5% ethidium bromide-agarose gel electrophoresis.
Data presentation and statistical analysis.
All the results were expressed as the mean ± 1 standard error of the mean (SEM) from three independent experiments; n refers to the number of mice tested. Statistical significance was calculated by using the nonparametric Mann-Whitney U test. Multiple comparisons were performed by using the Neuman-Keuls multiple-comparison test. A P of <0.05 was considered significant.
RESULTS
C. rodentium infection induces iNOS mRNA expression in the colon.
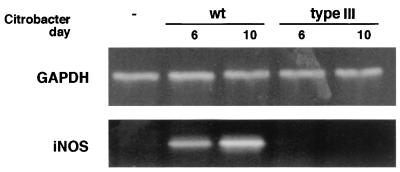
C57BL/6 mice were infected with either wild-type C. rodentium or the type III secretion mutant ΔescD. This mutation eliminates the ability of C. rodentium as well as EPEC to form pedestals on tissue culture cells. Following oral inoculation into mice, ΔescD only transiently colonized the colon and did not cause any appreciable tissue damage or disease (data not shown). RT-PCR for iNOS was performed on tissues taken from uninfected mice as well as from mice infected 6 or 10 days previously with the two isogenic C. rodentium strains. As shown in Fig. 1, iNOS mRNA levels were significantly increased by day 6 p.i. in mice infected with wild-type C. rodentium and expression was further increased by day 10 p.i. In contrast, no increase in iNOS mRNA expression was observed in tissues from mice that received the ΔescD mutant, indicating that the induction of iNOS mRNA required both the stable infection of the colon and C. rodentium possessing a functional type III secretion system.
FIG. 1.
Infection by wild-type (wt) C. rodentium but not the type III secretion mutant ΔescD leads to increased iNOS mRNA expression in colonic tissues. RT-PCR for iNOS demonstrated that infection of immunocompetent C57BL/6 mice with wild-type C. rodentium leads to a progressive and significant elevation in iNOS expression, as seen on days 6 and 10 p.i., compared to that in uninfected (−) mice. In contrast, infection with the ΔescD mutant did not induce iNOS mRNA expression over the same time course. The housekeeping gene GAPDH was used to normalize RNA abundance.
Immunoreactive iNOS expression is localized to colonic epithelial cells.
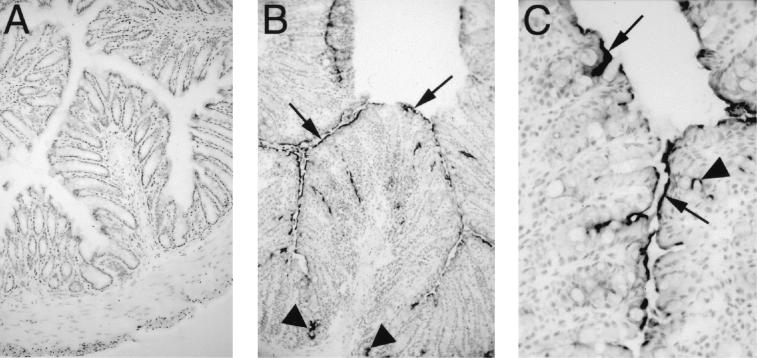
Based on the increased iNOS expression seen by RT-PCR, immunostaining was used to identify the cells expressing iNOS in the colons of mice infected with wild-type C. rodentium. Few if any cells in uninfected colonic tissues were immunoreactive for iNOS (Fig. 2A). In contrast, by day 6 p.i., more than 50% of the superficial epithelial cells in the infected distal colon expressed iNOS (data not shown). At this time, there was little inflammatory infiltrate and only a few iNOS-positive mononuclear cells could be detected within the lamina propria and submucosa. Compared to the number of epithelial cells that expressed iNOS, this cell population was negligible. By day 10 p.i., the inflammatory and pathological response to infection had increased, with edema and epithelial hyperplasia thickening the colonic mucosa. At this time, the number of iNOS-expressing cells had increased, as had the intensity of the staining throughout the infected colon. Again, 50 to 60% of the superficial epithelial cells were immunoreactive for iNOS. In addition, many of the less mature epithelial cells found midway along the length of the colonic crypts as well as at their base (Fig. 2B) expressed iNOS. Higher magnification revealed that iNOS expression by epithelial cells was focused along their apical membrane (Fig. 2C). While the number of inflammatory cells in the infected colon was greater on day 10 p.i., there were still only a small number that stained positively for iNOS. A similar pattern of iNOS expression was also seen at days 14 and 18 p.i., but between days 21 and 24 p.i., iNOS expression, while still observed on epithelial cells, had begun to subside (data not shown).
FIG. 2.
C. rodentium infection leads to iNOS expression by colonic epithelial cells. Immunohistochemistry for immunoreactive iNOS was performed on colonic tissues taken from uninfected mice (A), as well as mice at day 10 p.i. (B and C). Little if any immunoreactive iNOS was detectable in the colons of C57BL/6 mice prior to infection (panel A) (original magnification, ×100). By day 10 p.i., iNOS expression was seen along much of the mucosal surface (arrows) of infected C57BL/6 mice, including deep in the crypts (arrowheads) and by some scattered cells in the lamina propria (panel B) (original magnification, ×100). Under higher magnification, iNOS expression could be seen focused at the apical surface of many epithelial cells (arrows). A few lamina propria mononuclear cells were also found to express iNOS (arrowheads) (panel C) (original magnification, ×400).
Plasma nitrate and nitrite levels increase during infection.
Since elevated iNOS expression was seen in the colon during infection, the levels of NO found in the plasma were also assessed. As NO rapidly degrades to nitrate and nitrite, we measured total levels of these end products in plasma by converting nitrate to nitrite and then measuring nitrite by using the Griess reaction. Uninfected mice had plasma nitrate levels of 86.8 ± 12.6 μM. At the peak of the infection (day 10 p.i.), levels had significantly increased to 147.6 ± 11.2 μM (P < 0.05) (n = 4 in three independent infections).
SNP and GSNO are bacteriostatic for C. rodentium in vitro.
