Abstract
The trace fossil record is important in determining the timing of the appearance of bilaterian animals. A conservative estimate puts this time at ≈555 million years ago. The preservational potential of traces made close to the sediment–water interface is crucial to detecting early benthic activity. Our studies on earliest Cambrian sediments suggest that shallow tiers were preserved to a greater extent than typical for most of the Phanerozoic, which can be attributed both directly and indirectly to the low levels of sediment mixing. The low levels of sediment mixing meant that thin event beds were preserved. The shallow depth of sediment mixing also meant that muddy sediments were firm close to the sediment–water interface, increasing the likelihood of recording shallow-tier trace fossils in muddy sediments. Overall, trace fossils can provide a sound record of the onset of bilaterian benthic activity.
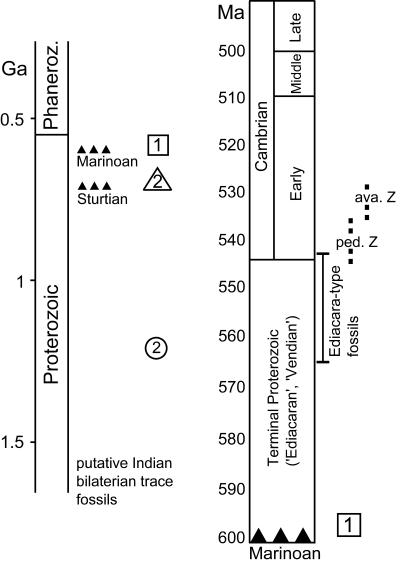
The appearance and subsequent diversification of bilaterian animals is a topic of current controversy (refs. 1–7; Fig. 1). Three principal sources of evidence exist: body fossils, trace fossils (trails, tracks, and burrows of animal activity recorded in the sedimentary record), and divergence times calculated by means of a molecular “clock.” The body fossil record indicates a geologically rapid diversification of bilaterian animals not much earlier than the Precambrian–Cambrian boundary, the so-called Cambrian explosion. The largely terminal Proterozoic Ediacaran biota remain problematic to questions of bilaterian origins (7–10). Molecular estimates (1) suggest that diversification of bilaterian groups may have commenced more than 1,000 million years ago. However, the molecular clock studies provide a considerable spread in the results, with some coming close (11) or even very close§ to the pattern seen from body fossils (Fig. 1). An increase in diversity and complexity of trace fossils across the Neoproterozoic–Cambrian boundary has long been recognized (12–14). The oldest widely accepted trace fossils are no older than 555 million years old (5, 15). Broadly speaking, terminal Proterozoic trace fossils are simple, unbranched, less than a few millimeters in diameter, and were made close to the sediment–water interface. In the Cambrian, morphological diversity increased, size range expanded, and depth of sediment penetration increased modestly (16).
Figure 1.
Generalized stratigraphy of the Proterozoic and Cambrian contrasting the timing of bilaterian appearance as deduced from body fossils, trace fossils, and molecular clock data. The oldest trace fossils accepted here are ≈555 million years old. Reports of older traces including more than 1-billion-year-old traces from India (37) and Australia (38) are considered doubtful. The numbers refer to bilaterian divergence dates based on selected molecular clock studies: 1, appearance of first bilaterians; 2, deuterostome-protostome split; circle, ref. 1; triangle, ref. 11; square§ ped Z, T. pedum zone; ava Z., R. avalonensis zone.
Although not accepted universally, bilaterians may be primitively benthic, and many of their morphological features could only have evolved in a moderately large animal with a benthic lifestyle (5, 6, 15). Such animals would have had the ability to burrow, and it requires special pleading to argue that they would not have produced trace fossils (5, 15). The appearance of macroscopic bilaterians, thus, is arguably recorded by terminal Proterozoic trace fossils. Conditions for the preservation of relatively surficial infaunal activity should have been particularly favorable because of the shallow depth of bioturbation at this time (5, 17). However, precise mechanisms of trace fossil preservation are poorly known, in particular with respect to preservation of traces made close to the sediment–water interface, which in general do not normally get preserved.
