Abstract
The interaction of primase monomers within the hexameric gene 4 helicase-primase of bacteriophage T7 has been examined by using two genetically distinct gene 4 proteins. The T7 56-kDa gene 4 protein differs from the full-length 63-kDa protein in that it lacks the N-terminal zinc motif essential for the recognition of primase recognition sites. A second gene 4 protein, gp4-K122A, is unable to catalyze the synthesis of phosphodiester bonds as the result of an amino acid change in the catalytic site. Although each protein alone is inactive, the two together catalyze the synthesis of RNA primers. Reconstitution of activity depends on hexamer formation. We propose that the zinc motif of one subunit in the hexamer interacts with the catalytic sites of adjacent subunits.
Among the replication proteins of bacteriophage T7, the gene 4 protein plays a central role by providing both helicase and primase activities. Helicase and primase functions are located in the carboxyl terminal and amino terminal halves of the protein, respectively (Fig. 1) (1). A C-terminal segment of the protein (residue no. 241–566) forms hexamers and exhibits helicase activity (2). Likewise, a primase fragment (residue no. 1–271) has full primase activity on oligonucleotide templates although it is unable to oligomerize, to bind DNA tightly, or to scan primase recognition sites on a large DNA molecule (3).
Figure 1.
Organization of T7 gene 4 protein. Gene 4 protein consists of a primase and a helicase domain, connected by a linker region. The primase domain contains two subdomains: a zinc motif and a catalytic core. The gene 4 proteins used in this study are schematically presented. A 63-kDa protein; 56-kDa protein, a truncated gene 4 protein that lacks the N-terminal 63-aa residue containing the zinc motif; gp4-K122A, a gene 4 protein in which K122 at the catalytic site is replaced by alanine; PF, a primase fragment that contains only the primase domain from amino acid 1 to 271; and PF-K122A, a primase fragment in which K122 is replaced by alanine. Signs next to the abbreviated protein names denote the ability to catalyze the synthesis of primers.
Earlier studies suggested that the primases of the DnaG family are composed of three regions: an N-terminal zinc motif, a catalytic RNA polymerase domain, and a C-terminal domain that interacts with DNA helicase (4–6). The N-terminal zinc motif plays a major role in the recognition of specific sequences. The T7, T4, and Escherichia coli primases initiate synthesis from a trinucleotide recognition site, 5′-GTC-3′, 5′-G(C/T) C-3′, and 5′-CTG-3′, respectively (1). The first 3′-nucleotide of the sequence is essential for recognition but is not copied into the product. Interestingly, an in-frame translation initiation site located within T7 gene 4 gives rise to a truncated 56-kDa gene 4 protein lacking the N-terminal 63-aa residue found in the 63-kDa protein (Fig. 1) (7). The 56-kDa protein cannot catalyze template-directed RNA synthesis (8) although it does form hexamers and exhibit helicase activity (9). It can, however, catalyze the synthesis of random diribonucleotides in a DNA-independent reaction. The 56-kDa protein alone cannot support growth of T7 phage whereas the 63-kDa protein can (10).
Several conserved motifs (motifs II through VI), in addition to the zinc motif (motif I), have been identified among prokaryotic primases (11). X-ray crystal structures of the E. coli DnaG primase show that the conserved residues create a central crevice (12, 13). These studies also confirm that motifs IV, V, and VI, implicated in the coordination of NTPs and divalent metal cations, form a TOPRIM fold found in topoisomerase (14). Positioned next to the metal binding site is a basic and hydrophobic depression, proposed to interact with the backbone of the template (12, 13). We recently demonstrated that Lys-122 and Lys-128 located between motif III and IV of the T7 primase play an essential role (15). Gene 4 primases in which either of these residues has been substituted with alanine are unable to catalyze the synthesis of phosphodiester bonds. However, these altered primases do recognize the primase recognition sequences and anneal an exogenous primer to the correct recognition site, and then transfer the primer to T7 DNA polymerase (15).
The third subdomain of the prokaryotic DNA primases resides in the C-terminal region of the primase and interacts with the DNA helicase of each organism. The T7 DNA helicase-primase differs from that of most other prokaryotic systems in that the two activities do not reside in separate proteins that physically interact but rather reside within a single polypeptide chain (7).