The bacteriostatic and bactericidal effects of RNI have been characterized for a number of bacterial species but not for A/E lesion-causing pathogens. The induction of iNOS expression and the elevation of circulating NO levels during C. rodentium infection indicated that this pathogen would encounter elevated NO levels as it infected its host. Therefore, the effects of the NO donor SNP and the NO releaser GSNO on C. rodentium growth were tested in vitro. As shown in Fig. 3A and B, both SNP and GSNO exhibited a dose-dependent inhibition of bacterial growth. SNP proved more effective than GSNO at inhibiting bacterial growth, although this may partially reflect the reduced basal bacterial growth seen at the low pH (pH 5.0) required for GSNO efficacy (58). At their most effective doses, SNP inhibited bacterial growth from an almost 1,000-fold increase down to less than 40-fold whereas GSNO inhibited growth from 450-fold down to approximately 60-fold. It should be noted that SNP in particular had a significant inhibitory effect on C. rodentium growth even at lower concentrations (100 μM).
FIG. 3.
The NO donor SNP and the NO releaser GSNO are bacteriostatic for C. rodentium in vitro. The addition of SNP (A) or GSNO (B) to bacterial cultures led to a dose-dependent suppression of bacterial growth over a period of 4 h. The cultures were grown in LB at pH 7.0 for SNP and pH 5.0 for GSNO. The asterisks denote a significant reduction in bacterial growth compared to that in untreated cultures (∗, P < 0.05).
iNOS deficiency does not affect morbidity in mice during infection.
Based on the increased iNOS expression and NO levels during infection and the finding that chemical sources of NO had strong bacteriostatic effects on C. rodentium in vitro, we next assessed the role of iNOS during infection in vivo. The course of C. rodentium infection in iNOS-deficient mice was compared to that seen in wild-type C57BL/6 mice. Infection led to reduced activity, ruffled fur, loose stool, and perianal fecal staining in both wild-type and iNOS-deficient mice. A similar degree and timing of weight loss was seen in both mouse strains during infection (data not shown), with body weight dropping by as much as 15% from days 10 to 14 p.i. compared to that of age-matched uninfected mice. Infection also resulted in the deaths of a small number of mice between days 10 and 14 p.i. In a total of three infections, mortality of wild-type mice averaged 24 ± 12% whereas mortality was reduced in iNOS-deficient mice to an average of 8 ± 6%. Despite the trend towards reduced mortality in the iNOS-deficient mice, it did not reach statistical significance.
We also examined the circulating plasma NO levels under basal and infected conditions in the two mouse strains by assaying both the nitrate and nitrite levels. In contrast to the significant increase in plasma NO levels found in infected wild-type mice (see above), levels in uninfected (74.0 ± 9.4 μM) and infected (89.8 ± 7.0 μM) iNOS-deficient mice were not significantly different (n = 4 in three independent infections). As well, staining of colonic tissues from infected and uninfected iNOS-deficient mice for immunoreactive iNOS (data not shown) confirmed their lack of iNOS protein expression.
iNOS expression accelerates bacterial clearance.
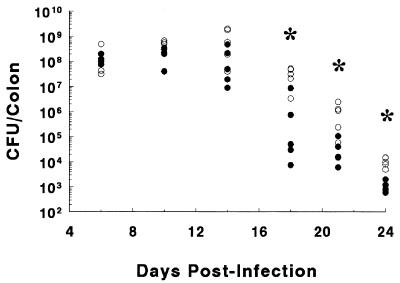
We next compared the course of bacterial colonization and clearance in wild-type and iNOS-deficient mice by homogenizing whole colons and plating the homogenate in serial dilutions on selective media. Both mouse strains were heavily colonized by C. rodentium by day 6 p.i., with bacterial numbers increasing on days 10 and 14 p.i. (Fig. 4). While there was a trend towards greater numbers in the iNOS-deficient mice at the earlier time points (days 6 to 14 p.i.), the differences did not reach statistical significance. By day 18 p.i., however, the numbers of C. rodentium bacteria within the colon began to drop and this clearance occurred more rapidly in the wild-type mice than in the iNOS-deficient mice. Bacterial counts were significantly greater (by 10- to 100-fold) in the iNOS-deficient mice than those in the wild-type mice at days 18, 21, and 24 p.i. (P < 0.05).
FIG. 4.
Following oral infection, both wild-type and iNOS-deficient mice were heavily colonized by C. rodentium by day 6 p.i. The numbers of bacteria recovered from the colons of individual infected wild-type C57BL/6 mice (filled circles) and iNOS-deficient mice (open circles) on days 6, 10, 14, 18, 21, and 24 p.i. are presented. Each group contained four or five mice. The asterisks denote the recovery of significantly more bacteria (mean value) recovered from iNOS-deficient mice compared to that recovered from wild-type mice at days 18, 21, and 24 p.i. (∗, P < 0.05.).
C. rodentium cells are found deeper in the crypts of iNOS-deficient mice.
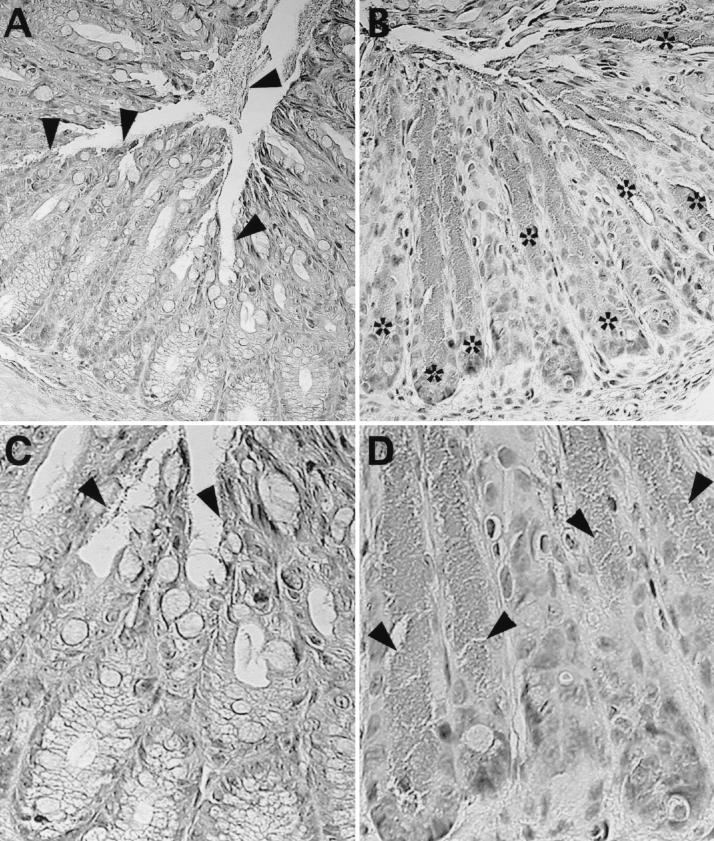
As recently described for the infection of IL-12- and IFN-γ-deficient mice (57), the absence of these cytokines leads to deeper penetration of C. rodentium into the colonic crypts of infected mice. Since the location and intensity of iNOS expression in the colonic crypts changed over the course of infection, we examined whether iNOS expression influenced bacterial localization within individual crypts. Bacterial colonization of both wild-type and iNOS-deficient mice occurred by day 6 p.i. (Fig. 4), but at this time, the infection was superficial, with bacteria rarely penetrating deeply into crypts. At day 10 p.i., most C. rodentium cells remained in superficial locations in the colons of wild-type mice (Fig. 5.) An occasional crypt was found where the infection had progressed deeper, to a depth halfway down the crypt (3.3 ± 1.2 crypts/cross section) or, rarely, to the base of the crypt (0.5 ± 0.4 crypts/cross section). At the same time, the depth of infection was much greater in the iNOS-deficient mice (Fig. 5), with many crypts partially (24.6 ± 12.9 crypts/cross section) or totally (13.0 ± 9.4 crypts/cross section) filled with bacteria all the way to their base. This effect was transient, since by day 14 p.i., bacteria in both mouse strains were again found to be in superficial locations, and this localization remained apparent at later time points.
