Abstract
Single mitochondria show the spontaneous fluctuations of ΔΨm. In this study, to examine the mechanism of the fluctuations, we observed ΔΨm in single isolated heart mitochondria using time-resolved fluorescence microscopy. Addition of malate, succinate, or ascorbate plus TMPD to mitochondria induced polarization of the inner membrane followed by repeated cycles of rapid depolarizations and immediate repolarizations. ADP significantly decreased the frequency of the rapid depolarizations, but the ADP effect was counteracted by oligomycin. On the other hand, the rapid depolarizations did not occur when mitochondria were polarized by the efflux of K+ from the matrix. The rapid depolarizations became frequent with the increase in the substrate concentration or pH of the buffer. These results suggest that the rapid depolarizations depend on the net translocation of protons from the matrix. The frequency of the rapid depolarizations was not affected by ROS scavengers, Ca2+, CsA, or BA. In addition, the obvious increase in the permeability of the inner membrane to calcein (MW 623) that was entrapped in the matrix was not observed upon the transient depolarization. The mechanisms of the spontaneous oscillations of ΔΨm are discussed in relation to the matrix pH and the permeability transitions.
INTRODUCTION
The control of the ΔΨm is an important process for maintaining cellular homeostasis. In a polarized state, mitochondria utilize ΔΨm to generate the energy for transporting charged molecules and ions. For example, ATP synthesis is driven by the translocation of H+ across the inner membrane through the ATP synthase. Thermogenesis is caused by the reentry of H+ into the matrix, which is mediated by mitochondrial uncoupling proteins (Ricquier and Bouillaud, 2000). Because the electric potential on the matrix side is negative, mitochondria accumulate Ca2+ to buffer the increase in Ca2+ concentration in the cytosol (Boitier et al., 1999). This accumulated Ca2+ can be released to amplify the Ca2+ level transiently upon depolarization by the permeability transition (Ichas et al., 1997). Depolarization is also reported to reduce the generation of ROS (Arsenijevic et al., 2000; Nishikawa et al., 2000).
For these reasons, extensive studies on ΔΨm have been performed and have produced results that help us to understand mitochondrial behaviors (Baracca et al., 2003; Bernardi, 1992; Chacon et al., 1994; Ichas et al., 1997; Krauss et al., 2002; Zoratti and Szabó, 1994). Since most of these studies were performed with mitochondrial ensembles, the results obtained represented mitochondrial behaviors that were averaged over a large number of mitochondria. In addition to averaged behaviors, ΔΨm of single mitochondria has been measured in several studies to obtain more detailed information. These experiments have revealed the persistence and changes over time of ΔΨm (De Giorgi et al., 2000; Loew et al., 1993, 1994; Zorov et al., 2000), as well as intracellular heterogeneity of the values (Collins et al., 2002; Smiley et al., 1991). Interestingly, different from the synchronized oscillations in ion flux across the inner membrane (Chance and Yoshioka, 1966; Gooch and Packer, 1974; Selivanov et al., 1998), single mitochondria can independently undergo repetitive transient depolarizations (Hüser et al., 1998; Jacobson and Duchen, 2002; Nakayama et al., 2002; Vergun et al., 2003). Since rhodamine-based cationic fluorescent dyes used in these experiments can also act at a high concentration as photosensitizers that produce the ROS when the dyes are illuminated, the spontaneous transient depolarizations of mitochondria observed were considered to be a mitochondrial response to ROS (Hüser et al., 1998; Jacobson and Duchen, 2002). Recent experiments with a low concentration of the dyes, however, demonstrate that the spontaneous transient depolarizations can occur independently of ROS (Nakayama et al., 2002; Vergun et al., 2003).
Here we demonstrate that the acute decrease in the proton concentration beneath the inner membrane due to proton pumping induces transient opening of a pH sensitive and proton conductive channel, resulting in the transient depolarization. Based on this observation, we propose the model for repeated cycles of depolarizations and immediate repolarizations.
EXPERIMENTAL PROCEDURES
Preparation of mitochondria
Porcine heart mitochondria were isolated by differential centrifugation (Palmer et al., 1977) and suspended in preparation buffer containing 10 mM Tris-HCl, 250 mM sucrose, 1 mM EDTA, 0.1 mM DTT, pH 7.4. For microscopic measurements, mitochondria were adsorbed onto a glass-bottom culture dish coated with Cell-Tak at ∼106 mitochondria/dish (Nakayama et al., 2002). They were then kept for 90 min in Tris buffer and were washed twice. All procedures described above were performed at 0°C–4°C. Each preparation was assayed for ATP production (Wibom et al., 1990) upon addition of 5 mM Na-malate, giving a rate of 415 ± 17 μmol ATP/g of protein/min (the mean ± SE, n = 6), which was compatible with the previous results (Nakayama et al., 2002; Wibom et al., 1990; Wibom and Hultman, 1990). Protein content was determined using the protein assay with BSA as a standard.
