Abstract
Nanosecond, megavolt-per-meter, pulsed electric fields induce phosphatidylserine (PS) externalization, intracellular calcium redistribution, and apoptosis in Jurkat T-lymphoblasts, without causing immediately apparent physical damage to the cells. Intracellular calcium mobilization occurs within milliseconds of pulse exposure, and membrane phospholipid translocation is observed within minutes. Pulsed cells maintain cytoplasmic membrane integrity, blocking propidium iodide and Trypan blue. Indicators of apoptosis—caspase activation and loss of mitochondrial membrane potential—appear in nanoelectropulsed cells at later times. Although a theoretical framework has been established, specific mechanisms through which external nanosecond pulsed electric fields trigger intracellular responses in actively growing cells have not yet been experimentally characterized. This report focuses on the membrane phospholipid rearrangement that appears after ultrashort pulse exposure. We present evidence that the minimum field strength required for PS externalization in actively metabolizing Jurkat cells with 7-ns pulses produces transmembrane potentials associated with increased membrane conductance when pulse widths are microseconds rather than nanoseconds. We also show that nanoelectropulse trains delivered at repetition rates from 2 to 2000 Hz have similar effects, that nanoelectropulse-induced PS externalization does not require calcium in the external medium, and that the pulse regimens used in these experiments do not cause significant intra- or extracellular Joule heating.
INTRODUCTION
Phosphatidylserine (PS), a key phospholipid component of the eukaryotic cell membrane, is found almost exclusively on the inner leaflet of the plasma membrane lipid bilayer in normal, healthy cells. This asymmetric distribution is maintained by the dynamic counterbalancing of two enzymatic activities (Verhoven et al., 1995), a Ca2+-inhibited, ATP-dependent aminophospholipid translocase, which moves PS from the external to the internal face of the membrane (Seigneuret and Devaux, 1984), and a Ca2+-dependent aminophospholipid scramblase (Basse et al., 1996; Comfurius et al., 1996), which facilitates the randomization of the PS distribution between the two lipid layers. PS externalization is an important physiological signal. Cells with exposed PS (apoptotic cells and aging erythrocytes, for example) are marked for phagocytosis and disposal (Fadok et al., 2001), and externalized PS plays a key role in platelet activation and blood coagulation (Balasubramanian and Schroit, 2003).
Although the net energy required to move PS from one side of the lipid bilayer to the other is small (approximately kT), a large energy barrier is associated with the transport of the charged phospholipid head group through the hydrophobic interior of the membrane. Translocation activation energies for stable, homogeneous membranes are on the order of 100 kJ/mol (Homan and Pownall, 1988), and rate constants for spontaneous transverse phospholipid migration are measured in hours (Kornberg and McConnell, 1971; Lipka et al., 1991). The cell membrane is far from homogeneous, however, and a number of factors, singly or in concert, may lower this activation energy or otherwise facilitate membrane restructuring and PS translocation. These include thermal- (Heimburg, 1998) and electric field-induced (Sugar, 1979) lipid phase transitions, lipid raft distribution and the dynamic restructuring of cytoskeletal-membrane attachments (Kunzelmann-Marche et al., 2002), membrane-spanning foreign (Eisenberg et al., 1973) and native peptides (Wu and Hubbell, 1993), including rhodopsin (Hessel et al., 2001), release of Ca2+ from internal stores (Martinez et al., 1999), and proximity of depolarized mitochondria (Blom et al., 2003).
Early (Dressler et al., 1983) and more recent (Haest et al., 1997) studies of electroporation showed that microsecond-pulsed electric fields (20 μs, 0.3–0.9 MV/m, delivered at 0°C in a nonnutrient buffer) can cause PS externalization in erythrocyte membranes. Because these conditions enhance the porative effects of the external field, with associated rearrangements of the membrane bilayer, it is not surprising that PS appears on the cell surface after pulse exposure. At the site of pore formation the two faces of the membrane become contiguous, bypassing the hydrophobic barrier to translayer movement of the charged phospholipid head groups. Lateral diffusion (Sonnleitner et al., 1999) can now carry PS molecules from the interior to the exterior face of the cell at micrometer-per-second rates (Fujiwara et al., 2002). Ultrashort, high-field pulses (7–300 ns, 2.5–30 MV/m) also induce PS translocation, but without significant poration of the external membrane, in both nonnutrient buffer suspensions (Beebe et al., 2002) and with cells in nutrient medium (Vernier et al., 2003a). The mechanism for this nanoelectropulse-induced translocation has not been elucidated.
Unlike electroporative pulses with durations in the microsecond range, which produce voltages across the cytoplasmic membrane with no direct effects on the cell interior, pulses shorter than the charging time constant of the plasma membrane (∼100 ns in physiological media for cells with a radius of a few micrometers), or more precisely pulses with rise and fall times faster than the membrane charging time constant, produce lower potentials across the external membrane and an electric field in the cytoplasm, expressed as voltages across the intracellular membranes of the nucleus, mitochondria, and other organelles (Sher et al., 1970; Schoenbach et al., 2001). For nanosecond pulses the cytoplasmic membrane appears electrically transparent, and, if the pulse amplitude is large enough, depolarizing and porating potentials can appear across internal structures and dissipate before the plasma membrane charges to hyperpolarizing voltages (Vernier et al., 2003a).
Preliminary evidence suggests several possible mechanisms for the loss of membrane phospholipid asymmetry after ultrashort pulsed electric field exposure. Depending on cell size and pulse parameters, nanoelectropulse-induced PS translocation could result entirely from intracellular effects such as calcium bursts (Vernier et al., 2003b), or from nonporative dipole interactions with membrane components (Miller, 2002), or as a consequence of diffusion facilitated by formation of nanosecond-duration, nanometer-diameter membrane openings of the external membrane (Taupin, et al., 1975; Popescu and Victor, 1991), or some combination of these. To evaluate these and other hypotheses it is essential to know the electrophysical boundaries of the pulsed field exposures that produce membrane phospholipid translocation.