FIG. 5.
C. rodentium bacteria at day 10 p.i. penetrate deeper into the colonic crypts of iNOS-deficient mice than in wild-type mice. (A) During the course of C. rodentium infection in wild-type C57BL/6 mice, the majority of the bacteria were found attached to superficial epithelial cells (arrowheads) and within the lumen. There is little evidence of bacteria penetrating deeply into colonic crypts. (C) Under higher magnification, one can see individual bacteria (arrowheads) on the surface of these superficial epithelial cells while the base of the crypts are uninfected. (B) In contrast, bacteria infecting iNOS-deficient mice at day 10 p.i. were found to penetrate deeper into crypts, sometimes reaching even the base of the glands. The asterisks identify crypts that are heavily infected with C. rodentium reaching to the base of the crypt. (D) At higher magnification, one can see several crypts filled down to their base with C. rodentium (arrowheads). This effect was transient with the infection in iNOS-deficient mice, again becoming superficial at later time points in the infection. Original magnification for all, ×400.
Colonic pathology is similar in wild-type and iNOS-deficient mice.
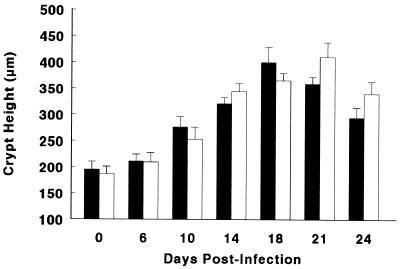
The colons of wild-type and iNOS-deficient mice were examined for changes in colonic weight as an indirect measure of epithelial hyperplasia, mucosal inflammation, and hyperemia (common features of C. rodentium infection). There was little increase in colon weight over the first 6 days of infection in either mouse strain, but between days 6 and 10 p.i., the tissue weight at least doubled that found in uninfected mice (Table 1). In the days following, the colon weights remained at this plateau and started to decrease only at day 21 p.i. Similarly, when we directly measured colonic crypt heights, we saw a similar dramatic increase in crypt heights in both wild-type and iNOS-deficient mice (Fig. 6). In both colon weight and crypt height measurements, while there was a trend towards greater pathology during the later stages of infection in the iNOS-deficient mice, the differences did not reach statistical significance.
TABLE 1.
Colon weights of the mice in this study
| Strain | Mean colon wt (mg)a at day:
|
||||||
|---|---|---|---|---|---|---|---|
| 0 | 6 | 10b | 14b | 18b | 21b | 24b | |
| Wild type | 102 ± 6 | 123 ± 11 | 188 ± 20 | 193 ± 16 | 190 ± 17 | 173 ± 19 | 168 ± 11 |
| iNOS (−/−) | 100 ± 4 | 120 ± 14 | 206 ± 26 | 208 ± 24 | 243 ± 28 | 203 ± 16 | 174 ± 9 |
C. rodentium infection results in increased colon weights in both wild-type and iNOS-deficient mice. Values represent the mean colon weight ± 1 SEM from three independent experiments, each with groups of four to five mice.
Significantly increased colon weight in both the wild-type and iNOS-deficient strains compared to that of uninfected mice of the same strain (P < 0.05).
FIG. 6.
Both wild-type C57BL/6 mice (solid bars) and iNOS-deficient mice (open bars) undergo significant increases in colonic crypt heights during C. rodentium infection. No significant differences in the mean crypt heights (in micrometers) were found between these two mouse strains over the first 24 days of infection. The data represent the means from three independent experiments in which each group contained five mice, and error bars represent standard errors.
Immunostaining for the virulence factor Tir to identify C. rodentium-infected cells.
Despite the observation that a number of colonic epithelial cells expressed iNOS during C. rodentium infection, it remained to be determined if the expression by these cells was directly induced by C. rodentium attachment or, alternatively, if it reflected an activation of the epithelial cells by the host immune response. This necessitated the development of an approach to identify infected cells. While staining for C. rodentium lipopolysaccharides (LPS) can be used to identify these pathogens in colonic tissue sections (61), such staining identifies all C. rodentium cells within the colon, many of which are not actually attached to host cells. Considering that C. rodentium can cover most of the colonic mucosal surface during the peak of its infection, a more specific marker for identifying infected cells was required. We therefore chose to stain for Tir, as this virulence factor is translocated inside host cells during the process of intimate attachment. By omitting detergents in our staining procedure, we found that we selectively stained Tir, as demonstrated in Fig. 7A. By double staining for C. rodentium with sera against LPS (serotype O152) as well as Tir, Tir staining was found beneath, but not inside, adherent bacteria, indicating that this is a suitable marker for identifying cells in colonic tissue sections that have been infected by C. rodentium.