Fluorescence imaging of ΔΨm in single mitochondria
For fluorescence imaging of ΔΨm, mitochondria were stained with tetramethylrhodamine ethyl ester (TMRE), a potentiometric fluorescent dye (Duchen et al., 1998; Hüser and Blatter, 1999; Nakayama et al., 2002). Before the measurements, mitochondria were incubated with 2 nM TMRE in sucrose buffer (10 mM Tris-HCl, 250 mM sucrose, 2 mM KH2PO4, 1 mM EDTA, 0.1 mM DTT, pH 7.4) for 30 min in the presence of 1 mg/ml BSA. When the effects of ADP (0.5 mM), oligomycin (1 μM), CsA (1 μM), BA (2 μM), catalase (1500 units/ml), or GSH (2 mM) were examined, mitochondria were stained with TMRE in the presence of these reagents. When the effects of TMPyP or H2O2 were examined, these reagents were added 10 min before the microscopic observation. When valinomycin (20 nM), an ionophore for K+, was added to polarize the inner membrane without proton pumping, mitochondria were first stained with TMRE (2 nM) for 30 min in KCl buffer (10 mM Tris-HCl, 140 mM KCl, 2 mM KH2PO4, 1 mM EDTA, 0.1 mM DTT, pH 7.4) in the presence of 1 mg/ml BSA. Then, KCl buffer was replaced with sucrose buffer containing 2 nM TMRE and 1 mg/ml BSA to reduce the concentration of K+ in the buffer 10 min before the measurements. In this case, 2 mM KH2PO4 in sucrose buffer was replaced with 2 mM NaH2PO4. For all experiments, the volume of buffer was 1 ml, so that the volume of buffer was >106 times larger than that of the total mitochondria. This allowed us to estimate that changes in TMRE concentration of the buffer due to polarization or depolarization of mitochondria are <1%, even when we assume that mitochondria polarize from 0 mV to −240 mV. The glass-bottom culture dish was placed on the stage of an inverted epifluorescence microscope (IX-70; Olympus; Tokyo, Japan) equipped with a 40× objective lens (Uapo40×/340, NA = 0.9; Olympus). Fluorescence was elicited by illumination with a 75 W xenon lamp through a 15 nm band-pass filter centered at 535 nm. Fluorescence at >580 nm was collected with a cooled CCD camera (PentaMAX 1317K 5 MHz; Princeton Instruments; Trenton, NJ). A series of image frames was acquired at intervals of 3 s, with binning pixels 2 × 2, under computer control. The exposure time for each frame was 1 s. During the remaining 2 s, the excitation light was cut off with a mechanical shutter to avoid mitochondrial damage that could possibly result from illumination. The intensity of illumination was also reduced to 25% with a neutral density filter to avoid photodynamic injury to mitochondria. The readout was digitized to 12 bits and analyzed with image-processing software (MetaMorph; Universal Imaging; Downingtown, PA). All procedures described above were performed at room temperature.
For the analysis of individual mitochondria, the fluorescence intensity was averaged over an area of 0.6 μm2 of the mitochondrion. The fluorescence intensity of TMRE in the buffer was measured in the same field as the mitochondria but was obtained at a position where the fluorescence intensity was not affected by mitochondrial TMRE.
Measurements of calcein release from mitochondria
To examine the permeability of the inner membrane to calcein, we measured the fluorescence of calcein entrapped in the matrix (Hüser et al., 1998; Petronilli et al., 1999). At first, mitochondria attached on a polystyrene dish were incubated with 3 μM calcein-AM in sucrose buffer containing 0.5 mM ADP for 30 min at room temperature and then were washed with an appropriate buffer twice. The fluorescence of calcein was measured in the same manner as that used for measurements of TMRE except for the optical filters and a dichroic mirror. Excitation was selected with a 20 nm band-pass filter centered at 480 nm. Fluorescence was collected between 515 and 550 nm. The intensity of illumination was reduced to 12% with a neutral density filter. For simultaneous measurements of calcein and TMRE fluorescence, mitochondria were loaded with 3 μM calcein-AM in the presence of 40 nM TMRE. Time intervals between images were lengthened to 6 s because we mechanically switched the optical filters for calcein and TMRE. Also, the intensity of illumination for both dyes was reduced to 2% to avoid photodynamic injury of mitochondria.
We also observed the calcein fluorescence in the supernatant of mitochondria attached on a polystyrene dish. After loading calcein-AM to mitochondria as mentioned above, the mitochondria were washed twice and placed in an appropriate buffer for a few minutes. Then, we added malate to mitochondria and immediately pipetted an appropriate amount of the supernatant into test tubes. Ten minutes after adding malate, we again pipetted the supernatant from the same dish into other test tubes. All these procedures were performed in the dark to eliminate the possibility of photodynamic injury of the mitochondria. The buffer containing calcein released from the mitochondria was measured using a spectrofluorimeter (FP-6500; JASCO; Tokyo, Japan) with excitation at 494 nm and emission at 517 nm. To compensate for the difference in the amount of mitochondria attached on dishes, calcein fluorescence measures were normalized by calcein fluorescence just after addition of malate.