Few detailed studies of the broader implications of trace fossils through this interval have been made (but see refs. 16–18), and the question remains: How accurate is the terminal Proterozoic–Cambrian trace fossil record? To address this question, we have examined several aspects of the terminal Proterozoic–Cambrian ichnological record including: (i) trace fossil preservation, (ii) preserved depth of bioturbation, (iii) nature of ichnofabric (all aspects of the trace fossil record include features such as mottled bedding resulting from sediment mixing where discrete trace fossils cannot be identified), and (iv) nature of the substrate, which has been recognized as a factor in trace fossil preservation (19).
Materials and Methods
This paper is based on field and laboratory examination of a large number of terminal Proterozoic and Cambrian siliciclastic units, trace fossils, and sedimentary structures. We primarily focus on Lower Cambrian units but also examine some younger as well as older units. These include the lowermost Cambrian Chapel Island formation (≈400 m measured); units of the Cambro-Ordovician Bell Island group (≈120 m) and Lower Ordovician Wabanna group (20 m) in Newfoundland; the Lower Cambrian Torneträsk (100 m) and Grammajukku (15 m) formations and the Mickwitzia sandstone (10 m) in Sweden; the lowermost Cambrian Uratanna formation (150 m) in south Australia; the Cambro-Ordovician Bynguano formation (30 m) in New South Wales, Australia; the Lower Cambrian Lontova (10 m) and Lükati (15 m) formations in Estonia; the Lower Cambrian Wood Canyon (100 m), Pioche (20 m), and Harkless (5 m) formations in western United States; and the terminal Proterozoic Huns Member (50 m) in southern Namibia.
We examined parts of these units that represent deposition on the shelf below fair-weather wave base and above maximum storm-wave base as determined by independent sedimentological criteria. The measured portions of these units (indicated in parentheses) are characterized by heterolithic bedding ranging from the centimeter to decimeter scale. Detailed logs were made of all sections, and selected intervals were described at the centimeter scale. Sedimentology, ichnofabrics, and trace-fossil taxa were described in relation to the sedimentary context (e.g., preservation). Rock samples were taken at decimeter intervals where possible, cut into slabs, and polished. Selected slabs were x-rayed. The terminal Proterozoic–Cambrian interval in many regions is characterized by terrigenous clastic deposition, and with units from four continents we feel that we have a fair representation of this environment.
Ichnological Record of Lowermost Cambrian Strata.
The greatest diversity of Cambrian trace fossils occurs in shelf settings that have a heterolithic bedding characterized by moderately thin, generally centimeter-scale, interbedded sandstone, siltstone, and mudstone. In this setting, even in the earliest Cambrian strata, trace fossils are relatively common—so much so, in fact, that they form an important part of lowermost Cambrian stratigraphy and define the base of the Cambrian system (14, 20). Carbonate successions are not common through this time interval, and deep-basinal settings are equally rare.
Of the units that we examined, the Lower Cambrian formations exhibit a number of shared ichnological and sedimentological characteristics. [We include with these the terminal Proterozoic Huns formation of Namibia, because it has treptichnids (21), which are at least comparable morphologically to Treptichnus pedum, the zone fossil for the base of the Cambrian (20)]. These characteristics are ubiquitous in Cambrian strata of the T. pedum and Rusophycus avalonensis zones (Fig. 1) but in most ways also characterize virtually all Lower Cambrian strata representing shelf settings. These characteristics include the following.
- 1.