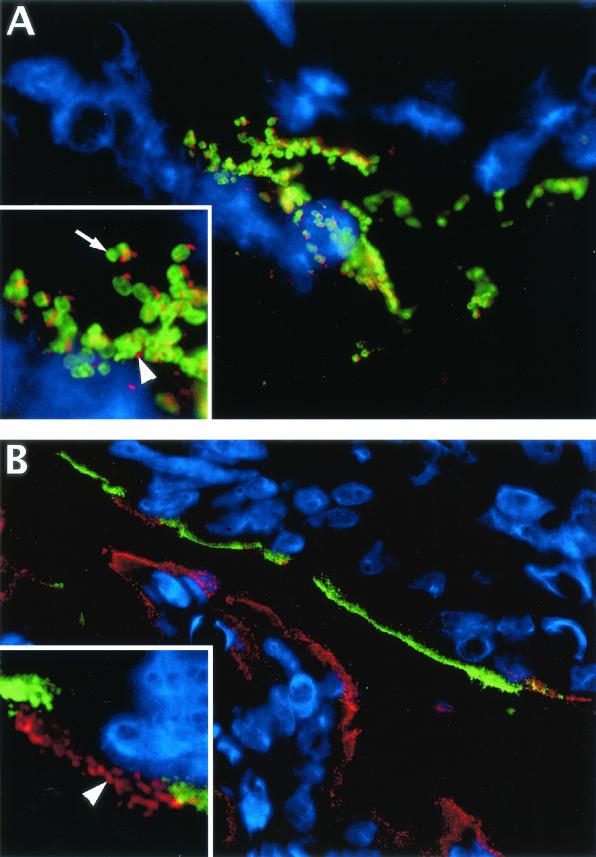
FIG. 7.
Immunofluorescence staining shows that infected cells in colonic tissue sections can be identified by their immunoreactivity for Tir but that Tir generally does not colocalize to cells expressing iNOS. (A) Colonic tissue section from a C. rodentium-infected mouse (day 10 p.i.) showing the lumenal surface of the colonic mucosa. Most host cells in this field of view (using DAPI to stain their nuclei blue) are superficial epithelial cells, while C. rodentium LPS is shown in green and Tir is shown in red. Large clusters of C. rodentium bacteria can be seen attached to these superficial epithelial cells during infection (original magnification, ×1,000). In the inset, at a higher magnification, one can see that Tir staining is not found inside the bacteria (arrow) but rather that it occurs outside of the bacteria (arrowhead), presumably translocated from the bacteria into the underlying epithelial cell and focused at the tip of actin pedestals. (B) This field of view shows the superficial colonic mucosa in both the upper and lower parts of the field, with the lumen in the center. By using the staining of Tir as a marker for infected cells, double immunostaining for Tir (red) and iNOS (green) shows that there is very little colocalization between cells expressing Tir and those expressing iNOS. The majority of the epithelial cells on the mucosal surface on the lower half of the picture are infected (Tir positive) but do not express iNOS, while the cells at the top express iNOS but are not infected and do not express Tir (original magnification, ×630). (Inset) At a higher magnification, one can see individual Tir foci in red (arrowhead) on the apical surface of one or two infected cells. These infected cells are the only iNOS-negative cells in a long stretch of uninfected epithelial cells expressing iNOS (green) at their apical surface.
iNOS is predominantly expressed by uninfected but not infected epithelial cells.
Using antibodies against both Tir and iNOS, we next examined colonic tissue sections to determine whether Tir-positive, C. rodentium-infected epithelial cells also expressed iNOS. Similar to our immunoperoxidase staining in Fig. 2, immunofluorescence staining of infected tissues identified many epithelial cells in the infected colon and particularly cells at the base of the colonic crypts as being iNOS positive. However, since C. rodentium predominantly infected superficial epithelial cells in immunocompetent C57BL/6 mice (Fig. 5), we focused only on those epithelial cells in the uppermost third of the crypts for the iNOS and Tir colocalization studies. Surprisingly, there was very little colocalization between the two markers on these superficial epithelial cells (Fig. 7B). While 87% of uninfected (Tir-negative) cells were strongly iNOS positive at day 10 p.i., epithelial cells that were mildly infected (fewer than 10 Tir foci/cell) seldom expressed iNOS (23%) and those that were more heavily infected were even less likely to express iNOS (Table 2). While Tir-positive, iNOS-negative epithelial cells were often found in stretches along several adjacent crypts, this staining pattern was also seen in individual cells. As shown in the Fig. 7B insert, there were long stretches of iNOS-expressing epithelial cells that were occasionally interspersed by Tir-positive, iNOS-negative epithelial cells. This pattern was seen on days 6, 10, and 14 p.i. At later time points, the number of bacteria in the colon had decreased to levels that made it difficult to identify more than a few scattered infected cells per tissue cross section.
TABLE 2.
iNOS expression by uninfected and infected colonic epithelial cells
| Cell status | % iNOS-expressing cellsa at day:
|
||
|---|---|---|---|
| 6 | 10 | 14 | |
| Uninfected | 75 ± 7 | 87 ± 10 | 90 ± 15 |
| Mildly infectedb | 21 ± 5 | 23 ± 6 | 29 ± 4 |
| Heavily infectedb | 14 ± 3 | 13 ± 4 | 18 ± 6 |
Infected cells were separated into groups of mildly infected cells (<10 foci of Tir/cell) and heavily infected cells (>10 foci of Tir/cell). Values represent the mean percentage of epithelial cells that express detectable iNOS ± 1 SEM from three independent experiments, each with groups of four mice.
Significantly lower percentages of iNOS-expressing cells at days 6, 10, and 14 than those of uninfected cells at the same time points (P < 0.05).
DISCUSSION
Following their ingestion, enteric bacterial pathogens must not only survive the harsh environment of the gastrointestinal tract but also overcome or avoid an array of mucosal defenses in order to replicate and successfully colonize their hosts. Strategies used by Shigella, Salmonella, and other bacterial pathogens to accomplish these goals include the invasion of the intestinal epithelium (47) and, in some cases, using the mucosa as a portal of entry to spread to distal sites by subsequently infecting professional phagocytes (14). As a result, most studies examining host defenses against these intestinal pathogens have focused on the responses of epithelial cells to bacterial invasion (10, 47) as well as on the antimicrobial mechanisms activated within phagocytes (6, 63). In contrast, there are many important diarrheagenic pathogens such as EPEC and EHEC that do not invade the intestinal epithelium but instead remain at and colonize the mucosal surface by attaching to the surface of epithelial cells (56). By remaining in this position throughout the duration of their infection, these microbes interact almost exclusively with underlying epithelial cells and have little contact with professional inflammatory or immune cells (62). Little is known about the host defense mechanisms and actions that are exerted against A/E pathogens.