MATERIALS AND METHODS
Cell lines and culture conditions
Human Jurkat T lymphocytes (ATCC TIB-152) were grown in RPMI 1640 (Irvine Scientific, Irvine, CA) containing 10% heat-inactivated fetal bovine serum (FBS; Gibco, Carlsbad, CA), 2 mM L-glutamine (Gibco), 50 units/mL penicillin (Gibco), and 50 μg/mL streptomycin (Gibco) at 37°C in a humidified, 5% carbon dioxide atmosphere.
Pulse generator and pulse exposures
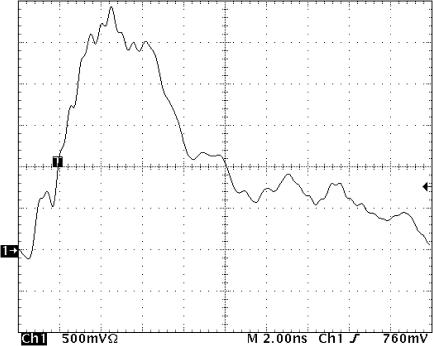
A MOSFET-based, inductive-adding pulse generator with a balanced, coaxial-cable pulse-forming network and spark-gap switch designed and assembled at the University of Southern California, provided electrical pulses (Fig. 1) to cell suspensions (2 × 107 cells/mL) at room temperature in growth medium in rectangular electroporation cuvettes with a 1-millimeter electrode separation (Bio-Rad, Hercules, CA).
FIGURE 1.
Nanoelectropulse from spark gap-switched cable discharge pulse generator. Pulse was 2500 V, 7 ns, with 2-ns rise time, delivered to cells suspended in growth medium (RPMI 1640).
For microscopic observations, cells were placed in a rectangular channel 100 μm wide, 30 μm deep, and 12 mm long, with gold-plated electrode walls, microfabricated with photolithographic methods on a glass microscope slide. A fast MOSFET MicroPulser (Behrend et al., 2003) was mounted on the microscope stage for delivery of pulses directly to the microchamber electrodes in ambient atmosphere at room temperature (Fig. 2).
FIGURE 2.
Microscope slide-based, MOSFET-driven pulse generator. (a) Pulse generator and pulse exposure chamber mounted on the stage of a fluorescence microscope. (b) Pulse waveform of 370 V, 30 ns, delivered to cell suspension in lithographically fabricated microchamber.
Phosphatidylserine externalization assay
Translocation of PS and the degree of membrane permeabilization were measured by flow cytometric analysis (FACStar with CellQuest software, Becton-Dickinson, San Jose, CA) of annexin V-FITC (fluorescein isothiocyanate) binding and propidium iodide uptake. Staining reagents and buffer were from a commercial kit (Annexin V-FITC Apoptosis Detection Kit, BD Pharmingen, San Diego, CA). Data represent the results of at least three independent experiments. EGTA is molecular biology grade (Calbiochem, San Diego, CA).
Temperature-sensitive fluorescence microphotometry
Observations of live cells during pulse exposures were made with a Zeiss Axiovert 200 fluorescence microscope. Cells were loaded with the temperature-sensitive fluorochrome europium thenoyltrifluoroacetonate (Eu-TTA; Acros Organics, Morris Plains, NJ; λex = 360 +/− 20 nm, λem = 605 +/− 30 nm) at 50 μM in growth medium for 1 h at 37°C, then washed and resuspended in growth medium. Images were captured and analyzed with a LaVision PicoStar HR12 camera and software (LaVision, Goettingen, Germany).
RESULTS
Pulsed electric field threshold for PS translocation (annexin V binding)
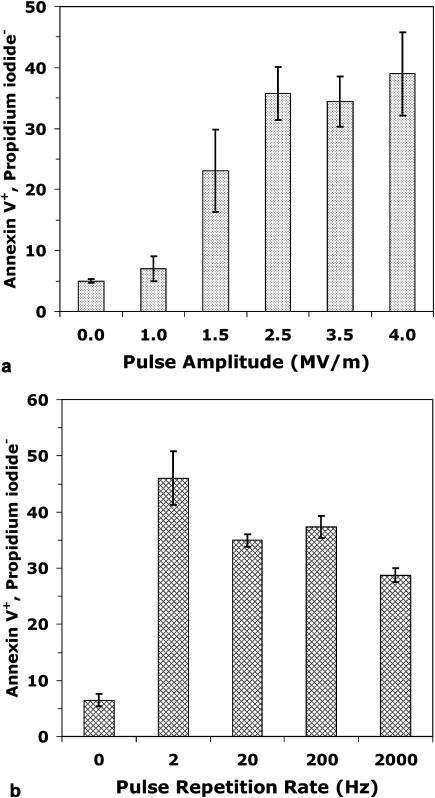
Annexin V binding, a standard gauge of PS externalization in apoptosis assays (Koopman et al., 1994), provides a measure for nanoelectropulse-induced membrane phospholipid translocation. Jurkat cells exposed to 50 7-ns pulses delivered at 20 Hz with field strengths from 0 to 1.0 MV/m bind annexin V at background levels similar to unexposed controls (Fig. 3 a). The 50-pulse dose was chosen for these experiments as an end point, based on previous observations, at which a maximum percentage of the cell population responds without producing the increasing levels of membrane-damaged (necrotic) cells observed at higher pulse counts (Vernier et al., 2003a).