Measurements of NADH by a mitochondrial ensemble
The autofluorescence of NAD(P)H in mitochondria was measured using a spectrofluorimeter (FP-6500; JASCO) with excitation at 340 nm and emission at 460 nm. Before the addition of malate, mitochondria (0.2 mg of protein/ml) were incubated for 10 min at room temperature in an appropriate buffer. Changes in NAD(P)H fluorescence upon addition of malate were monitored for 10 min.
Drugs and solutions
Malate, succinate, or valinomycin was applied by pressure ejection from micropipettes. All solutions were applied to mitochondria with appropriate pH correction. Ethanol was used as the solvent for rotenone, oligomycin, antimycin A, CsA, BA, and valinomycin. It was used at a maximum concentration of 0.1% and, when tested alone, did not have significant effects under these experimental conditions. The concentration of TMRE was determined spectrophotometrically with ɛ549nm = 109 mM−1cm−1. All experiments with TMRE were performed in the presence of 1 mg/ml BSA because nonuniform adsorption of TMRE occurred on a cover slip at the bottom of the dish if BSA was not included. The fluorescence intensity of TMRE must be obtained as a signal that is proportional to the concentration of TMRE in mitochondria and in the medium to evaluate ΔΨm by TMRE fluorescence. Therefore, nonuniform adsorption interferes with the analysis of ΔΨm.
TMRE and calcein-AM were obtained from Molecular Probes (Eugene, OR). Rotenone and oligomycin were from Sigma (St. Louis, MO). BCA protein assay protein assay reagent was from Pierce Chemical (Rockford, IL). A luciferase-luciferin kit was from Wako Pure Chemical (Osaka, Japan). TMPyP was from Calbiochem (San Diego, CA). Other chemicals were of the highest purity available commercially.
Statistical analysis
We averaged the data produced using mitochondria prepared from at least three independent samples of porcine hearts. The results were expressed as the mean ± SE and analyzed by ANOVA followed by Bonferroni correction. The difference was considered statistically significant at P < 0.05.
RESULTS
Membrane potential of single mitochondria upon addition of malate
To monitor the time course of changes in ΔΨm, we observed TMRE fluorescence in individual mitochondria. Mitochondrial TMRE fluorescence was normalized by the background fluorescence (TMRE fluorescence in the medium) to obtain the fluorescence intensity that was dependent on the membrane potential (Nakayama et al., 2002). Before addition of malate, the normalized fluorescence intensity (R) was 1.21 in the absence of ADP. In most mitochondria, ΔΨm was stable, whereas a small population of mitochondria (<10%) showed transient depolarizations followed by immediate repolarizations. Upon addition of malate (5 mM), the mitochondria became brighter, with R = 1.64 (Fig. 1 A), indicating further polarization of the inner membrane. At the highly polarized state upon addition of malate, most mitochondria underwent rapid and transient depolarizations (Fig. 1 B) and repeated cycles of depolarizations followed by immediate repolarizations (Fig. 1 C). These oscillations are not synchronized among mitochondria, and the patterns of the oscillations significantly depend on individual mitochondria.
FIGURE 1.
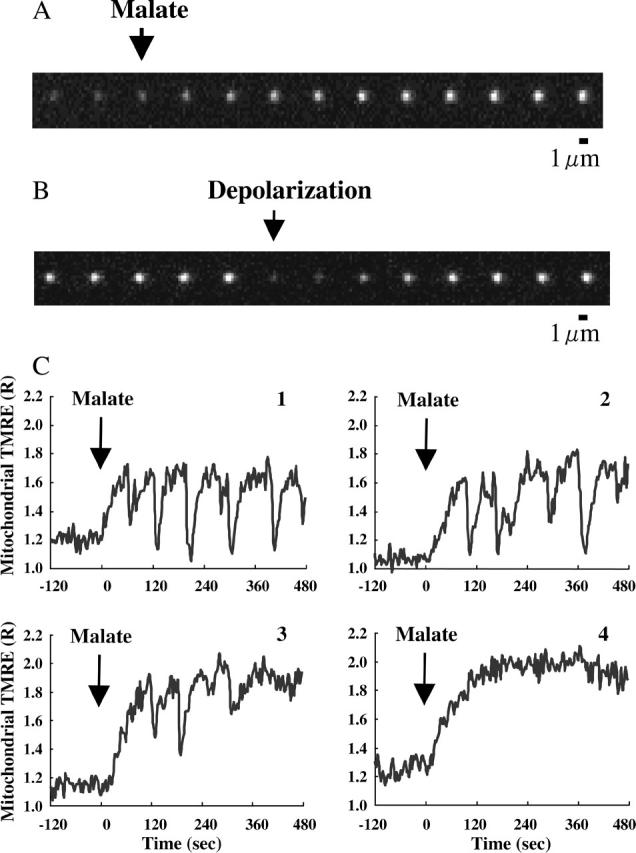
Time-resolved TMRE fluorescence of single mitochondria in response to addition of malate. TMRE fluorescence was monitored in the absence of ADP. (A and B) ΔΨm-dependent fluorescence images of TMRE in single mitochondria. The time interval between images is 3 s. (A) The arrow marks the addition of malate (5 mM). (B) The arrow marks the rapid depolarization observed after addition of malate. (C) The typical responses of ΔΨm observed in single mitochondria to addition of malate. The vertical axis represents the fluorescence intensity of TMRE in single mitochondria, normalized by the TMRE fluorescence in buffer. At t = 0, 5 mM malate was added (arrow). The fluctuations of ΔΨm significantly depend on individual mitochondria. Mitochondria 1–3 are fluctuating, whereas mitochondrion 4 does not show the fluctuation of ΔΨm.