This study demonstrates that one host defense mechanism encountered by the A/E pathogen C. rodentium during its infection of the mouse colon is the upregulation of iNOS expression by uninfected rather than infected colonic epithelial cells. iNOS-derived NO is a central effector molecule in the innate immune response to many pathogens. While NO and other RNI have a number of immunoregulatory functions, including inhibiting lymphocyte proliferation, altering cytokine and prostaglandin production, and inducing or inhibiting apoptosis (11), their primary function in host defense appears to be to damage and destroy pathogens (44). A number of cell types can produce RNI; however, macrophages generate the highest output, considerably outpacing all other cell types, including polymorphonuclear cells. Studies examining the antimicrobial actions of NO have therefore focused on the actions of macrophages against intracellular pathogens. Within macrophages, iNOS expression results in the generation of high concentrations of NO in close proximity to pathogens (44). At these concentrations, NO possesses potent antimicrobial activity and plays an important host-protective role against many intracellular pathogens, including Mycobacterium tuberculosis (44) and Leishmania major (65). While there is significant evidence for iNOS contributing to host defense against several intracellular pathogens, its role against extracellular mucosal pathogens is less clear (56). This is particularly true for A/E pathogens that are extracellular, actually residing outside the host itself. As a result of this protected location, they are protected from many of the actions of the host's inflammatory cells. Furthermore, EPEC and probably other A/E pathogens are capable of impairing the phagocytic ability of macrophages (21) through a phosphatidylinositol 3-kinase-dependent mechanism (5). Even so, simply preventing their uptake by macrophages may not fully protect EPEC and other pathogens, as RNI can be released by host cells into the external environment, killing extracellular parasites and bacteria such as E. coli (44).
Considering the number of factors limiting the ability of professional inflammatory cells to defend against A/E pathogens, it was interesting to find that colonic epithelial cells rather than phagocytes were the major cellular source of iNOS during C. rodentium infection. Such a host response is not unique, as iNOS expression has been shown in the gastric mucosa during Helicobacter pylori infection (17) and in the bladder epithelium of uropathogenic E. coli-infected mice (42). Similar to the results found with these diverse mucosal pathogens, iNOS expression by epithelial cells in the colon was focused along their apical surface. Since C. rodentium intimately attaches to the colonic epithelium, it seemed plausible that iNOS expression was directly induced by bacterial attachment. There are several studies demonstrating that epithelial cells respond to A/E pathogen attachment by activating inflammatory signaling pathways. EPEC and EHEC infections in vitro trigger NF-κB activation and the release of IL-8 (50-52). Similarly, EPEC infection has been shown to activate mitogen-activated protein kinase pathways in host cells (7, 8, 53). While there are no data demonstrating that A/E pathogens directly trigger iNOS expression in epithelial cells, Salmonella and other invasive pathogens do trigger a massive increase in iNOS expression in epithelial cells during the process of cellular invasion (10, 66).
Intestinal epithelial cells have been shown to express iNOS not only during infection but also in response to other challenges such as intestinal inflammation. This has been seen in humans (19, 34) and in experimental models (2, 35, 41) as well as in tissue culture following cytokine and endotoxin stimulation (36). Studies in vitro have conclusively demonstrated not only that epithelium-derived iNOS is functional but that the NO produced by epithelial cell monolayers is released predominantly from the apical surface (66). In the colon, this should result in the release of NO predominantly into the colonic lumen. Accordingly, this is what was found in ulcerative colitis patients in whom extensive iNOS expression by colonic epithelial cells was associated with lumenal NO levels elevated more than 20-fold over normal levels (19, 38, 46). The NO released by epithelial cells also can exert cytotoxic effects, as seen during Bordetella pertussis infection in the lung, where NO released from nonciliated epithelial cells causes the destruction of adjacent ciliated cells (13). Since A/E pathogens are in such intimate contact with the epithelium, they should be exposed to damaging levels of NO released from epithelial cells. Whether this is sufficient to kill bacteria or would merely limit their growth depends on the inherent susceptibility to RNI of the pathogen in question, as some bacteria possess mechanisms to limit the effects of NO (6). One way to test this is to use chemical sources of NO in culture. While the susceptibility of EPEC and EHEC to NO has yet to be examined, in previous studies, the NO donor SNP and the NO releaser GSNO have both been shown to have bacteriostatic effects on Salmonella and other gram-negative bacterial pathogens (39, 44, 58).
Studies examining the susceptibility of C. rodentium to RNI in vitro found that both SNP and GSNO had significant bacteriostatic effects on its growth in culture. At the highest doses tested, bacterial growth was reduced by more than 97% from an almost 1,000-fold increase in numbers over 4 h to less than a 40-fold increase. GSNO, which works best at the pH of the phagosome (pH 5.0), also suppressed C. rodentium growth, even though the baseline bacterial growth was reduced at this low pH. More importantly, SNP in particular had significant bacteriostatic effects on C. rodentium at fairly low concentrations (100 μM). These results suggested that iNOS and NO could be potent players in host defense in the C. rodentium model; therefore, iNOS-deficient mice were infected to determine the effect the loss of this source of NO had on the course of C. rodentium infection in vivo. Curiously, iNOS deficiency only transiently altered the infection, permitting C. rodentium to penetrate deeper into the colonic crypts of iNOS-deficient mice, as well as causing a moderate delay in bacterial clearance. iNOS expression had even less of an effect on macroscopic and histologic changes in the infected colon. Considering the widespread and consistent expression of iNOS in the colon of C. rodentium-infected wild-type mice, this was an unexpected result, particularly if adherent bacteria were in direct contact with the epithelial cells expressing iNOS. To determine if this was the case, it was necessary to examine whether infected cells were actually expressing iNOS. In order to test this, we needed to differentiate infected from uninfected epithelial cells in colonic tissues. To achieve this goal, we used a novel approach, staining a translocated bacterial effector protein in infected tissues. Based on its location at the tip of actin pedestals (22), we chose the bacterial effector Tir as a marker to identify C. rodentium-infected colonic epithelial cells. Through double-label immunostaining for both Tir and iNOS, we observed that iNOS expression was limited almost exclusively to uninfected epithelial cells. In contrast, those cells directly infected by C. rodentium were predominantly iNOS negative. This lack of colocalization between iNOS and Tir was seen throughout the course of the infection.
These results suggest that the small protective role identified for iNOS in vivo was probably mediated by uninfected rather than infected cells. More intriguing was the apparently selective expression of immunoreactive iNOS by uninfected rather than infected epithelial cells. This result strongly indicates that iNOS induction on epithelial cells was not caused by direct contact with C. rodentium but rather due to stimulation by bacterial LPS and/or the host inflammatory response. The lack of iNOS expression seen following infection with the type III secretion mutant ΔescD was probably due to the limited ability of this strain to colonize or induce an inflammatory response in the host rather than a direct effect of type III secretion. While there are several possible reasons behind the lack of iNOS and Tir colocalization to the same epithelial cells, it seems unlikely that it is simply due to C. rodentium preferentially infecting cells not expressing iNOS. Within a given section of colon, one can assume that the epithelial cells lining infected crypts should receive at least as much exposure to LPS and cytokines as those epithelial cells lining uninfected crypts. Despite this, iNOS expression was rarely seen in those crypts that were infected by C. rodentium. Furthermore, the observation that, amidst large stretches of iNOS-expressing epithelial cells, the few isolated cells that were infected were iNOS negative suggests that this pathogen may be taking an active role in limiting its exposure to NO.