The crystal structures of the helicase and primase domains of the gene 4 protein are each known (16, 17) (M. Kato and T. Ellenberger, personal communication). An electron microscopic image analysis of the T7 gene 4 protein suggests that the zinc motif has a high degree of flexibility and that the primase active site is located on the outside of the hexamer (18). Furthermore, cross-linking studies of E. coli DnaG protein show that the catalytic center is topographically close to both the zinc motif and to the C-terminal helicase-interacting subdomain (19). Our studies based on the ability of the primase to access recognition sites distal to the site to which it is bound also led us to conclude that the primase active site resides on the outside of the hexamer (20).
The uncertainty regarding the precise arrangement of the helicase and primase domains raises several intriguing questions. Are all six primase subunits within a hexameric assembly functional? How is the translocation of the helicase compatible with nucleotide polymerization by the primase in the opposite direction? Why has phage T7 evolved two gene 4 proteins, one lacking a zinc motif? Here, we demonstrate an interaction of the zinc motif of one subunit with the catalytic site of another within a hexameric assembly.
Materials and Methods
Oligonucleotides, enzymes, and strains were as described (15).
Preparation of Gene 4 Proteins.
Gene 4 proteins were overproduced in E. coli strain HMS174(DE3) that contained the appropriate gene 4-expressing plasmid. Construction of plasmids and purification of proteins were described (3, 15). pET-g4P-K122A that expresses the primase fragment containing a substitution of lysine to alanine at position 122 was constructed by inserting the mutated DNA fragment into pET-g4P between BstBI and NdeI sites (3).
Primase Oligoribonucleotide Synthesis Assay.
Primase activity was determined directly by measuring the amount of oligoribonucleotides synthesized on a DNA template containing a primase recognition site (21). The standard reaction (10 μl) contained the indicated amount of DNA template, 0.1 mM each of ATP and CTP, 0.1 μCi [α-32P]CTP, 40 mM Tris⋅HCl (pH 7.5), 10 mM MgCl2, 10 mM DTT, 50 mM potassium glutamate, and gene 4 protein (1 Ci = 37GBq). After incubation at 37°C for 20 min, the reaction was terminated by the addition of 3 μl of sequencing dye (98% formamide/10 mM EDTA, pH 8.0/0.1% xylenecyanol FF/0.1% bromophenol blue) and loaded onto a 25% denaturing polyacrylamide sequencing gel containing 3 M urea. Radioactive oligoribonucleotide products were analyzed by using a Fuji BAS 1000 Bioimaging analyzer.
Results
In Vitro Complementation of Inactive Primases.
For the T7 DNA primase to catalyze template-directed synthesis of oligoribonucleotides, both a zinc motif and a catalytic active site are required (1). Both the 56-kDa protein, lacking the zinc motif, and the gp4-K122A primase containing an inactive catalytic site are unable to catalyze template-dependent oligoribonucleotide synthesis independently. We considered the possibility that the two gene 4 proteins could interact such that each functional domain complements the respective inactive domain of the other.
In the experiment shown in Fig. 2, we measured DNA primase activity by using combinations of gene 4 proteins. A functional primase produces predominantly tetraribonucleotides, rACCC, at the recognition site, 5′-GGGTC-3′ on a 15-nt template. Primase activity was determined by measuring the amount of radioactive [α-32P]CMP incorporated into rACCC as a function of the amount of gene 4 protein. The 56-kDa gene 4 protein itself does not have detectable primase activity (Fig. 2, lane 1). However, addition of gp4-K122A to the 56-kDa protein resulted in the synthesis of primers, and the amount of synthesis was dependent on the concentration of the added protein (Fig. 2, lanes 2–4). Conversely, when the amount of gp4-K122A was fixed and increasing amounts of 56-kDa gene 4 protein were added, a similar amount of primer synthesis was observed (Fig. 2, lanes 5–8). Amounts of primer synthesized by the mixed proteins were slightly higher with excess 56-kDa gene 4 protein as compared to gp4-K122A (① vs. ②), suggesting that primer synthesis occurs at the catalytic site of the 56-kDa protein. When compared to 63-kDa protein, the mixture of 56-kDa protein and gp4-K122A exhibited ≈30–60% of primase activity on a short DNA template. The protein mixture also produced oligoribonucleotides from the same primase recognition sites on M13mp18 single-stranded DNA template as did the 63-kDa protein (data not shown).
Figure 2.