FIGURE 3.
Megavolt-per-meter field threshold and effect of pulse repetition rate on PS externalization. Flow cytometric fraction of cells binding annexin V (standard assay for PS translocation) with intact membranes (no propidium iodide influx) after 50-pulse exposures to 7-ns pulses at amplitudes (a) and pulse repetition rates (b) indicated on the x axes. Pulses in (a) were delivered at 20 Hz. Pulses in (b) were 2.5 MV/m.
1.5 MV/m pulses produce an intermediate level of PS externalization, above background but less than the maximum response observed with higher amplitudes, indicating that the field threshold for PS translocation with 7-ns pulses lies somewhere between 1.0 and 1.5 MV/m. At higher field strengths a plateau in the response is observed. Pulses of 2.5, 3.5, and 4.0 MV/m induce PS translocation in approximately the same fraction of the population—30–40% of the cells in these experiments (Fig. 3 a). Fields greater than 4 MV/m delivered in 50-pulse doses cause dissolution of a significant fraction of the cells (not shown).
The data in Fig. 3 is from samples assayed immediately after pulse exposure. Similar results are obtained from samples at 2 and 5 h after pulsing.
Pulse-induced PS externalization at different pulse repetition rates
Varying the pulse repetition rate over three orders of magnitude for 50-pulse, 7-ns, 2.5-MV/m exposures reveals a measurable decline in PS translocation at 2000 Hz, the highest rate tested, and an enhancement at 2 Hz, the slowest rate tested. These observations place constraints on the mechanism for nanoelectropulse-induced PS externalization, which we discuss below.
Pulse-induced PS externalization does not require Ca2+ in external medium
Although it has been demonstrated that neither Ca2+ nor propidium iodide nor Na+ enters the cell after nanoelectropulse exposures in quantities detectable by fluorescence microscopic methods (Vernier et al., 2003b), it is possible that nanosecond-duration, nanometer-diameter pores are formed during the pulses, and that the large Ca2+ concentration gradient across the plasma membrane ([Ca2+]e = 750 μM; [Ca2+]i = 100 nM) drives an influx of Ca2+ through these nanopores sufficient to stimulate enough phospholipid scramblase activity to produce the PS translocation that we observe.
If this were true, then reducing the amount of available Ca2+ in the external medium would be expected to inhibit nanoelectropulse-induced PS externalization. The data in Table 1 does not support this hypothesis. Adding 5 mM EGTA to the medium before pulse exposure does not reduce the amount of PS translocation detected by flow cytometric analysis of annexin V binding. Divalent cation chelating agents are known to increase the resistance of cells to electric pulses of longer duration (microseconds, milliseconds), perhaps through direct effects on the membrane phospholipid bilayer itself or on its associations with elements of the cytoskeleton (Mussauer et al., 1999), but those protective mechanisms do not prevent nanosecond pulse-induced phospholipid redistribution.
TABLE 1.
PS externalization does not require Ca2+ in the external medium
| Exposure | Percent of cells with externalized PS (annexin V+) | 95% Confidence limits |
|---|---|---|
| 0 pulses | 10.6 | 6.2 |
| 50 pulses | 42.0 | 5.4 |
| 50 pulses + EGTA | 49.5 | 7.2 |
The presence of 5 mM EGTA in the external medium does not inhibit nanoelectropulse-induced PS translocation (annexin V binding) after 50 7-ns, 2.5-MV/m pulses. Data represent the average of six samples (10,000 cells each) from three independent experiments.
Temperature-dependent Eu-TTA fluorescence shows lack of nanoelectropulse-induced cell heating
Temperature-driven decreases in the fluorescence lifetime of 7-nitrobenz-2-oxa-1,3-diazol-4-yl (NBD) (Chapman et al., 1995), thermal quenching of rhodamine fluorescence (Kato et al., 1999), and the much more temperature-sensitive phosphorescence of Eu-TTA (Zohar et al., 1998) have been utilized as cell “thermometers.” We observed Eu-TTA fluorescence in solution and in loaded cells in a microfabricated electrode chamber during micro- and nanoelectropulse application. Long pulses (1 μs, 1.0 MV/m, 30 μJ per pulse) produce spikes of decreased fluorescence (increased temperature) for Eu-TTA in the extracellular medium (Fig. 4 a) corresponding to temperature increases of ∼1 K. Short pulses (30 ns, 2.5 MV/m, 5 μJ per pulse) produce no detectable Eu-TTA fluorescence decrease (heating), measured photometrically in Eu-TTA stained cells (Fig. 4 b) or in the extracellular medium (data not shown), indicating a temperature change of less than 0.1 K.
FIGURE 4.
Temperature-sensitive Eu-TTA fluorescence—absence of nanoelectropulse-induced Joule heating in cells. (a) Long pulses (1 μs; 1.0 MV/m) produce temperature-induced step decreases in the fluorescence emission of 50 μM Eu-TTA in culture medium. Each data point represents the integrated fluorescence emission from a selected area in a uniformly illuminated microscope field of view (growth medium only—no cells). (b) Short pulses (30 ns, 2.5 MV/m) produce no detectable Eu-TTA fluorescence decrease (heating) in Eu-TTA stained cells. Each data point represents the integrated fluorescence emission from one of three single cells.
DISCUSSION
Field threshold and plateau
The plasma membranes of cells exposed to ultrashort, high-field electric pulses are not electroporated by conventional measures (propidium iodide influx, for example), but an important early effect of the pulsed-field treatment is translocation of the normally inner membrane phospholipid PS, a physiologically and physically significant modification of the membrane lipid bilayer that has been associated with electroporation in other studies (Haest et al., 1997). It has not been established whether this redistribution of membrane phospholipids is the result of a direct interaction between the pulsed field and components of the membrane, or a secondary consequence of an intracellular triggering event or set of events. Our observations provide some limits against which hypothetical mechanisms and models can be tested.