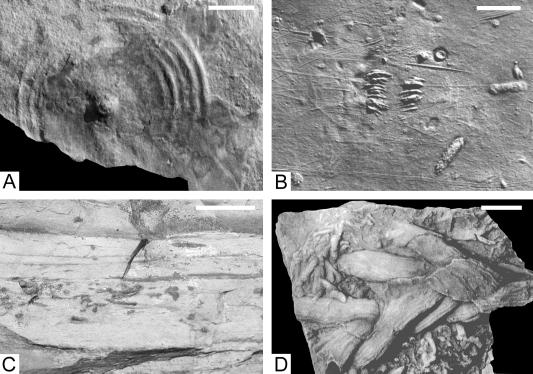
Preserved depth of bioturbation. In modern settings, burrows, tracks, and trails produced near the surface have virtually no chance of preservation, because the potential trace fossils are destroyed by those animals that subsequently burrow deeply and completely into the sediment. Earliest Cambrian sediments preserve a range of trace fossils that are interpreted as representing shallow tiers—that is, the burrows did not extend more than a few centimeters at most below the sediment–water interface. There is very limited evidence for deeper penetration in any of the included portions of these units. For example, in Newfoundland, Arenicolites, a simple U-shaped burrow, and Planolites, a simple unbranched burrow, very rarely reach depths of 4–5 cm.These shallow-tiered burrows include various treptichnids (including T. pedum; Fig. 2D). They consisted of additions of curved elements; the burrows themselves were open and the tops extended to the sediment–water surface. The geometry and style of preservation of these trace fossils suggest that they formed less than a few centimeters below the sediment–water surface (17). These trace fossils are characteristic of Lower Cambrian shallow marine terrigenous clastic rocks. Other burrows include Gyrolithes, a corkscrew-type trace fossil that has a preserved depth of 1–2 cm. The diameter of this burrow is on the order of millimeters, and it is interpreted to have been an open-burrow system. Gyrolithes is abundant in the Chapel Island formation of Newfoundland and in the Lower Cambrian units of Baltica (see figure 6C in ref. 17).
- 2.
Quality of preservation. Although the treptichnid burrows were constructed close to the sediment–water interface they have sharp walls without actively reinforced margins, and in certain cases delicate surface ornamentation is preserved (Fig. 2D). Compaction of the burrows also is relatively minor. Several other trace fossils of shallow emplacement show excellent preservation of detail including the vertical spiral burrow Gyrolithes and shallow Rusophycus (Fig. 2B). This quality of preservation is ubiquitous in the Lower Cambrian units examined.
- 3.
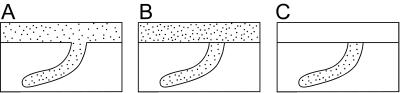
Styles of preservation. In most shallow marine settings, burrows preserved on the base of sandstone beds are created by animals that burrow through the sand to the interface with the underlying finer-grained sediment (22). In Lower Cambrian strata, particularly, but also not uncommonly in Middle and Upper Cambrian strata, a fundamentally different style of preservation seems particularly common. Cambrian sand-filled burrows are generally preserved in one of two manners. The burrow may be cast by a source bed to which it remains attached, or it may be cast by sand that bypassed the sea floor, and thus the cast is attached to the base of a different bed, or may even be preserved as a sand-filled burrow completely in silt (ref. 17; Figs. 2C and 3). This is a common type of preservation of treptichnids, Gyrolithes, and Palaeophycus/Planolites-type burrows and is the most common style of trace-fossil preservation in all the Lower Cambrian units examined.This type of preservation requires that burrows are open and, given the preservation of shallow-tier trace fossils in this manner, that the muddy sediment was rather resistant to erosion, allowing the trapping of sand in burrows rather than the destruction of the burrows.
- 4.
Nature of Ichnofabric. Animals that mix sediment, in general, do not leave well defined discrete trace fossils. Instead, the record produced is one of some degree of homogenization, where primary sedimentary structures are not preserved. The final texture has a mottled appearance, which is direct evidence of a mixed layer. In sedimentary rocks of the T. pedum zone, we have virtually no evidence of such homogenization. In R. avalonensis strata, isolated homogenized beds occur less than 1 cm in thickness.
Figure 2.