Based on the data in this study, we hypothesize that C. rodentium infection is capable of modulating iNOS expression through the epithelial cells in its natural host, mice. In fact, a number of bacterial pathogens have recently been shown to manipulate or divert innate host defenses including iNOS and NO production (6). Best described are the mechanisms used by bacterial pathogens to subvert the microbicidal actions of macrophages. For example, Vazquez-Torres and Fang recently demonstrated the ability of Salmonella enterica serovar Typhimurium to divert NADPH (phagocyte oxidase) from the Salmonella-containing vacuole (63) whereas the bacterial pathogen Burkholderia pseudomallei has been shown to interfere with iNOS expression in an infected macrophage cell line (60). While less is known about the interactions between bacterial pathogens and the antimicrobial mechanisms expressed by epithelial cells, there is increasing evidence that pathogens do subvert the host's epithelial defenses. The H. pylori arginase rocF was recently found to inhibit NO production by eukaryotic cells (18), presumably as a strategy for survival in the face of epithelium-based NO production in the infected gastric mucosa. In addition, studies examining biopsy samples from Shigella flexneri-infected patients found a striking reduction in the expression of several antimicrobial peptides (defensins) in the infected epithelium (27). In the cases of both macrophages and epithelial cells, it appears that bacterial pathogens that interact for any length of time with these host cells commonly act to subvert their antimicrobial defenses. EPEC has already been described as being able to block phagocytosis (5, 21) and inhibit cytokine release from lymphocytes (31, 32). These abilities, taken together with the fact that A/E pathogens are intimately bound to epithelial cells for several days and can cause lengthy infections (12, 22), suggest that these pathogens have probably developed some means to avoid epithelial antimicrobial defenses, including NO-dependent killing. Future studies will be required to determine if the lack of iNOS expression on C. rodentium-infected cells is mediated through the specific actions of translocated bacterial effectors. Alternatively, since iNOS is associated with the actin cytoskeleton (64), the extensive cytoskeletal rearrangements caused by these infections may prevent the recruitment of iNOS from the cytoplasm to the apical surface of epithelial cells.
In conclusion, as we begin to understand the mucosal defenses involved in protecting the host during A/E pathogen infections, it appears that colonic epithelial cells do play an active role in host defense against these pathogens. Although this study specifically examined iNOS expression, several other antimicrobial effectors, including defensins, can be expressed by colonic epithelial cells. In fact, during the course of these studies, Simmons et al. (57) found that β-defensin-3 and iNOS, as well as the cytokines IL-12 and IFN-γ, were upregulated during C. rodentium infection. Unfortunately, the contribution of β-defensin-3 to host defense was not examined; however, compared to the significant roles seen for IL-12 and IFN-γ, iNOS was found to play little role during the course of infection and was not examined further. Our more extensive evaluation over the full time course of C. rodentium infection did reveal a modest contribution by iNOS to host defense but, more importantly, led us to the intriguing observation that the activation of iNOS expression on epithelial cells was generally absent on infected cells. While determining the basis for this specific observation will require further studies using tissue culture models, it is important to note the novelty and applicability of this approach. This is the first study where a translocated bacterial virulence factor has been stained in infected tissue sections, allowing us to differentiate between the host response in the whole infected tissue and the response at the single-infected-cell level. Future studies should assist in our understanding of the intricate and complex interactions between A/E pathogens and their hosts. Identifying what antimicrobial mechanisms these pathogens actually encounter and how they may subvert host cell functions should prove of benefit, not only in combating this important family of human and veterinary pathogens but in managing public health in general.
Acknowledgments
We thank Valerie Templeman for technical assistance and Carrie Rosenberger and Cathy Horne for critical reading of the manuscript.
This work was supported by operating grants from the Canadian Institute for Health Research (CIHR) and the Howard Hughes Medical Institute. B.A.V. is supported by a Canadian Digestive Diseases Foundation/Medical Research Council of Canada (MRC) postdoctoral fellowship and is an honorary Isaak Walton Killam fellow. W.D. was supported by an MRC postdoctoral fellowship. B.B.F. is a Howard Hughes International Research Scholar and a CIHR Distinguished Investigator.
Editor: A. D. O'Brien
REFERENCES
- 1.Abe, A., U. Heczko, R. G. Hegele, and B. B. Finlay. 1998. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J. Exp. Med. 188:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asfaha, S., C. J. Bell, J. L. Wallace, and W. K. MacNaughton. 1999. Prolonged colonic epithelial hyporesponsiveness after colitis: role of inducible nitric oxide synthase. Am. J. Physiol. 276:G703-G710. [DOI] [PubMed] [Google Scholar]
- 3.Barthold, S. W., G. L. Coleman, R. O. Jacoby, E. M. Livestone, and A. M. Jonas. 1978. Transmissible murine colonic hyperplasia. Vet. Pathol. 15:223-236. [DOI] [PubMed] [Google Scholar]
- 4.Celli, J., W. Deng, and B. B. Finlay. 2000. Enteropathogenic Escherichia coli (EPEC) attachment to epithelial cells: exploiting the host cell cytoskeleton from the outside. Cell. Microbiol. 2:1-9. [DOI] [PubMed] [Google Scholar]