Role of proton pumping for transient depolarizations
Addition of malate to mitochondria leads to the generation of NAD(P)H, and the consequent NADH donates electrons to the mitochondrial electron transfer chain where the electron transfer is coupled to proton pumping. To investigate the mechanisms by which malate induces the transient depolarizations of the inner membrane, we classified the actions of malate into two steps 1), from addition of malate to NAD(P)H accumulation, and 2), the electron transfer and proton pumping. To examine the effects of these two steps on the transient depolarizations separately, we polarized the mitochondrial membrane in the presence of rotenone, an inhibitor of NADH dehydrogenase.
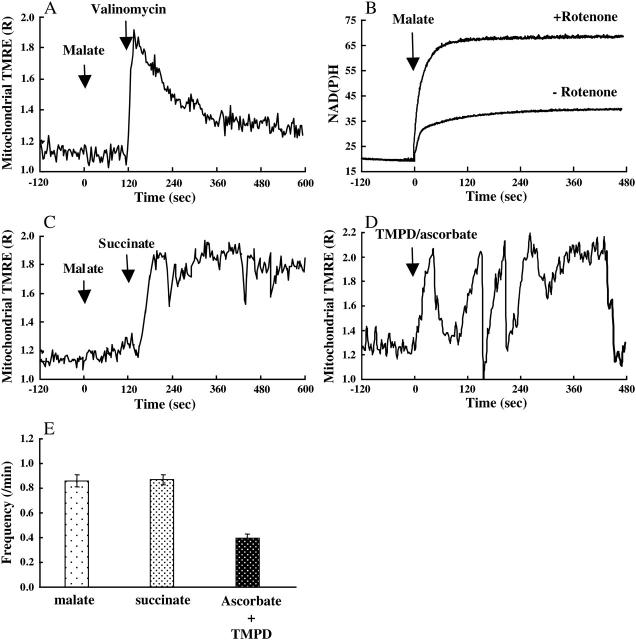
At first, we added malate to accumulate NADH in the presence of rotenone and then polarized the inner membrane without proton translocation by using valinomycin, a potassium ionophore (Nakayama et al., 2002) since addition of valinomycin induces the efflux of potassium ion from the matrix to the buffer surrounding mitochondria under these conditions. Addition of malate (5 mM) did not induce polarization (Fig. 2 A) but did enhance the accumulation of NAD(P)H (Fig. 2 B). Most of the mitochondria did not show the transient depolarizations in this state. Further addition of valinomycin resulted in a rapid polarization of the mitochondrial membrane. The rapid polarization was followed by a gradual depolarization, not by a rapid and transient depolarization (Fig. 2 A). These results indicate that the processes occurring from addition of malate to NAD(P)H accumulation do not induce the transient and rapid depolarizations.
FIGURE 2.
Electron transfer and oscillations of ΔΨm. (A–C) Rotenone (1 μM) was added to mitochondria that were bathed in KCl buffer 10 min before the measurements. (A) The response of ΔΨm observed in single mitochondria. At t = 0, malate was added to accumulate NADH in mitochondria. At t = 120, valinomycin was added to polarize the inner membrane without the pumping of protons. The vertical axis is the same as in Fig. 1 C. (B) Autofluorescence of NAD(P)H in the mitochondria suspended in KCl buffer containing 0.5 mM ADP. At t = 0, malate was added. (C) The time course of succinate-induced polarization of a single mitochondrion. At t = 0, malate was added. At t = 120, succinate was added to polarize the inner membrane by proton pumping. The vertical axis is the same as in Fig. 1 C. (D) Changes in ΔΨm upon addition of ascorbate + TMPD in the presence of antimycin A. (E) Frequencies of the rapid depolarizations of the inner membrane. Frequencies were measured in sucrose buffer in the presence of malate (5 mM), succinate (5 mM) + rotenone (1 μM), or ascorbate (2.5 mM) + TMPD (0.25 mM) + antimycin A (2 μM).