The data in Fig. 3 is consistent with the hypothesis that nanoelectropulse-induced PS externalization results from a single, discrete process or set of closely related parallel processes rather than an assortment of unrelated mechanisms, with at least one step in this putative process requiring the application of an external field greater than 1 MV/m for several nanoseconds. For 50 7-ns pulses, fields from 2.5 to 4.0 MV/m produce approximately the same response, the result one would expect for a relatively simple mechanism with an activation energy provided by pulses greater than 1 MV/m. At the high-field end of this plateau, above 4 MV/m, more destructive effects of the pulsed fields become significant with a 50-pulse dose.
A value for the peak transmembrane potential during a 7-ns, 1-MV/m trapezoidal pulse with 2-ns rise and fall times can be estimated from a simple dielectric shell model of the cell (Plonsey and Altman, 1988)
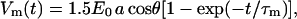 |
(1) |
where E0 is the applied electric field, a is the cell radius, θ is the position angle relative to the electric field, t is the time after the initiation of the pulse, and τm is the membrane charging time constant.
With physical parameters reasonable for the Jurkat T cells and suspension medium used in these experiments (a = 5 μm, θ = 0, τm = 100 ns), and t = 7 ns, Vm,peak for E0 = 1.5 MV/m is ∼500 mV. Values at other pulse amplitudes scale accordingly (Table 2). Although it must be stressed that these cell physical parameters are approximate, that the dielectric shell model embodies only the most general electrophysical properties of the system, and that a real population of cells is heterogeneous in size, shape, and physiological state, this 500 mV is close to the transmembrane voltage associated with long-pulse electroporation (Benz et al., 1979).
TABLE 2.
Transmembrane potentials from simple dielectric shell model
| Pulsed electric field (MV/m) | Vm,peak, 2-ns pulse | Vm,peak, 7-ns pulse |
|---|---|---|
| 0.0 | 0.00 | 0.00 |
| 0.5 | 0.04 | 0.18 |
| 1.0 | 0.07 | 0.37 |
| 1.5 | 0.11 | 0.56 |
| 2.0 | 0.15 | 0.73 |
| 2.5 | 0.19 | 0.91 |
| 3.0 | 0.22 | 1.10 |
| 3.5 | 0.26 | 1.28 |
| 4.0 | 0.30 | 1.46 |
Peak transmembrane voltages after 2- and 7-ns trapezoidal pulses, calculated from Eq. 1 for an ideal membrane across which the potential may increase without limit. Increased conductivity (poration) in hyperpolarized biological membranes clamps the transmembrane potential at ∼1 V.
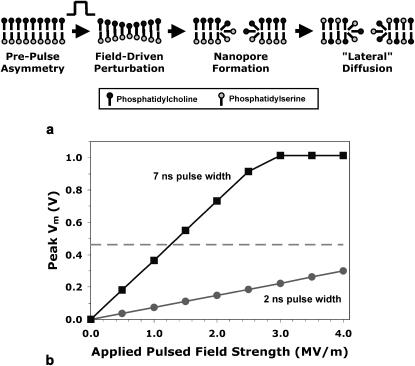
The conductivity of biological lipid bilayers increases by orders of magnitude when the transmembrane potential approaches 1 V (Zimmermann, 1982). Most models of electroporation imply that higher external electric fields lead to the appearance of more conductive pores, clamping the membrane voltage at a critical, porating voltage (Weaver, 2003). Since exact values for the transmembrane potential at the onset of increased conductivity and for the maximum, clamped voltage depend on the cell type and the composition of the medium, it is reasonable to consider the hypothesis that under the conditions of our experiments the critical membrane voltage is approached with 1-MV/m pulses and exceeded with larger pulsed fields, generating in the membrane a population of nanometer-diameter, nanosecond-duration pores (Popescu et al., 1991; Weaver and Chizmadzhev, 1996). Although these pores would be expected to close quickly after the trailing edge of the pulse under physiological conditions (Zimmermann, 1996), they would provide a path while they are open for diffusion of PS from the inner leaflet of the membrane to the outer surface (Fig. 5 a). Repeated cycles of pulse-induced nanoporation would expose successively more and more inner-membrane PS to these paths to the external face of the cell. If these localized membrane perturbations persist longer than the pulse repetition rate, then the response to multiple pulses would likely be nonlinear.
FIGURE 5.
Model for nanoelectropulse-induced PS externalization. (a) Diagram showing nanoelectropulse-induced pore formation and “lateral” diffusion of PS from the inner to the outer leaflet of the membrane lipid bilayer. (b) Peak plasma membrane voltage (Vm) versus pulsed field strength for 2- and 7-ns trapezoidal pulses calculated for Jurkat T cells from Eq. 1 and clamped at 1 V to represent the effect of the opening of conducting pores. Horizontal dashed line indicates approximate threshold for PS externalization from the data represented in Fig. 3. This model predicts that 2-ns pulses will not produce PS translocation at fields <4 MV/m.
A plot of the calculated values from Table 2, modified to indicate the clamping of the transmembrane potential at 1 V, is shown in Fig. 5 b. Note that for a given field strength, shorter pulses charge the membrane to lower voltages (assuming that the pulse durations are substantially less than the membrane charging time constant). If the hypothesis above is valid, then 2-ns pulses (which necessarily have rise and fall times of 1 ns or less) should cause only minimal PS externalization at 2.5–4.0 MV/m, because the external membrane would charge to <0.5 V, but at fields >4 MV/m, the effects seen with 7-ns pulses should be observed.