- 5.Celli, J., M. Olivier, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J. 20:1245-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakravortty, D., I. Hansen-Wester, and M. Hensel. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular salmonella from reactive nitrogen intermediates. J. Exp. Med. 195:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czerucka, D., S. Dahan, B. Mograbi, B. Rossi, and P. Rampal. 2001. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect. Immun. 69:1298-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Grado, M., C. M. Rosenberger, A. Gauthier, B. A. Vallance, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli infection induces expression of the early growth response factor by activating mitogen-activated protein kinase cascades in epithelial cells. Infect. Immun. 69:6217-6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng, W., Y. Li, B. A. Vallance, and B. B. Finlay. 2001. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 69:6323-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckmann, L., J. R. Smith, M. P. Housley, M. B. Dwinell, and M. F. Kagnoff. 2000. Analysis by high density cDNA arrays of altered gene expression in human intestinal epithelial cells in response to infection with the invasive enteric bacteria Salmonella. J. Biol. Chem. 275:14084-14094. [DOI] [PubMed] [Google Scholar]
- 11.Eisenstein, T. K. 2001. Implications of Salmonella-induced nitric oxide (NO) for host defense and vaccines: NO, an antimicrobial, antitumor, immunosuppressive and immunoregulatory molecule. Microbes Infect. 3:1223-1231. [DOI] [PubMed] [Google Scholar]
- 12.Fagundes Neto, U., V. D. Ferreira, F. R. Patricio, V. L. Mostaco, and L. R. Trabulsi. 1989. Protracted diarrhea: the importance of the enteropathogenic E. coli (EPEC) strains and Salmonella in its genesis. J. Pediatr. Gastroenterol. Nutr. 8:207-211. [PubMed] [Google Scholar]
- 13.Flak, T. A., and W. E. Goldman. 1999. Signalling and cellular specificity of airway nitric oxide production in pertussis. Cell. Microbiol. 1:51-60. [DOI] [PubMed] [Google Scholar]
- 14.Fleckenstein, J. M., and D. J. Kopecko. 2001. Breaching the mucosal barrier by stealth: an emerging pathogenic mechanism for enteroadherent bacterial pathogens. J. Clin. Investig. 107:27-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsythe, R. M., D. Z. Xu, Q. Lu, and E. A. Deitch. 2002. Lipopolysaccharide-induced enterocyte-derived nitric oxide induces intestinal monolayer permeability in an autocrine fashion. Shock 17:180-184. [DOI] [PubMed] [Google Scholar]
- 16.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 17.Fu, S., K. S. Ramanujam, A. Wong, G. T. Fantry, C. B. Drachenberg, S. P. James, S. J. Meltzer, and K. T. Wilson. 1999. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology 116:1319-1329. [DOI] [PubMed] [Google Scholar]
- 18.Gobert, A. P., D. J. McGee, M. Akhtar, G. L. Mendz, J. C. Newton, Y. Cheng, H. L. Mobley, and K. T. Wilson. 2001. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc. Natl. Acad. Sci. USA 98:13844-13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godkin, A. J., A. J. De Belder, L. Villa, A. Wong, J. E. Beesley, S. P. Kane, and J. F. Martin. 1996. Expression of nitric oxide synthase in ulcerative colitis. Eur. J. Clin. Investig. 26:867-872. [DOI] [PubMed] [Google Scholar]
- 20.Goffaux, F., B. China, L. Janssen, V. Pirson, and J. Mainil. 1999. The locus for enterocyte effacement (LEE) of enteropathogenic Escherichia coli (EPEC) from dogs and cats. Adv. Exp. Med. Biol. 473:129-136. [DOI] [PubMed] [Google Scholar]
- 21.Goosney, D. L., J. Celli, B. Kenny, and B. B. Finlay. 1999. Enteropathogenic Escherichia coli inhibits phagocytosis. Infect. Immun. 67:490-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goosney, D. L., S. Gruenheid, and B. B. Finlay. 2000. Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu. Rev. Cell. Dev. Biol. 16:173-189. [DOI] [PubMed] [Google Scholar]
- 23.Hecht, G. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VII. Enteropathogenic Escherichia coli: physiological alterations from an extracellular position. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G1-G7. [DOI] [PubMed] [Google Scholar]
- 24.Higgins, L. M., G. Frankel, I. Connerton, N. S. Goncalves, G. Dougan, and T. T. MacDonald. 1999. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285:588-591. [DOI] [PubMed] [Google Scholar]
- 25.Higgins, L. M., G. Frankel, G. Douce, G. Dougan, and T. T. MacDonald. 1999. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 67:3031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill, S. M., A. D. Phillips, and J. A. Walker-Smith. 1991. Enteropathogenic Escherichia coli and life threatening chronic diarrhoea. Gut 32:154-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Islam, D., L. Bandholtz, J. Nilsson, H. Wigzell, B. Christensson, B. Agerberth, and G. Gudmundsson. 2001. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat. Med. 7:180-185. [DOI] [PubMed] [Google Scholar]
- 28.Kallas, M. R., F. R. Patricio, and U. Fagundes Neto. 1995. Morphometrics of the small intestine in children with diarrhea due to classical enteropathogenic Escherichia coli and to environmental asymptomatic enteropathy. Rev. Assoc. Med. Bras. 41:162-166. [PubMed] [Google Scholar]
- 29.Kaper, J. B. 1998. Enterohemorrhagic Escherichia coli. Curr. Opin. Microbiol. 1:103-108. [DOI] [PubMed] [Google Scholar]
- 30.Kenny, B., R. DeVinney, M. Stein, D. Reinscheid, E. Frey, and B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 31.Klapproth, J. M., M. S. Donnenberg, J. M. Abraham, and S. P. James. 1996. Products of enteropathogenic E. coli inhibit lymphokine production by gastrointestinal lymphocytes. Am. J. Physiol. 271:G841-G848. [DOI] [PubMed] [Google Scholar]
- 32.Klapproth, J. M., I. C. Scaletsky, B. P. McNamara, L. C. Lai, C. Malstrom, S. P. James, and M. S. Donnenberg. 2000. A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect. Immun. 68:2148-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi, O., H. Miwa, S. Watanabe, M. Tsujii, R. N. Dubois, and N. Sato. 2001. Cyclooxygenase-2 downregulates inducible nitric oxide synthase in rat intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G688-G696. [DOI] [PubMed] [Google Scholar]
- 34.Kolios, G., N. Rooney, C. T. Murphy, D. A. Robertson, and J. Westwick. 1998. Expression of inducible nitric oxide synthase activity in human colon epithelial cells: modulation by T lymphocyte derived cytokines. Gut 43:56-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kubes, P. 2000. Inducible nitric oxide synthase: a little bit of good in all of us. Gut 47:6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahde, M., R. Korhonen, and E. Moilanen. 2000. Regulation of nitric oxide production in cultured human T84 intestinal epithelial cells by nuclear factor-kappa B-dependent induction of inducible nitric oxide synthase after exposure to bacterial endotoxin. Aliment. Pharmacol. Ther. 14:945-954. [DOI] [PubMed] [Google Scholar]