Trace fossils and sedimentary structures from Lower Cambrian subtidal settings. (A) Kullingia-type scratch circle (Torneträsk Formation, northern Sweden, Swedish Geological Survey, Uppsala, 8624). (B) Sole of thin (≈2-cm) storm bed preserving delicate Rusophycus and tool marks (Mickwitzia sandstone, Swedish Museum of Natural History, Stockholm, RM X3313). Rare vertical burrows have penetrated this sole. (C) Field photograph of sediment from the T. pedum zone on Burin Peninsula, Newfoundland, showing sand-filled burrows in silt, without a preserved connection to source of sand. (D) A Treptichnus with sharply defined burrow margins in portions with preserved longitudinal ornamentation (Arumbera sandstone, central Australia). (Scale bars, 10 mm.)
Figure 3.
Schematic representations of contrasting preservational styles for sand-filled open burrows constructed in a muddy/silty sediment. (A) Passive fill of an open burrow in which burrow fill is continuos with source of casting sediment. (B) Passive fill in which there is a distinct break between the burrow fill and overlying sediment (adhering preservation of ref. 17). (C) Passively filled burrow that is surrounded by silty sediment (floating preservation of ref. 17).
Bioturbation in Younger Strata.
The above characteristics are found ubiquitously in strata of T. pedum and R. avalonensis zones. Current correlation suggests that they correspond to the Nemakit–Daldynian and the Tommotian stages, Siberian units that remain useful for global correlation (Fig. 1).
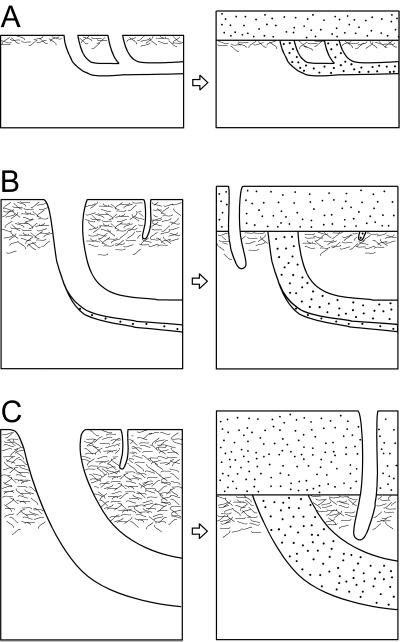
Not until the Atdabanian (Figs. 1 and 4) does a well developed mixed layer appear. The very shallowest tier burrows such as T. pedum and Gyrolithes are no longer preserved routinely. However, the styles and quality of preservation are consistent with a (very) shallow mixed layer and the presence of a firm substrate just beneath it. In strata of Cambro-Ordovician age, exquisite preservation of relatively shallow tiers (e.g., Rusophycus and Trichophycus emplaced no deeper than 5–10 cm) is common. The mixed layer would have been less than 5 cm.
Figure 4.
Schematic representations of the ichnofabric and sediment response to storm-related erosion in T. pedum zone (A), Atdabanian (B), and the late Cambrian (C). Sand is indicated by the stippled pattern. The area indicated by white reflects muddy sediments. The mixed layer is depicted by dense, fine patterning and increases in thickness (e.g., depth) from time A to time C. The mixed layer is more prone to erosion by strong currents, resulting in greater depth of erosion and loss of more deeply emplaced open burrows. Note that the depth of mixing in C exceeds the depth of the open burrow in A.
In high-energy sand-dominated shallow marine environments, however, the depth of bioturbation is greater. As early as the Atdabanian and probably the Tommotian, vertical trace fossils such as Skolithos and Diplocraterion extended to depths of decimeters and commonly created a relatively dense fabric (23). Although these burrows would not have mixed sediment, they would have allowed the oxygenation of sediment to depths of several tens of centimeters.