Pulse rate effects
If successive pulses in an n-pulse train produce entirely independent and lasting effects (such as opening and closing of nanopores with associated sites of facilitated PS diffusion onto the external face of the membrane), then it is reasonable to expect that the measured effects are cumulative, increasing with n, and that is what is observed for pulses delivered at 20 Hz (Vernier et al., 2003a). Decreasing the time between pulses to a value less than the time required for opening, closing, and “healing” a nanopore might eliminate this independence and modify the cumulative response, since membrane integrity would not be fully restored before each successive pulse. (Because a 7-ns pulse duration is a negligible fraction of the pulse-to-pulse period for pulse frequencies even up to a megahertz, varying the pulse repetition rate from 2 to 2000 Hz, as in these experiments, may be considered equivalent to varying the time between pulses from 500 ms to 0.5 ms.) Without a detailed knowledge of the actual pulse-induced membrane changes it is not clear whether faster pulse delivery rates (less time between pulses) will enhance or diminish the effects of a pulse exposure, but the possibility of an observable difference in the magnitude of the response when the pulse delivery period overlaps the time for membrane recovery cannot be excluded. The small decrease in PS externalization at 2000 Hz relative to 20 and 200 Hz (Fig. 2) may signify such a change, indicating a nanopore lifetime on the order of microseconds.
Given a nominal lateral diffusion coefficient of 10 μm2 s−1 for membrane phospholipids (Sonnleitner, et al., 1999), nanopores with a mean lifetime on the order of hundreds of nanoseconds or more would be open long enough to provide sites for PS migration onto the cell surface (Anezo et al., 2003). Reported times for pulsed electric field-induced membrane pore formation and lifetime vary widely. Conductivity increases in artificial membranes can be measured as early as 10 ns after pulse exposure (Benz and Zimmermann, 1980), consistent with simulations of field-driven pore opening within nanoseconds (Tieleman et al., 2003). Electropores may last from 7 to 500 μs (Hibino et al., 1993), or even into the range of 3 ms to 1 s (Melikov et al., 2001), depending on pulse characteristics and exposure conditions. All of these numbers are consistent with nanoelectropulse-facilitated externalization of PS.
Calcium-independent mechanisms
Since it is known that intracellular calcium release facilitates PS translocation, it is tempting to associate the nanoelectropulse-induced calcium bursts reported elsewhere (Vernier et al., 2003b) with PS externalization, keeping in mind that a simple rise in intracellular calcium concentration may not be sufficient for PS translocation (Wurth and Zweifach, 2002; Orrenius et al., 2003). Although neither pulse-induced nanomolar calcium influx nor intracellular calcium release and immediate reuptake can be excluded, the data in Table 1, and additional unpublished observations in our laboratory, do not support a direct role for calcium in nanoelectropulse-induced PS externalization.
Possible thermal effects
Long (μs), high-field (kV/m) electric pulses can cause significant heating in biological material (Pliquett et al., 1996). Nanosecond pulses used in the studies reported here have a high power density (5 TW m−3) but a low energy density (100 kJ m−3), so that if distributed uniformly each pulse will raise the temperature of cell suspensions by milliKelvins or less, depending on the thermal isolation of the sample. It is possible, however, that the pulse energy is not deposited uniformly. For example, field-driven ionic currents through nanoelectropores or existing voltage-activated ion channels could develop very localized high temperatures (intra- and circumpore increased intramolecular agitation and/or a shift of molecular velocity distributions to the right). The essential heterogeneity of dielectric properties of biological membranes points to the possibility of nanometer-scale “heating,” although heating has previously been shown to be only a secondary factor for electric field effects on conductive channels (Chen et al., 1998). The observations with Eu-TTA reported here represent the first attempt to measure the temperature of cells during nanoelectropulse exposure. They are not calibrated measurements, but rather comparisons of short-pulse (30 ns) with long-pulse (1000 ns) exposures, and we were able to detect no pulse-induced temperature changes greater than tens of milliKelvins.
Nanoelectropulse-induced phosphatidylserine translocation
It seems probable that the overturning of molecular layers may be a phenomenon of considerable biological significance. If the monolayers contain dipoles or ionic charges as well as hydrophobic and hydrophilic groups, the overturning of the layer may cause large changes of electric potential. Conversely, change of potential or of chemical composition of the liquid on one side of a membrane may cause an overturning of one or more of the monolayers and so change the properties of the film (Langmuir, 1938).
Langmuir was not aware of the asymmetry of the eukaryotic membrane lipid bilayer, but his appreciation for the significance of membrane lipid rearrangements proved to be a guiding and lasting insight. Even today we do not fully understand how the asymmetry is maintained, and when, why, and how it is disturbed. Nanoelectropulse studies may become a component of the ongoing search for the answers to these questions.
PS externalization appears very soon after nanoelectropulse exposure (within seconds; manuscript in preparation). Along with other early postpulse events—intracellular calcium bursts (Vernier et al., 2003b) and changes in the appearance of nuclear material (Chen et al., 2004)—immediate PS translocation is likely to be closely linked to the primary electrophysical interaction between the nanosecond-pulsed electric field and the biological cell, and thus may provide a handle for identifying and characterizing the mechanisms through which cells respond to external ultrashort electric pulses. Phosphatidylserine exposure not only serves as a signal that mediates cell clearance in immune and apoptotic processes, it also indicates a modification of the normal cytoskeletal-membrane associations that maintain and regulate the physiological integrity of the membrane (Kunzelmann-Marche et al., 2001; Manno et al., 2002). Appropriate pulse regimens require further investigation because they may permit separation of the immediate, electrophysically mediated translocation from the longer term externalization associated with apoptosis.