- 37.Li, Y., E. Frey, A. M. Mackenzie, and B. B. Finlay. 2000. Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect. Immun. 68:5090-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ljung, T., M. Herulf, E. Beijer, H. Jacobsson, J. Lundberg, Y. Finkel, and P. M. Hellstrom. 2001. Rectal nitric oxide assessment in children with Crohn disease and ulcerative colitis. Indicator of ileocaecal and colorectal affection. Scand. J. Gastroenterol. 36:1073-1076. [DOI] [PubMed] [Google Scholar]
- 39.Lu, S., P. B. Killoran, F. C. Fang, and L. W. Riley. 2002. The global regulator ArcA controls resistance to reactive nitrogen and oxygen intermediates in Salmonella enterica serovar Enteritidis. Infect. Immun. 70:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luperchio, S. A., J. V. Newman, C. A. Dangler, M. D. Schrenzel, D. J. Brenner, A. G. Steigerwalt, and D. B. Schauer. 2000. Citrobacter rodentium, the causative agent of transmissible murine colonic hyperplasia, exhibits clonality: synonymy of C. rodentium and mouse-pathogenic Escherichia coli. J. Clin. Microbiol. 38:4343-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacNaughton, W. K., S. S. Lowe, and K. Cushing. 1998. Role of nitric oxide in inflammation-induced suppression of secretion in a mouse model of acute colitis. Am. J. Physiol. 275:G1353-G1360. [DOI] [PubMed] [Google Scholar]
- 42.Mysorekar, I. U., M. A. Mulvey, S. J. Hultgren, and J. I. Gordon. 2002. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J. Biol. Chem. 277:7412-7419. [DOI] [PubMed] [Google Scholar]
- 43.Nataro, J., and J. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nathan, C., and M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newman, J. V., B. A. Zabel, S. S. Jha, and D. B. Schauer. 1999. Citrobacter rodentium espB is necessary for signal transduction and for infection of laboratory mice. Infect. Immun. 67:6019-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perner, A., I. Nordgaard, P. Matzen, and J. Rask-Madsen. 2002. Colonic production of nitric oxide gas in ulcerative colitis, collagenous colitis and uninflamed bowel. Scand. J. Gastroenterol. 37:183-188. [DOI] [PubMed] [Google Scholar]
- 47.Philpott, D. J., S. E. Girardin, and P. J. Sansonetti. 2001. Innate immune responses of epithelial cells following infection with bacterial pathogens. Curr. Opin. Immunol. 13:410-416. [DOI] [PubMed] [Google Scholar]
- 48.Robins-Browne, R. M., A. M. Tokhi, L. M. Adams, V. Bennett-Wood, A. V. Moisidis, E. O. Krejany, and L. E. O'Gorman. 1994. Adherence characteristics of attaching and effacing strains of Escherichia coli from rabbits. Infect. Immun. 62:1584-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salzman, A. L., T. Eaves-Pyles, S. C. Linn, A. G. Denenberg, and C. Szabo. 1998. Bacterial induction of inducible nitric oxide synthase in cultured human intestinal epithelial cells. Gastroenterology 114:93-102. [DOI] [PubMed] [Google Scholar]
- 50.Savkovic, S., A. Koutsouris, and G. Hecht. 1998. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 51.Savkovic, S., A. Koutsouris, and G. Hecht. 1996. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 64:4480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 53.Savkovic, S. D., A. Ramaswamy, A. Koutsouris, and G. Hecht. 2001. EPEC-activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G890-G898. [DOI] [PubMed] [Google Scholar]
- 54.Schauer, D. B. 1994. Murine colonic hyperplasia, p. 197-208. In V. L. Miller, J. B. Kaper, D. A. Portnoy, and R. R. Isberg (ed.), Molecular genetics of bacterial pathogenesis. American Society for Microbiology, Washington, D.C.
- 55.Schauer, D. B., and S. Falkow. 1993. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect. Immun. 61:4654-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simmons, C. P., S. Clare, and G. Dougan. 2001. Understanding mucosal responsiveness: lessons from enteric bacterial pathogens. Semin. Immunol. 13:201-209. [DOI] [PubMed] [Google Scholar]
- 57.Simmons, C. P., N. S. Goncalves, M. Ghaem-Maghami, M. Bajaj-Elliott, S. Clare, B. Neves, G. Frankel, G. Dougan, and T. T. MacDonald. 2002. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-gamma. J. Immunol. 168:1804-1812. [DOI] [PubMed] [Google Scholar]
- 58.St. John, G., N. Brot, J. Ruan, H. Erdjument-Bromage, P. Tempst, H. Weissbach, and C. Nathan. 2001. Peptide methionine sulfoxide reductase from Escherichia coli and Mycobacterium tuberculosis protects bacteria against oxidative damage from reactive nitrogen intermediates. Proc. Natl. Acad. Sci. USA 98:9901-9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tacket, C. O., M. B. Sztein, G. Losonsky, A. Abe, B. B. Finlay, B. P. McNamara, G. T. Fantry, S. P. James, J. P. Nataro, M. M. Levine, and M. S. Donnenberg. 2000. Role of EspB in experimental human enteropathogenic Escherichia coli infection. Infect. Immun. 68:3689-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Utaisincharoen, P., N. Tangthawornchaikul, W. Kespichayawattana, P. Chaisuriya, and S. Sirisinha. 2001. Burkholderia pseudomallei interferes with inducible nitric oxide synthase (iNOS) production: a possible mechanism of evading macrophage killing. Microbiol. Immunol. 45:307-313. [DOI] [PubMed] [Google Scholar]
- 61.Vallance, B. A., W. Deng, L. A. Knodler, and B. B. Finlay. 2002. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect. Immun. 70:2070-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vazquez-Torres, A., and F. C. Fang. 2001. Salmonella evasion of the NADPH phagocyte oxidase. Microbes Infect. 3:1313-1320. [DOI] [PubMed] [Google Scholar]
- 64.Webb, J. L., M. W. Harvey, D. W. Holden, and T. J. Evans. 2001. Macrophage nitric oxide synthase associates with cortical actin but is not recruited to phagosomes. Infect. Immun. 69:6391-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei, X. Q., I. G. Charles, A. Smith, J. Ure, G. J. Feng, F. P. Huang, D. Xu, W. Muller, S. Moncada, and F. Y. Liew. 1995. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408-411. [DOI] [PubMed] [Google Scholar]
- 66.Witthoft, T., L. Eckmann, J. M. Kim, and M. F. Kagnoff. 1998. Enteroinvasive bacteria directly activate expression of iNOS and NO production in human colon epithelial cells. Am. J. Physiol. 275:G564-G571. [DOI] [PubMed] [Google Scholar]
- 67.Zhu, C., T. S. Agin, S. J. Elliott, L. A. Johnson, T. E. Thate, J. B. Kaper, and E. C. Boedeker. 2001. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infect. Immun. 69:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]