Oligoribonucleotide synthesis catalyzed by mixtures of gene 4 proteins. A fixed amount (50 ng) of the indicated gene 4 protein was mixed with increasing amount (0, 50, 100, or 200 ng) of the indicated other gene 4 protein. The mixture was incubated with 10 μM DNA template 5′-GGGTCA10-3′ in the standard reaction for 30 min at 37°C. Note that dTTP was omitted. Reaction products were separated and analyzed as described in Materials and Methods. In the autoradiograph of the gel, the major product of the reaction, tetraribonucleotide ACCC, is denoted on the left.
Requirement of Hexamer Formation for Reconstitution of Primase Activity.
Reconstitution of primase activity from a mixture of the two inactive primases implies an interaction between the two proteins to bring essential parts of the primase together. We could envision an interaction of adjacent N-terminal primase domains within the configuration of the hexamer.
(i) Requirement for helicase domain.
Gene 4 protein forms a hexamer via its C-terminal helicase domain and linker region. Consequently, each subunit in the hexamer will have a primase domain located at the N terminus of the helicase domain. Therefore, the functional interaction of the distinct primases may be dependent on hexamer formation to bring the two domains into proximity. To test this hypothesis, we have modified one of the distinct gene 4 proteins so that it cannot form hexamers. The primase fragment of gene 4 protein has full primase activity but is unable to form hexamers (3). We introduced the single amino acid change, K122A, into the primase catalytic site of the primase fragment and then examined its ability to complement the 56-kDa protein. As shown in Fig. 2, lanes 9–12, a mixture of the primase fragment and the 56-kDa protein does not have primase activity.
(ii) Stimulation by dTTP.
We omitted dTTP from the assays shown in Fig. 2 to simplify interpretation. However, in view of the requirement for the helicase domain to reconstitute primase activity, we investigated the effect of dTTP on reconstitution. The ability of gene 4 protein to form hexamers is promoted by the presence of dTTP (22). In the assays shown in Fig. 3A, a mixture of gp4-K122A and the 56-kDa protein was incubated with various concentrations of dTTP or its nonhydrolyzable analog, β,γ-methylene dTTP. The amount of primers synthesized by the two proteins was increased proportional to the concentration of dTTP up to 125 μM. The addition of β,γ-methylene dTTP increased primase activity but the same amounts of primer products were produced over the entire concentration range. Small amounts of the analog, as low as 8 μM, were sufficient to enhance primase activity. This analog has been shown to lock the gene 4 proteins into its hexamer configuration (22). The effects of dTTP and its analog confirm that hexamer formation is required for reconstitution.
Figure 3.
Effect of dTTP and its analog β,γ-methylene dTTP on primase activity. (A) The reconstituted primases consisting of 0.15 μM each of the 56-kDa protein and gp4-K122A were incubated with 25 μM template 5′-GGGTCA10-3′ in the standard reaction with various concentrations of either dTTP or its nonhydrolyzable analog β,γ-methylene dTTP for 20 min at 37°C. Reaction products were separated and analyzed as described in Materials and Methods. Incorporation of [α-32P]CMP into the product was plotted against the concentration of the dNTPs. (B) Inhibition of primase activity by preincubation with β,γ-methylene dTTP. The indicated gene 4 protein (0.08 μM) was incubated with 1 mM β,γ-methylene dTTP for the indicated period at room temperature before the addition of an equal amount of the other gene 4 protein. After an additional 5-min incubation, primase reactions were initiated by the addition of 10 μM template 5′-GGGTCA10-3′, and rNTPs, and carried out for 20 min at 37°C. Incorporation of [α-32P]CMP into the product was plotted against the preincubation time with the dTTP analog.
To reconstitute primase activity, hexamer formation must occur between gp4-K122A and the 56-kDa protein. Preincubation of gene 4 protein with the nonhydrolyzable dTTP analog before the addition of the other gene 4 protein should yield hexamers of only one protein. The reaction shown in Fig. 3B initially contained either gp4-K122A or 56-kDa gene 4 protein. The protein was first preincubated with 1 mM β,γ-methylene dTTP and then the other gene 4 protein was added. After 5 min incubation to ensure hexamer formation, the reaction was initiated by the addition of template, ATP, and CTP. Preincubation of either protein with the dTTP analog reduced primase activity. The longer the protein was preincubated with the dTTP analog, the less primers were synthesized.
Two Modes for Primer Synthesis Catalyzed by Gene 4 Protein.