Discussion
The combination of features described above has implications for Cambrian-substrate properties and the fidelity of the trace-fossil record. In earliest Cambrian strata, representing deposition in storm-influenced shallow marine environments representing most of the record, the quality of preservation is excellent, and shallow tiers are commonly preserved; no ichnofabric other than that produced by discrete trace fossils is well developed, implying no mixing. Ubiquitous preservation of shallow tiers requires minimal erosion of surficial fine-grained sediment, which is accomplished only if that sediment is at least somewhat cohesive.
The features that we describe such as the preservation of sharp burrow margins with delicate scratch marks preserved and the low degree of compaction characterize firm-ground conditions (24). Thus, earliest Cambrian sediments were firm close to the sediment–water interface. A firm ground indicates stiff but uncemented sediment. In modern settings, firm grounds are exposed at the surface after erosion of upper layers; firm conditions at depth generally result from advanced dewatering and compaction (19). In the Early Cambrian, compaction would not be an important process, but rather silty, muddy sediment would be deposited and in the absence of bioturbators would tend to dewater more rapidly (see below). This process alone would result in a cohesive sediment surface. That Cambrian sediments were firm near the surface is suggested also by the presence of particular sedimentary structures that must have been formed close to the sediment–water interface including “Kullingia” scratch circles which form when a tethered organism is rotated by currents (Fig. 2A; ref. 25). The circles are formed in silts or fine sands and are cast by overlying coarser material. Their preservation requires that sediment just beneath the sediment–water interface is firm enough to imprint delicate concentric structures and also to withstand the erosion of currents in subtidal, shallow marine settings. Scratch circles are most common in lowermost Cambrian terrigenous clastic strata including the Chapel Island, Uratanna, and Torneträsk formations as well as the Mickwitzia sandstone of the units that we have examined, and they are reported from the Khmelnitskiy formation of the Ukraine.
Animals have a significant impact on substrate properties (18, 19, 26). We suggest that the relative firmness of earliest Cambrian muddy, surficial sediment is linked to the low levels of bioturbation and in particular to the low levels of motile deposit feeding in fine-grained sediments. None of the trace fossils common to lowermost Cambrian strata are interpreted to represent motile deposit feeding, but rather represent permanent or semipermanent dwelling structures (17), the formation of which would not have involved much sediment reworking.
Bioturbation influences the physical properties of sediments, although these effects are rather complex and differ as a consequence of sediment type and type of infaunal activity (26–28). In muddy sediments bioturbation may reduce surface-sediment shear strength by creating a more open sediment fabric, leading to increased porosity and water content (29, 30). Dense populations of modern burrowing bivalves in subtidal silty-clay facies at Buzzards Bay, MA, for example, caused a distinctly reworked surface-sediment fabric (30). The fabric had sand-sized pellets and clasts of mud as well as a zone of dense minerals, apparently largely caused by the activity of Nucula proxima. Increased water content and an irregular surface also made these sediments prone to resuspension by weak currents. In experiments with the bivalve Nucula in sediments of silt grade, constant burrowing and subsurface deposit feeding lead to at least doubled physical resuspension at specific shear values, perhaps caused by disruption of the cohesive nature of the sediment adjacent to each individual (31). Insecticide applied to areas of an intertidal mudflat in the Humber estuary, England, to study the effect of macro- and meiofauna on sediment properties resulted in a 300% increase of the critical erosion threshold for the sediment surface, which was attributed to an 8% reduction in water content (32). Reduced surface topography and an increase in microphytobenthos (as a consequence of reduced grazing) may have contributed also (32).