In this work we have identified a specific, physiologically significant molecular event—translocation of PS from the inner leaflet of the cell membrane lipid bilayer to the exterior face of the cell—that is associated directly with the application of ultrashort (nanosecond), high-field (megavolt-per-meter) electric pulses. We have established a threshold amplitude for this pulsed electric field, below which PS externalization does not occur, and we have shown that this threshold field strength corresponds closely to the induced transmembrane potential (≥0.5 V) that causes increased conductance when much longer (microsecond) pulses are applied (conventional electroporation). It is probably not coincidental that the potential energy of the negatively charged head group of PS at the 0.5–1.0 V membrane potential associated with the threshold electric field for PS translocation corresponds with the activation energy for phospholipid transbilayer migration—∼100 kJ/mol (1 eV).
Our data suggest that nanoelectropulse-induced PS translocation is not the consequence of known, calcium-stimulated enzymatic activities and point to a directly physical interaction between the external field and the components of the plasma membrane. At the same time we provide evidence that is consistent with only an insignificant amount of pulse-induced localized heating of the membrane, arguing against the notion that PS translocation results from enthalpically driven lipid-phase changes and subsequent layer mixing.
The sum of the evidence presented here leads to the conclusion that since nanoelectropulse-induced PS externalization apparently requires the establishment of a transmembrane voltage that is similar in magnitude to the membrane voltage associated with electric field-induced membrane poration, the initial steps in the two processes may be similar even though the end points are different. Although the critical transmembrane potential required for poration develops even during the 7-ns pulses used in these experiments, several lines of evidence indicate that nanosecond-pulsed field exposures do not create openings in the plasma membrane like those associated with electroporation. One membrane-based molecular event occurs—PS translocation—but the abrupt cessation of the field halts further progression toward the opening of conductive pores through which ions can pass.
Our results are consistent, however, with the formation of nanopores—nanosecond-duration, nanometer-diameter openings in the membrane that are not measurably conductive but that would provide a site for PS lateral diffusion to the external face of the cell. At the limits of our pulse repetition rate data we see evidence for a nanopore (or subpore defect) lifetime on the order of microseconds—consistent with values for membrane pore lifetime in conductive media in the electroporation literature, although it must be emphasized that we do not see evidence for actual membrane poration.
These observations of the effects of nanosecond pulses on the membrane challenge the competing physical models of electroporation. These models must accommodate field-dependent PS translocation after a fast-rising pulse edge, either through facilitated phospholipid diffusion at the perimeter of pulse-induced pores, or through direct translation of the PS head group through a pulse-perturbed section of membrane, or through an extremely rapid mechanical rearrangement in response to Maxwellian flexure.
In addition to the basic cell biology and bioelectrics information that can be obtained through explorations of these possibilities, an investigation of nanoelectropulse-induced PS externalization may lead to practical applications. The ability to flip this phospholipid switch with remotely delivered nanosecond electric pulses, and to be able to do so selectively for different cell types (Vernier et al., 2003a) depending on their dielectric properties (Polevaya et al., 1999) suggests the development of pulse exposure recipes (with pulse trains of varying amplitude, duration, and pattern), eventually at the clinical level, for eliminating undesirable cell populations by inducing them either to advertise for or to initiate directly their own destruction.
Acknowledgments
We gratefully recognize Harold Soucier for flow cytometry, Matthew Behrend and Joseph Yampolsky for pulse generator design and assembly, Mya Thu, Katherine Chiu, and Yushun Zhang for cell culture and technical expertise, and Sarah Salemi and Reza Khosravani for contributions to the Eu-TTA observations.
This work was made possible by support from the Air Force Office of Scientific Research and the Army Research Office.
References
- Anezo, C., A. H. de Vries, H. D. Holtje, D. P. Tieleman, and S. J. Marrink. 2003. Methodological issues in lipid bilayer simulations. J. Phys. Chem. B. 107:9424–9433. [Google Scholar]
- Balasubramanian, K., and A. J. Schroit. 2003. Aminophospholipid asymmetry: a matter of life and death. Annu. Rev. Physiol. 65:701–734. [DOI] [PubMed] [Google Scholar]
- Basse, F., J. G. Stout, P. J. Sims, and T. Wiedmer. 1996. Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J. Biol. Chem. 271:17205–17210. [DOI] [PubMed] [Google Scholar]
- Beebe, S. J., P. M. Fox, L. J. Rec, K. Somers, R. H. Stark, and K. H. Schoenbach. 2002. Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: Apoptosis induction and tumor growth inhibition. IEEE Trans. Plasma Sci. 30:286–292. [Google Scholar]