The primase activity of the 63-kDa gene 4 protein is increased by the presence of dTTP on a long DNA template. The stimulatory effect of dTTP on the 63-kDa protein derives from its ability to promote the formation of hexamers, which bind to the template and increase the affinity of the primase for primase recognition sites (23). The primase fragment is unable to form hexamers and thus no stimulation by dTTP is detected (3). A similar phenomenon is observed when the 3′-flanking sequence of a primase recognition site in DNA template is <5 nt, presumably because there is insufficient 3′-flanking sequence to which hexamers can bind (21).
(i) Effect of hexamer formation on interaction of primase domains.
We have used mixtures of 56-kDa protein and gp4-K122A in conjugation with the full-length protein and the primase fragment to explore the contribution of hexamer formation to primase activity (Fig. 4A). Primase activities of proteins were measured by using two templates; one template (5′-GGGTCA10-3′) is sufficiently long to allow hexamers to bind, and the other template (5′-GGGTCA-3′) is too short for binding. We attribute the stimulation of primase activity by dTTP to be indicative of hexamer formation (3). Primase activity catalyzed by the 63-kDa protein is not stimulated by dTTP on the 6-mer template whereas it is stimulated twofold on the 15-mer (Fig. 4A). Likewise, the primase fragment shows no stimulation by dTTP on both templates. On the other hand, an equimolar mixture of the 56-kDa protein and gp4-K122A has minimal activity in the absence of dTTP but has unequivocal activity in the presence of dTTP, especially on the longer 15-mer template. Note that despite its low magnitude, the protein mixture exhibits a primase activity in the absence of dTTP, similar to that shown in Fig. 2. Primase activity of the mixture of gene 4 proteins is stimulated at least eightfold on the 15-mer. These results demonstrate the two modes of primer synthesis, one mediated via the hexamer in the presence of dTTP and the other by a nonhexameric protein.
Figure 4.
Two modes of primer synthesis catalyzed by gene 4 protein. (A) Effect of 3′-flanking sequence on the primase activity of gene 4 protein. Both the 6-nt (5′-GGGTCA-3′) and 15-nt (5′-GGGTCA10-3′) templates have the same primase recognition site but differ in the 3′-side flanking length. The indicated gene 4 proteins were incubated with the template in the standard reaction with or without 0.1 mM dTTP for 20 min at 37°C. The protein concentration of either the 63-kDa protein or the primase fragment was 0.1 μM, and total concentration of equimolar mixture of the 56-kDa protein and gp4-K122A was 0.3 μM. Incorporation efficiency of [α-32P]CMP into the product is presented as height of bars in graphs. The presence or absence of dTTP is denoted. (B) Effect of defective gene 4 proteins on the primase activity of 63-kDa gene 4 protein. On the two different sets of templates described above, increasing concentrations of the 63-kDa protein alone were incubated in the standard reaction at 37°C for 20 min (▵). Either the 56-kDa protein (○) or gp4-K122A (●) was added to the 63-kDa protein to yield a total 0.3 μM protein. The mixtures of proteins used for the 15-nt template were incubated for 10 min at 37°C before the primer synthesis reaction. Incorporation of [α-32P]CMP into the product is plotted against the 63-kDa protein concentration. (C) Effect of mixing ratio on primer synthesis catalyzed by the 56-kDa protein and g-K122A. Reaction condition was the same as the one used for the 15-mer template in B.
(ii) Effect of defective primases on the primase activity of the 63-kDa protein.
In the experiment shown in Fig. 4B, mixtures of the 63-kDa protein with either 56-kDa protein or gp4-K122A were formed and primer synthesis was measured on both the 6-mer template without dTTP and the 15-mer template with dTTP. The former template without dTTP assures the absence of the hexamer mode of synthesis whereas the latter template with dTTP promotes hexamer formation. In the mixtures, the ratio of proteins was varied but the total amount of protein was maintained constant. Primer synthesis catalyzed by the 63-kDa gene 4 protein alone or by mixtures with either 56-kDa protein or gp4-K122A on the 6-mer increases linearly with the amount of the 63-kDa protein (Fig. 4B). In this mode of primer synthesis, which is not mediated by hexamers, synthesis depends on the concentration of the 63-kDa protein, and neither inactive 56-kDa protein nor gp4-K122A makes any contribution to the activity.