Next, we polarized mitochondria with succinate in the presence of rotenone to examine the effects of proton pumping on the transient depolarizations. Since succinate donates electrons to the electron transfer chain via succinate dehydrogenase, addition of succinate induces the translocation of protons by proton pumps in the electron transfer chain, even in the presence of rotenone. Addition of succinate (5 mM) induced polarization of the inner membrane followed by rapid and transient depolarizations (Fig. 2 C). Also, we observed repetitive transient depolarizations upon addition of ascorbate (2.5 mM) and TMPD (0.25 mM) to mitochondria bathed in the buffer containing 2 μM antimycin A, an inhibitor of complex III in the electron transfer chain (Fig. 2 D). Under this condition only cytochrome c oxidase was able to pump protons from the matrix since addition of malate or succinate was confirmed not to induce polarization. In the presence of azide (5 mM), these respiration substrates (malate, succinate, and ascorbate + TMPD) did not polarize mitochondria. These results indicate that the translocation of protons by proton pumps plays a significant role in the transient depolarizations.
Definition of rapid depolarizations
The characteristics of malate-induced changes in ΔΨm were the rapid and transient depolarizations due to the translocation of protons. For further characterization, we analyzed the normalized fluorescence intensity (R) before addition of malate in the absence of ADP to clarify the level of noise. The amplitude of the noise in R was <0.17. Therefore, decreases in R having a minimal amplitude of 0.18 were defined as rapid depolarizations under these conditions. It should be noted that the rate (0.18 per frame) of the decrease in R was much larger than the rate of the decrease that was observed in mitochondria that were polarized with valinomycin (Fig. 2 A).
Frequency of rapid and transient depolarizations
Since the translocation of protons across the inner membrane induced transient depolarizations, we examined whether the activation or suppression of proton translocation changes the frequency of the rapid and transient depolarizations (Fig. 3 A). The frequency was counted with a computer program written in Visual Basic for Microsoft Excel. Before comparing the frequencies of the rapid depolarizations under several conditions, we confirmed that the percentages of the polarized mitochondria upon addition of malate did not depend on the conditions tested. This percentage was ∼80%.
FIGURE 3.

Frequencies of the rapid depolarizations of the inner membrane. (A) Effects of malate, ADP (0.5 mM) or oligomycin (1 μM) on the frequencies of the rapid depolarizations. Frequencies were measured in sucrose buffer at pH 7.4. Values represent the mean ± SE (n > 100). *, P < 0.05 vs. 5 mM malate without ADP and oligomycin. (B) Effects of pH on the frequencies. The frequencies were measured in the presence of 5 mM malate with neither ADP nor oligomycin. Values represent the mean ± SE (n > 100). *, P < 0.05 vs. pH 7.4. (C) Effects of CsA (1 μM) and BA (2 μM) on the frequencies. The control was measured in the presence of 5 mM malate with neither ADP nor oligomycin at pH 7.4. Values represent the mean ± SE (n > 100).
At first, we measured the frequencies of the rapid depolarizations in the presence of 0.2, 1, or 5 mM malate. As the malate concentration was increased from 0.2 mM to 5 mM, the frequency of the rapid and transient depolarizations increased from 0.41 ± 0.02/min (0.2 mM) to 0.86 ± 0.05 (5 mM) in the absence of ADP (Fig. 3 A). Since the increase in the concentration of malate should lead to the activation of proton translocation, this result suggests that the efflux of protons from the matrix stimulates the rapid and transient depolarizations.
Next, we assessed the effects of ADP on the rapid and transient depolarizations. The presence of ADP (0.5 mM) drastically decreased the measured frequency from 0.86 ± 0.05 to 0.17 ± 0.02 in the presence of 5 mM malate (Fig. 3 A). Oligomycin, an inhibitor of FoF1-ATPase, counteracted the ADP effects on the frequency. Since ADP allows protons to reenter the matrix through FoF1-ATPase, and since oligomycin blocks the entry, these findings indicate that the rapid and transient depolarizations are significantly activated by a net efflux of protons that results in the increase in pH beneath the inner membrane. It should be noted that we confirmed that the presence of oligomycin completely blocked ATP synthesis under these conditions and that oligomycin had no effects on the frequency in the absence of ADP.
Third, we changed the pH of the buffer to activate or suppress the translocation of protons from the matrix. For this purpose, we replaced the buffer at pH 7.4 with buffer at an appropriate pH just before the experiments. At a low pH, the translocation of protons should be suppressed due to the high concentration of protons in the buffer, and the proton translocation should be enhanced at a high pH. We measured the frequency of the rapid and transient depolarizations at pH 6.9, 7.4, and 8.3. The result indicated that the frequency increases significantly with pH of the buffer (Fig. 3 B). These results also suggest that the net translocation of protons from the matrix enhances the rapid and transient depolarizations.
Effects of Ca2+ and Mg2+ on the membrane potential
Ca2+ and Mg2+ have significant effects on ΔΨm (Aon et al., 2003; Bernardi et al., 1992; Rizzuto et al., 1998; Robb-Gaspers et al., 1998). For further characterization of the observed oscillations, therefore, we measured the effects of Ca2+ and Mg2+ on the oscillations of ΔΨm upon addition of 5 mM malate. The concentrations of Ca2+ and Mg2+ were adjusted with 1 mM EGTA and 1 mM EDTA, respectively. Mg2+ significantly suppressed the oscillations of ΔΨm. The frequency of the oscillations did not change when the concentration of Mg2+ was between 40 and 500 μM (Fig. 4 A). Different from Mg2+, the increase in Ca2+ concentration significantly decreased the percentage of polarized mitochondria (Fig. 4 B). In polarized mitochondria in the presence of Ca2+, oscillations of ΔΨm were observed and did not cease during observation for 8 min. Based on these results, we performed experiments in the absence of Ca2+ and Mg2+ to eliminate the effects of these divalent cations on the cycles, if not mentioned.