The presence of relatively firm, fine-grained sediments close to the sediment–water interface in the Early Cambrian has several important implications. First, relatively shallow tiers are represented rather faithfully in the Cambrian; thus, the early trace-fossil record fairly indicates of the origin of infaunal activity and therefore also the appearance of macroscopic bilaterian animals (cf. ref. 5). Although nonbilaterian metazoans can produce simple burrows and surface traces (33), this activity results in little or no sediment mixing. In the Neoproterozoic (and before), conditions would have been exceptionally favorable for preservation of all trace fossils including those formed near the sediment surface with little vertical component (e.g., Helminthoidichnites). Given these properties of Cambrian muddy substrate, we suggest that animals displacing sediment on the sediment surface would stand a relatively good chance of leaving a trace-fossil record. Trace fossils with a vertical component first appearing in the terminal Proterozoic (Huns formation) and elsewhere in the Lowermost Cambrian can be interpreted as representing the first bilaterians exploiting a vertical component of the sediment. The assumed characters of the last common ancestor of bilaterians were adaptations to a benthic lifestyle and included the ability to burrow (5, 15). Data from this study suggest that the substrate conditions of the Neoproterozoic and the earliest Cambrian would have been favorable particularly for the preservation of trace fossils; tiny millimeter-scale burrows are preserved in the Neoproterozoic–Lower Cambrian. Thus, burrowing by macroscopic bilaterians (or any other metazoans) during the Neoproterozoic that was not preserved is unlikely.
Second, firm grounds and limited bioturbation indicates little sediment mixing during the earliest Cambrian. The advent of sediment mixing, or development of the mixed layer (upper portion on the sediment that is being burrowed actively), has important implications for a number of issues critical to the terminal Proterozoic–Cambrian transition. The onset of bioturbation might have had a significant impact on sediment geochemistry and ocean geochemistry including shifts in the carbon and oxygen isotope record, nutrient cycling, and the distribution of organic material (34, 35). However, results from this study do not necessarily predict a sudden shift at the base of the Cambrian as a result of bioturbation or sediment mixing. A change could possibly be associated with the development of an early, very shallow, widespread mixed layer near the base of the Atdabanian when direct evidence of sediment mixing is preserved (17, 36). The time represented from the base of the Cambrian to the base of the Atdabanian is ≈20 million years, and thus the difference in timing of the development of the mixed layer is not insignificant. Bioturbation in part may have caused the gradual decline in δ13C from 700 to 530 million years ago (35). The results presented here, however, as well as data from the terminal Proterozoic (16) are not consistent with this hypothesis.
Acknowledgments
This article benefited from conversations with Nigel Hughes, Paul Myrow, Roland Goldring, Stephen Grant, and Guy Narbonne. This paper also benefited from two anonymous reviewers. We acknowledge partial funding for the field work from National Science Foundation Grant EAR-9219731 and National Geographic grants (to M.L.D.).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Peterson, K. J. & Takacs, C. M. (2001) Am. Zool. 41, 1554 (abstr.).
References
- 1.Wray G A, Levinton J S, Shapiro L H. Science. 1996;274:568–573. [Google Scholar]