The patterns of primase activity are strikingly different under conditions where hexamer formation is mediated. First, the rate of primer synthesis catalyzed by the 63-kDa protein alone has a hyperbolic increase with response to protein concentration (Fig. 4B). This hyperbolic response reflects the concentration dependence of the assembly of gene 4 protein monomers into a functional hexamer. Hexamers consisting of a mixture of the 63-kDa protein with either the 56-kDa protein or gp4-K122A differ significantly. The 56-kDa protein is as efficient as the 63-kDa protein in primer synthesis provided that there are sufficient 63-kDa monomers in the hexamer (Fig. 4B). Under this condition the zinc motif of a 63-kDa subunit can presumably interact with an adjacent active site of 56-kDa protein subunit to form an active primase. However, in hexamers containing gp4-K122A, only a 63-kDa subunit can function in primer synthesis and consequently the protein dependency more closely resembles that observed with a 63-kDa protein alone.
(iii) Effect of mixing ratio on primer synthesis catalyzed by the 56-kDa and gp-K122A.
To further explore the interaction between the 56-kDa protein and gp4-K122A in reconstituted primase, we mixed those proteins at various ratios, keeping the total amount of protein constant, and measured primer synthesis catalyzed by the mixtures. Primase activity of a mixture at a ratio of 5:1 (56-kDa gene 4 protein:gp4-K122A) is greater than that at a ratio of 1:5 (Fig. 4C). Because the same numbers of interfaces between the two proteins are expected at both ratios, the difference in activity suggests that the primase synthesis site is more than the composite sites of these interfaces. One possibility is that the zinc motif from a gp4-K122A subunit has sufficient flexibility to interact with two neighboring catalytic subunits of the 56-kDa protein at a ratio of 5:1. As a result, the efficiency for primer synthesis correlates with the number of catalytic sites that contact the zinc motif. This model also rules out the possibility that the mixtures of the two distinct proteins function as a dimer since both ratios (5:1 vs. 1:5 or 4:2 vs. 2:4) at which the protein mixtures provide the same numbers of dimers exhibit different levels of primer synthesis.
Other Enzymatic Activities of Reconstituted DNA Primase.
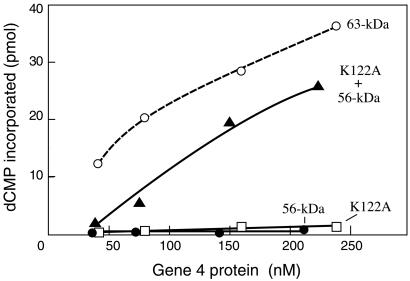
As shown in Fig. 5, the reconstituted primase was nearly as efficiently as 63-kDa gene 4 protein in providing primers for T7 DNA polymerase on M13 single-stranded DNA. We also found that mixtures of the two distinct proteins that restore primase activity caused neither a synergetic nor an inhibitory effect on helicase activities including dTTP hydrolysis, DNA binding, and DNA unwinding (data not shown).
Figure 5.
Reconstituted primase synthesizes functional primers for T7 DNA polymerase. The ability of gene 4 protein to prime DNA synthesis catalyzed by T7 DNA polymerase was determined by measuring the incorporations of dNTP into DNA product (15). The reaction contained 9.8 nM M13 single-stranded DNA, 0.3 mM of all four dNTPs, 0.1 μCi [α-32P]dCTP, 0.1 mM each of ATP and CTP, the increasing amounts of the indicated gene 4 protein, and 20 nM T7 DNA polymerase. After incubation for 10 min at 37°C, radioactivity incorporated into DNA products was measured and plotted against the concentration of gene 4 proteins used.
Discussion
Neither a 56-kDa gene 4 protein lacking a zinc motif nor a 63-kDa gene 4 protein with an inactive catalytic primase site (gp4-K122A) can catalyze the synthesis of primers. However, together they catalyze the synthesis of oligoribonucleotides that are functional primers for T7 DNA polymerase. This reconstituted catalytic activity requires hexamer formation between the two protein subunits as determined by a requirement for the C-terminal helicase domain and a stimulation of the reaction by dTTP.
Organization of T7 Gene 4 Protein.