- Behrend, M., A. Kuthi, X. Gu, P. T. Vernier, L. Marcu, C. M. Craft, and M. A. Gundersen. 2003. Pulse generators for pulsed electric field exposure of biological cells and tissues. IEEE Trans. Dielectr. Electr. Insulat. 10:820–825. [Google Scholar]
- Benz, R., F. Beckers, and U. Zimmermann. 1979. Reversible electrical breakdown of lipid bilayer membranes: a charge-pulse relaxation study. J. Membr. Biol. 48:181–204. [DOI] [PubMed] [Google Scholar]
- Benz, R., and U. Zimmermann. 1980. Pulse-length dependence of the electrical breakdown in lipid bilayer membranes. Biochim. Biophys. Acta. 597:637–642. [DOI] [PubMed] [Google Scholar]
- Blom, W. M., H. J. De Bont, and J. F. Nagelkerke. 2003. Regional loss of the mitochondrial membrane potential in the hepatocyte is rapidly followed by externalization of phosphatidylserines at that specific site during apoptosis. J. Biol. Chem. 278:12467–12474. [DOI] [PubMed] [Google Scholar]
- Chapman, C. F., Y. Liu, G. J. Sonek, and B. J. Tromberg. 1995. The use of exogenous fluorescent probes for temperature measurements in single living cells. Photochem. Photobiol. 62:416–425. [DOI] [PubMed] [Google Scholar]
- Chen, W., Y. Han, Y. Chen, and D. Astumian. 1998. Electric field-induced functional reductions in the K+ channels mainly resulted from supramembrane potential-mediated electroconformational changes. Biophys. J. 75:196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N., K. H. Schoenbach, J. F. Kolb, R. J. Swanson, A. L. Garner, J. Yang, R. P. Joshi, and S. J. Beebe. 2004. Leukemic cell intracellular responses to nanosecond electric fields. Biochem. Biophys. Res. Commun. 317:421–427. [DOI] [PubMed] [Google Scholar]
- Comfurius, P., P. Williamson, E. F. Smeets, R. A. Schlegel, E. M. Bevers, and R. F. A. Zwaal. 1996. Reconstitution of phospholipid scramblase activity from human blood platelets. Biochemistry. 35:7631–7634. [DOI] [PubMed] [Google Scholar]
- Dressler, V., K. Schwister, C. W. Haest, and B. Deuticke. 1983. Dielectric breakdown of the erythrocyte membrane enhances transbilayer mobility of phospholipids. Biochim. Biophys. Acta. 732:304–307. [DOI] [PubMed] [Google Scholar]
- Eisenberg, M., J. E. Hall, and C. A. Mead. 1973. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes. J. Membr. Biol. 14:143–176. [DOI] [PubMed] [Google Scholar]
- Fadok, V. A., A. de Cathelineau, D. L. Daleke, P. M. Henson, and D. L. Bratton. 2001. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 276:1071–1077. [DOI] [PubMed] [Google Scholar]
- Fujiwara, T., K. Ritchie, H. Murakoshi, K. Jacobson, and A. Kusumi. 2002. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol. 157:1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haest, C. W. M., D. Kamp, and B. Deuticke. 1997. Transbilayer reorientation of phospholipid probes in the human erythrocyte membrane. Lessons from studies on electroporated and resealed cells. Biochim. Biophys. Acta. 1325:17–33. [DOI] [PubMed] [Google Scholar]
- Heimburg, T. 1998. Mechanical aspects of membrane thermodynamics. Estimation of the mechanical properties of lipid membranes close to the chain melting transition from calorimetry. Biochim. Biophys. Acta. 1415:147–162. [DOI] [PubMed] [Google Scholar]
- Hessel, E., P. Mueller, A. Herrmann, and K.-P. Hofmann. 2001. Light-induced reorganization of phospholipids in rod disc membranes. J. Biol. Chem. 276:2538–2543. [DOI] [PubMed] [Google Scholar]
- Hibino, M., H. Itoh, and K. Kinosita. 1993. Time courses of cell electroporation as revealed by submicrosecond imaging of transmembrane potential. Biophys. J. 64:1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan, R., and H. J. Pownall. 1988. Transbilayer diffusion of phospholipids: dependence on headgroup structure and acyl chain length. Biochim. Biophys. Acta. 938:155–166. [DOI] [PubMed] [Google Scholar]
- Kato, H., T. Nishizaka, T. Iga, K. Kinosita, Jr., and S. Ishiwata. 1999. Imaging of thermal activation of actomyosin motors. Proc. Natl. Acad. Sci. USA. 96:9602–9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman, G., C. P. Reutelingsperger, G. A. Kuijten, R. M. Keehnen, S. T. Pals, and M. H. van Oers. 1994. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 84:1415–1420. [PubMed] [Google Scholar]
- Kornberg, R. D., and H. M. McConnell. 1971. Inside-outside transitions of phospholipids in vesicle membranes. Biochemistry. 10:1111–1120. [DOI] [PubMed] [Google Scholar]
- Kunzelmann-Marche, C., J.-M. Freyssinet, and M. C. Martinez. 2001. Regulation of phosphatidylserine transbilayer redistribution by store-operated Ca2+ entry—role of actin cytoskeleton. J. Biol. Chem. 276:5134–5139. [DOI] [PubMed] [Google Scholar]
- Kunzelmann-Marche, C., J. M. Freyssinet, and M. C. Martinez. 2002. Loss of plasma membrane phospholipid asymmetry requires raft integrity. Role of transient receptor potential channels and ERK pathway. J. Biol. Chem. 277:19876–19881. [DOI] [PubMed] [Google Scholar]
- Langmuir, I. 1938. Overturning and anchoring of monolayers. Science. 87:493–500. [DOI] [PubMed] [Google Scholar]
- Lipka, G., J. A. Op den Kamp, and H. Hauser. 1991. Lipid asymmetry in rabbit small intestinal brush border membrane as probed by an intrinsic phospholipid exchange protein. Biochemistry. 30:11828–11836. [DOI] [PubMed] [Google Scholar]