FIGURE 4.
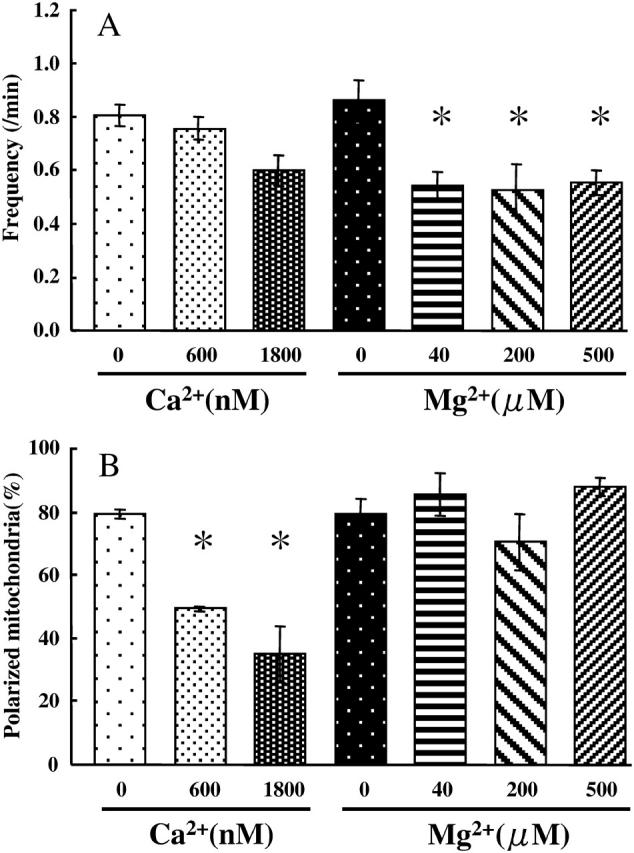
Effects of Ca2+ and Mg2+ on ΔΨm. The changes in ΔΨm were measured in the presence of 5 mM malate. The concentrations of Ca2+ and Mg2+ were adjusted with 1 mM EGTA and 1 mM EDTA, respectively. (A) Effects of Ca2+ and Mg2+ on the frequencies of the oscillations. Values represent the mean ± SE (n > 100). *, P < 0.05 vs. 0 μM Mg2+. (B) Effects of Ca2+ and Mg2+ on the percentages of mitochondria polarized upon addition of 5 mM malate. *, P < 0.05 vs. 0 μM Ca2+.
Effects of oxidative stress on the oscillations of the membrane potential
Mitochondria produce ROS with the electron transfer chain. In addition, rhodamine-based ΔΨm probes produce ROS upon illumination. Therefore, the ROS produced might induce the observed oscillations since ROS are reported to destabilize ΔΨm (Aon et al., 2003; Hüser et al., 1998; Jacobson and Duchen, 2002). To examine ROS effects on the oscillations, we first examined whether the presence of catalase (1500 units/ml) or GSH (2 mM) suppressed the oscillations. Results are shown in Fig. 5 A. These scavengers of H2O2 did affect neither the frequency of the oscillations nor the percentage of polarized mitochondria upon addition of 5 mM malate. In addition, TMPyP (250 μM), a membrane-permeant superoxide anion scavenger (Faulkner et al., 1994; Gardner et al., 1996), did not change the frequency of the oscillations and the percentage of polarized mitochondria. Next, we added H2O2 to sucrose buffer and measured the frequency of the oscillations evoked by 1 mM malate. Results are shown in Fig. 5 B. The oscillations were not enhanced by the presence of 100 or 1000 nM H2O2. The percentage of polarized mitochondria was not decreased by 100 nM H2O2, although 1000 nM H2O2 decreased the percentage by 10% (data not shown). The concentration of H2O2 produced by mitochondria in the absence of TMRE was <1 nM under these conditions. These results clearly show that the bulk H2O2 around mitochondria does not affect the oscillations of ΔΨm.
FIGURE 5.