A unique feature of T7 gene 4 protein is the coexistence of primase and helicase activities in a single polypeptide. Having these two functions physically associated provides advantages for DNA replication. First, DNA primase has immediate access to recognition sites on the single-stranded DNA that is extruded by helicase activity at the replication fork. Once primers are synthesized, they are directed to T7 DNA polymerase for the initiation of lagging strand synthesis. This simplicity of organization contrasts with that of other organisms such as E. coli and T4 phage where accessory proteins are required to constitute the replisome (24, 25). A second advantage is an enhancement of primase domain binding to a DNA template by the hexameric helicase domain. The primase domain, when it is tethered to the helicase domain, is positioned close to the DNA template with a high binding affinity conferred by the helicase domain. Third, translocation of gene 4 protein by the helicase domain transports the primase domain in its search for primase recognition sites. Such a dependency of primase activity on helicase activity also is observed in both the E. coli or T4 phage replication systems in which the two proteins are encoded by separate genes but form a stable complex (6, 26, 27). Finally, each primase subunit in the hexameric gene 4 protein is in close proximity to neighboring subunits. Such a packed conformation of the primase domain allows a cooperative effect by which a zinc motif also can interact with adjacent catalytic sites. Thus, hexamer formation of the gene 4 protein is not only critical to helicase activity but also advantageous for primase activity.
Interaction Between the Zinc Motif and RNA Polymerase Domain.
Sequence recognition of the DNA template by the zinc motif of DNA primase is the first step in primer synthesis. Subsequently, the recognized sequence must be placed within the catalytic domain. Reconstituted primase activity from two inactive primases is a direct demonstration that the interaction between the two proteins occurs between subunits in a hexameric conformation. A zinc motif from one subunit seems to be able to interact with catalytic sites in neighboring subunits. Consistent with this proposal, a preliminary crystallographic structure of T7 primase shows the existence of a flexible linker between the zinc motif and the catalytic core (M. Kato & T. Ellenberger, personal communication). Interaction of a zinc motif with a catalytic center in a hexamer is depicted in Fig. 6. In the model, a protruding zinc motif from one 63-kDa subunit contacts the primase active site of an adjacent 56-kDa subunit. Upon specific recognition of the primase recognition sequence by the zinc motif, the catalytic site initiates primer synthesis on the template. A flexible linker between the zinc motif and the catalytic core enables the zinc motif to make contact with other catalytic cores in hexameric conformation.
Figure 6.
Model for primer synthesis catalyzed by hexamer composed of 56- and 63-kDa gene 4 proteins.
Why Does Bacteriophage T7 Produce the 56-kDa Gene 4 Protein?
In E. coli infected by T7 phage, the 56-kDa and 63-kDa proteins are present in equimolar amounts (7). An intriguing question is why bacteriophage T7 encodes the 56-kDa protein because the full-length 63-kDa protein has both helicase and primase activities. What advantages arise for T7 phage when it expresses this dispensable gene product (28)? Although the 56-kDa protein does not function as a DNA primase by itself, we have shown that its catalytic site can function in primer synthesis upon forming a hexamer with gene 4 protein monomers containing a zinc motif. As long as one zinc motif per two catalytic sites is supplied, mixtures of the 56-kDa and 63-kDa gene 4 proteins exhibit similar level of activity (Fig. 4B). Therefore, expression of the 56-kDa protein can be an economic strategy for the phage to save production of unnecessary zinc motifs in the 63-kDa subunits in the hexamer. Indeed, it is suggested that less than three molecules of primase form a stable complex with one hexamer of helicase from T4 phage and Bacillus stearothermophilus (6, 27).
Alternatively, a clue can be sought from a possible enhancement of helicase activity by replacing 63-kDa subunits in functional gene 4 hexamer with 56-kDa protein. It is possible that there is a spatial clash between adjacent zinc motifs within a 63-kDa gene 4 hexamer. Finally, it is tempting to speculate that a less compact conformation of the N-terminal region of the gene 4 hexamer could be spatially reserved for interaction with other proteins.
Acknowledgments
We are grateful to Joonsoo Lee for suggesting experiments with mixture of proteins. We thank Ingrid Richardson, David Frick, and Donald Crampton for critical reading of manuscript. This work was supported by U.S. Public Health Service Grant GM 54397 (to C.C.R.).