- Manno, S., Y. Takakuwa, and N. Mohandas. 2002. Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl. Acad. Sci. USA. 99:1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, M. C., S. Martin, F. Toti, E. Fressinaud, J. Dachary-Prigent, D. Meyer, and J. M. Freyssinet. 1999. Significance of capacitative Ca2+ entry in the regulation of phosphatidylserine expression at the surface of stimulated cells. Biochemistry. 38:10092–10098. [DOI] [PubMed] [Google Scholar]
- Melikov, K. C., V. A. Frolov, A. Shcherbakov, A. V. Samsonov, and Y. A. Chizmadzhev. 2001. Voltage-induced nonconductive pre-pores and metastable single pores in unmodified planar lipid bilayer. Biophys. J. 80:1829–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, I. R. 2002. Effect of electric fields on the structure of phosphatidyl choline in a multibilayer system. Bioelectrochemistry. 57:145–148. [DOI] [PubMed] [Google Scholar]
- Mussauer, H., V. L. Sukhorukov, A. Hasse, and U. Zimmermann. 1999. Resistivity of red blood cells against high-intensity, short-duration electric field pulses induced by chelating agents. J. Membr. Biol. 170:121–133. [DOI] [PubMed] [Google Scholar]
- Orrenius, S., B. Zhivotovsky, and P. Nicotera. 2003. Calcium: Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 4:552–565. [DOI] [PubMed] [Google Scholar]
- Pliquett, U., E. A. Gift, and J. C. Weaver. 1996. Determination of the electric field and anomalous heating caused by exponential pulses with aluminum electrodes in electroporation experiments. Bioelectrochem. Bioenerg. 39:39–53. [Google Scholar]
- Plonsey, R., and K. W. Altman. 1988. Electrical stimulation of excitable cells—a model approach. Proc. IEEE. 76:1122–1129. [Google Scholar]
- Polevaya, Y., I. Ermolina, M. Schlesinger, B.-Z. Ginzburg, and Y. Feldman. 1999. Time domain dielectric spectroscopy study of human cells. II. Normal and malignant white blood cells. Biochim. Biophys. Acta. 1419:257–271. [DOI] [PubMed] [Google Scholar]
- Popescu, D., C. Rucareanu, and G. Victor. 1991. A model for the appearance of statistical pores in membranes due to self-oscillations. Bioelectrochem. Bioenerg. 25:91–103. [Google Scholar]
- Popescu, D., and G. Victor. 1991. The transversal diffusion coefficient of phospholipid molecules through black lipid membranes. Bioelectrochem. Bioenerg. 25:105–108. [Google Scholar]
- Schoenbach, K. H., S. J. Beebe, and E. S. Buescher. 2001. Intracellular effect of ultrashort electrical pulses. Bioelectromagnetics. 22:440–448. [DOI] [PubMed] [Google Scholar]
- Seigneuret, M., and P. F. Devaux. 1984. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc. Natl. Acad. Sci. USA. 81:3751–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher, L. D., E. Kresch, and H. P. Schwan. 1970. On the possibility of nonthermal biological effects of pulsed electromagnetic radiation. Biophys. J. 10:970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnleitner, A., G. J. Schutz, and T. Schmidt. 1999. Free Brownian motion of individual lipid molecules in biomembranes. Biophys. J. 77:2638–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar, I. P. 1979. A theory of the electric field-induced phase transition of phospholipid bilayers. Biochim. Biophys. Acta. 556:72–85. [DOI] [PubMed] [Google Scholar]
- Taupin, C., M. Dvolaitzky, and C. Sauterey. 1975. Osmotic pressure induced pores in phospholipid vesicles. Biochemistry. 14:4771–4775. [DOI] [PubMed] [Google Scholar]
- Tieleman, D. P., H. Leontiadou, A. E. Mark, and S. J. Marrink. 2003. Simulation of pore formation in lipid bilayers by mechanical stress and electric fields. J. Am. Chem. Soc. 125:6382–6383. [DOI] [PubMed] [Google Scholar]
- Verhoven, B., R. A. Schlegel, and P. Williamson. 1995. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J. Exp. Med. 182:1597–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernier, P. T., A. Li, L. Marcu, C. M. Craft, and M. A. Gundersen. 2003a. Ultrashort pulsed electric fields induce membrane phospholipid translocation and caspase activation: differential sensitivities of Jurkat T lymphoblasts and rat glioma C6 cells. IEEE Trans. Dielectr. Electr. Insulat. 10:795–809. [Google Scholar]
- Vernier, P. T., Y. Sun, L. Marcu, S. Salemi, C. M. Craft, and M. A. Gundersen. 2003b. Calcium bursts induced by nanosecond electric pulses. Biochem. Biophys. Res. Commun. 310:286–295. [DOI] [PubMed] [Google Scholar]
- Weaver, J. C. 2003. Electroporation of biological membranes from multicellular to nano scales. IEEE Trans. Dielectr. Electr. Insulat. 10:754–768. [Google Scholar]
- Weaver, J. C., and Y. A. Chizmadzhev. 1996. Theory of electroporation: a review. Bioelectrochem. Bioenerg. 41:135–160. [Google Scholar]
- Wu, G., and W. L. Hubbell. 1993. Phospholipid asymmetry and transmembrane diffusion in photoreceptor disc membranes. Biochemistry. 32:879–888. [DOI] [PubMed] [Google Scholar]
- Wurth, G. A., and A. Zweifach. 2002. Evidence that cytosolic calcium increases are not sufficient to stimulate phospholipid scrambling in human T-lymphocytes. Biochem. J. 362:701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, U. 1982. Electric field-mediated fusion and related electrical phenomena. Biochim. Biophys. Acta. 694:227–277. [DOI] [PubMed] [Google Scholar]
- Zimmermann, U. 1996. The effect of high intensity electric field pulses on eukaryotic cell membranes: fundamentals and applications. In Electromanipulation of Cells. U. Zimmermann and G. A. Neil, editors. CRC, Boca Raton, FL.
- Zohar, O., M. Ikeda, H. Shinagawa, H. Inoue, H. Nakamura, D. Elbaum, D. L. Alkon, and T. Yoshioka. 1998. Thermal imaging of receptor-activated heat production in single cells. Biophys. J. 74:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]