- 2.Fortey R A, Briggs D E G, Wills M A. Biol J Linn Soc. 1996;57:13–33. [Google Scholar]
- 3.Fortey R A, Briggs D E G, Wills M A. BioEssays. 1997;19:429–434. [Google Scholar]
- 4.Knoll A H, Carroll S B. Science. 1999;284:2129–2137. doi: 10.1126/science.284.5423.2129. [DOI] [PubMed] [Google Scholar]
- 5.Budd G E, Jensen S. Biol Rev Camb Philos Soc. 2000;75:253–295. doi: 10.1017/s000632310000548x. [DOI] [PubMed] [Google Scholar]
- 6.Conway Morris S. Cell. 2000;100:1–11. doi: 10.1016/s0092-8674(00)81679-7. [DOI] [PubMed] [Google Scholar]
- 7.Collins A G, Valentine J W. Evol Dev. 2001;3:432–442. doi: 10.1046/j.1525-142x.2001.01048.x. [DOI] [PubMed] [Google Scholar]
- 8.Gehling J G. Mem-Geol Soc India. 1991;20:181–224. [Google Scholar]
- 9.Seilacher A. J Geol Soc (London) 1992;149:607–613. [Google Scholar]
- 10.Fedonkin M A, Waggoner B M. Nature (London) 1997;388:868–871. [Google Scholar]
- 11.Ayala F J, Rzhetsky A, Ayala F J. Proc Natl Acad Sci USA. 1998;95:606–611. doi: 10.1073/pnas.95.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seilacher A. Neues Jahrb Geol Palaontol Abh. 1956;103:155–180. [Google Scholar]
- 13.Crimes T P. Geol Mag. 1987;124:97–119. [Google Scholar]
- 14.Gehling J G, Jensen S, Droser M L, Myrow P M, Narbonne G M. Geol Mag. 2001;138:213–218. [Google Scholar]
- 15.Valentine J W. Proc Natl Acad Sci USA. 1994;91:6751–6757. doi: 10.1073/pnas.91.15.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Droser M L, Gehling J G, Jensen S. Geology. 1999;27:625–628. [Google Scholar]
- 17.Droser M L, Jensen S, Gehling J G, Myrow P M, Narbonne G M. Palaios. 2002;17:3–15. [Google Scholar]
- 18.McIlroy D, Logan G A. Palaios. 1999;14:58–72. [Google Scholar]
- 19.Bromley R G. Trace Fossils, Biology, Taphonomy and Applications. London: Chapman & Hall; 1996. [Google Scholar]
- 20.Narbonne G M, Myrow P, Landing E, Anderson M A. Can J Earth Sci. 1987;24:1277–1293. [Google Scholar]
- 21.Jensen S, Saylor B Z, Gehling J G, Germs G J B. Geology. 2000;28:143–146. [Google Scholar]
- 22.Seilacher A. In: Trace Fossils. Crimes T P, Harper J C, editors. Liverpool, U.K.: Seel House; 1970. pp. 447–476. [Google Scholar]
- 23.Droser M L. Palaios. 1996;6:316–325. [Google Scholar]
- 24.Goldring R. In: Marine Palaenvironmental Analysis of Fossils. Boscence D W J, Allison P A, editors. London: Geol. Soc.; 1995. pp. 151–180. [Google Scholar]
- 25.Jensen S, Droser M L, Gehling J G. Paleobios. 2001;21,Suppl.:74–75. [Google Scholar]
- 26.Lee H, Swartz C. In: Contaminants and Sediments. Baker R A, editor. Vol. 2. Ann Arbor, MI: Ann Arbor Science; 1980. pp. 555–606. [Google Scholar]
- 27.Meadows P S, Tait J. Mar Biol (Berlin) 1989;101:75–82. [Google Scholar]
- 28.Jones S E, Jago C F. Mar Biol (Berlin) 1993;115:133–142. [Google Scholar]
- 29.Rhoads D C. In: Trace Fossils. Crimes T P, Harper J C, editors. Liverpool, U.K.: Seel House; 1970. pp. 391–406. [Google Scholar]
- 30.Rhoads D C, Young D K. J Mar Res. 1970;28:150–178. [Google Scholar]
- 31.Davis W R. J Exp Mar Biol Ecol. 1993;71:187–200. [Google Scholar]
- 32.de Deckere E M G T, Tolhurst T J, de Brouwer J F C. Estuarine Coastal Shelf Sci. 2001;53:665–669. [Google Scholar]
- 33.Collins A G, Lipps J H, Valentine J W. Paleobiology. 2000;26:47–55. [Google Scholar]
- 34.Brasier M. Nature (London) 1990;347:521–522. [Google Scholar]
- 35.Brasier M D, McIlroy D. J Geol Soc (London) 1998;155:5–12. [Google Scholar]
- 36.Droser M L, Bottjer D J. Geology. 1988;16:233–236. [Google Scholar]
- 37.Seilacher A, Bose P K, Pflüger F. Science. 1998;282:80–83. doi: 10.1126/science.282.5386.80. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen B, Bengtson S, Fletcher I R, McNaughton N J. Science. 2002;296:1112–1115. doi: 10.1126/science.1070166. [DOI] [PubMed] [Google Scholar]