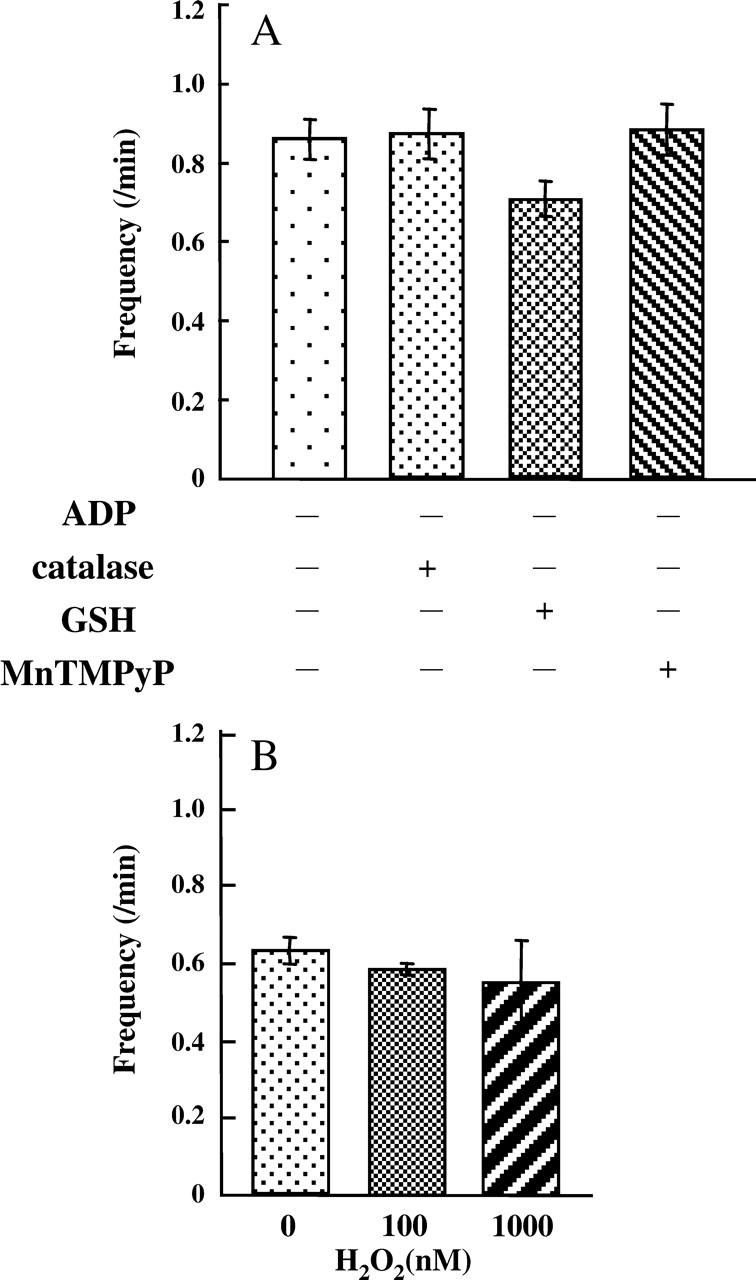
ROS effects on oscillations of ΔΨm. (A) The effects of catalase, GSH, and TMPyP on oscillations of ΔΨm evoked by 5 mM malate. (B) The effects of H2O2 on oscillations of ΔΨm evoked by 1 mM malate. Values represent the mean ± SE (n > 100).
Calcein release from mitochondria
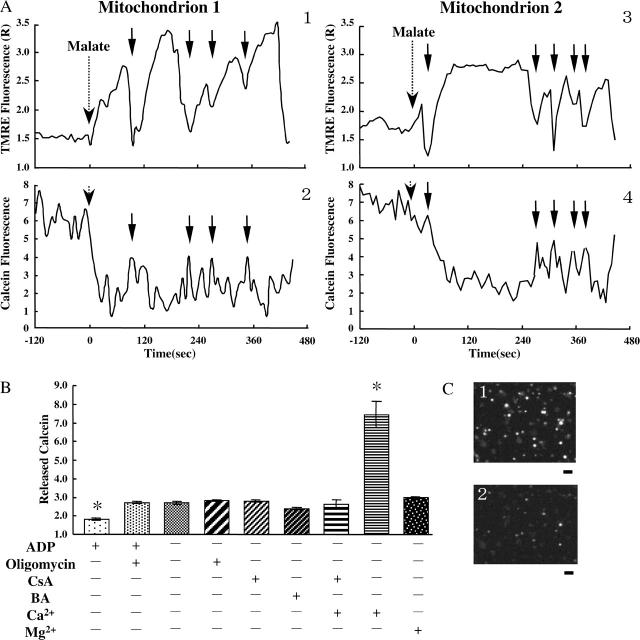
MPT is the increase in the permeability of the inner membrane to solutes up to 1.5 kDa (Hunter and Haworth, 1979; Ichas and Mazat, 1998; Petronilli et al., 1999). Since the rapid depolarization appears to require the opening of channels or pores with a large conductance, mitochondria might undergo MPT upon the rapid depolarizations. To examine whether or not the rapid depolarizations were due to MPT, we assayed the permeability of the inner membrane to calcein (MW 623) upon the rapid depolarizations by simultaneously observing calcein fluorescence and TMRE fluorescence in individual mitochondria. Results are shown in Fig. 6 A. Calcein fluorescence was significantly affected by the accumulation of TMRE in polarized mitochondria. Accumulation of TMRE in mitochondria induced the decrease in calcein fluorescence. In a similar way, the release of TMRE from mitochondria upon the depolarization resulted in the increase in calcein fluorescence. This is because the fluorescence spectrum of calcein overlaps the absorption spectrum of TMRE. For these reasons, we compared the levels of calcein fluorescence upon rapid depolarization. Calcein fluorescence recovered to the same level upon each depolarization, suggesting the amount of calcein released upon the rapid depolarizations was zero or infinitesimal.