References
- 1.Frick D N, Richardson C C. Annu Rev Biochem. 2001;70:39–80. doi: 10.1146/annurev.biochem.70.1.39. [DOI] [PubMed] [Google Scholar]
- 2.Guo S, Tabor S, Richardson C C. J Biol Chem. 1999;274:30303–30309. doi: 10.1074/jbc.274.42.30303. [DOI] [PubMed] [Google Scholar]
- 3.Frick D N, Baradaran K, Richardson C C. Proc Natl Acad Sci USA. 1998;95:7957–7962. doi: 10.1073/pnas.95.14.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun W, Tormo J, Steitz T A, Godson G N. Proc Natl Acad Sci USA. 1994;91:11462–11466. doi: 10.1073/pnas.91.24.11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird L E, Hakansson K, Pan H, Wigley D B. Nucleic Acids Res. 1997;25:2620–2626. doi: 10.1093/nar/25.13.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jing D H, Dong F, Latham G J, von Hippel P H. J Biol Chem. 1999;274:27287–27298. doi: 10.1074/jbc.274.38.27287. [DOI] [PubMed] [Google Scholar]
- 7.Dunn J J, Studier F W. J Mol Biol. 1983;166:477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein J A, Richardson C C. Proc Natl Acad Sci USA. 1988;85:396–400. doi: 10.1073/pnas.85.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein J A, Richardson C C. J Biol Chem. 1988;263:14891–14899. [PubMed] [Google Scholar]
- 10.Notarnicola S M, Richardson C C. J Biol Chem. 1993;268:27198–27207. [PubMed] [Google Scholar]
- 11.Ilyina T V, Gorbalenya A E, Koonin E V. J Mol Evol. 1992;34:351–357. doi: 10.1007/BF00160243. [DOI] [PubMed] [Google Scholar]
- 12.Keck J L, Roche D D, Lynch A S, Berger J M. Science. 2000;287:2482–2486. doi: 10.1126/science.287.5462.2482. [DOI] [PubMed] [Google Scholar]
- 13.Podobnik M, McInerney P, O'Donnell M, Kuriyan J. J Mol Biol. 2000;300:353–362. doi: 10.1006/jmbi.2000.3844. [DOI] [PubMed] [Google Scholar]
- 14.Aravind L, Leipe D D, Koonin E V. Nucleic Acids Res. 1998;26:4205–4213. doi: 10.1093/nar/26.18.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S J, Richardson C C. J Biol Chem. 2001;276:49419–49426. doi: 10.1074/jbc.M108443200. [DOI] [PubMed] [Google Scholar]
- 16.Sawaya M R, Guo S, Tabor S, Richardson C C, Ellenberger T. Cell. 1999;99:167–177. doi: 10.1016/s0092-8674(00)81648-7. [DOI] [PubMed] [Google Scholar]
- 17.Singleton M R, Sawaya M R, Ellenberger T, Wigley D B. Cell. 2000;101:589–600. doi: 10.1016/s0092-8674(00)80871-5. [DOI] [PubMed] [Google Scholar]
- 18.VanLoock M S, Chen Y J, Yu X, Patel S S, Egelman E H. J Mol Biol. 2001;311:951–956. doi: 10.1006/jmbi.2001.4932. [DOI] [PubMed] [Google Scholar]
- 19.Mustaev A A, Godson G N. J Biol Chem. 1995;270:15711–15718. doi: 10.1074/jbc.270.26.15711. [DOI] [PubMed] [Google Scholar]
- 20.Kusakabe T, Baradaran K, Lee J, Richardson C C. EMBO J. 1998;17:1542–1552. doi: 10.1093/emboj/17.5.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendelman L V, Richardson C C. J Biol Chem. 1991;266:23240–23250. [PubMed] [Google Scholar]
- 22.Patel S S, Hingorani M M. J Biol Chem. 1993;268:10668–10675. [PubMed] [Google Scholar]
- 23.Frick D N, Richardson C C. J Biol Chem. 1999;274:35889–35898. doi: 10.1074/jbc.274.50.35889. [DOI] [PubMed] [Google Scholar]
- 24.Yuzhakov A, Kelman Z, O'Donnell M. Cell. 1999;96:153–163. doi: 10.1016/s0092-8674(00)80968-x. [DOI] [PubMed] [Google Scholar]
- 25.Benkovic S J, Valentine A M, Salinas F. Annu Rev Biochem. 2001;70:181–208. doi: 10.1146/annurev.biochem.70.1.181. [DOI] [PubMed] [Google Scholar]
- 26.Dong F, von Hippel P H. J Biol Chem. 1996;271:19625–19631. doi: 10.1074/jbc.271.32.19625. [DOI] [PubMed] [Google Scholar]
- 27.Bird L E, Pan H, Soultanas P, Wigley D B. Biochemistry. 2000;39:171–182. doi: 10.1021/bi9918801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendelman L V, Notarnicola S M, Richardson C C. Proc Natl Acad Sci USA. 1992;89:10638–10642. doi: 10.1073/pnas.89.22.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]