FIGURE 6.
Calcein fluorescence inside and outside mitochondria. (A) Time-resolved TMRE (1, 3) and calcein (2, 4) fluorescence in single mitochondria. TMRE and calcein fluorescence was simultaneously monitored in the same mitochondrion. Typical behaviors observed for two different mitochondria (Mitochondrion 1 and 2) are shown. The vertical axes for TMRE fluorescence are the same as those of Fig. 1 C. The vertical axes for calcein fluorescence are shown in arbitrary unit. Malate was added at t = 0. Arrows show the time when the rapid depolarizations occurred. (B) Release of calcein under the various conditions. The vertical axis represents the calcein release from mitochondria to the medium. The detailed explanation of the vertical axis is described in the text. Values represent the mean ± SE (n > 3). *, P < 0.05 versus the sample without ADP, oligomycin, CsA, BA, Ca2+, and Mg2+. (C) Fluorescence images of calcein entrapped in individual mitochondria in the same microscopic field. Bar, 5 μm. (1) Before addition of malate; (2) after incubation with malate in the absence of ADP.
For further examination, we observed the fluorescence of calcein released from the matrix to the buffer during the incubation for 10 min with 5 mM malate. We measured calcein fluorescence in the supernatant upon addition of malate and after 10 min incubation. Since calcein fluorescence upon addition of malate is considered to be proportional to the amount of mitochondria attached on the dish, we calculated the ratio of the calcein fluorescence after 10 min incubation to that upon addition of malate for each dish and regarded this ratio as a measure of the calcein release from mitochondria. Results are shown in Fig. 6 B, and the vertical axis of Fig. 6 B is the ratio. Addition of 50 μM Ca2+ to mitochondria significantly enhanced the calcein release, and the enhancement of the calcein release was blocked by 1 μM CsA. These results indicate that MPT can be detected by using this technique. Calcein release from mitochondria in the absence of Ca2+ was much smaller than that upon MPT induced by Ca2+. Also, neither CsA nor BA suppressed the release of calcein in the absence of Ca2+. These results support the idea that the observed slight release of calcein might be induced with a different mechanism from MPT.
Almost all mitochondria partially released calcein to the supernatant during the incubation with malate (Fig. 6 C, 1 and 2). When the buffer without malate was added, the decrease in the intensity of calcein fluorescence in individual mitochondria was significantly suppressed (data not shown). In addition, the presence of ADP significantly suppressed the malate-induced slight release of calcein from mitochondria in the absence of oligomycin. These results suggest that calcein release from mitochondria was affected by malate and ADP.
DISCUSSION
In this study we found that 1), ascorbate + TMPD, malate, or succinate-induced polarization of the inner mitochondrial membrane is followed by cycles of rapid depolarizations and immediate repolarizations; 2), the frequency of the cycles increases when the net translocation of protons from the matrix to the intermembrane space is accelerated; 3), the rapid depolarizations do not occur when the inner membrane is polarized by the efflux of potassium from the matrix; 4), scavengers of ROS do not affect the frequency of the cycles; and 5), the amount of calcein released from mitochondria upon a single depolarization is infinitesimal.
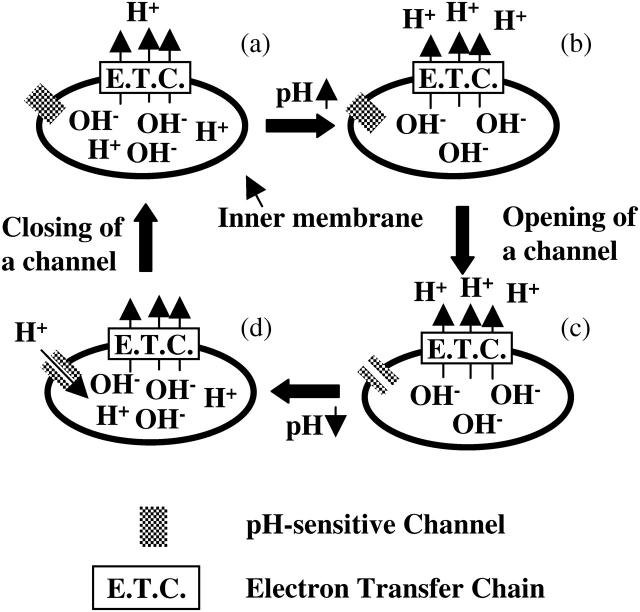
We observed that mitochondria repeat the cycles of rapid depolarizations and immediate repolarizations. Although these repetitive transient depolarizations have been reported both in cultured cells and isolated mitochondria (Collins et al., 2002; De Giorgi et al., 2000; Hüser et al., 1998; Jacobson and Duchen, 2002; Nakayama et al., 2002; Vergun et al., 2003), so far the reason the transient depolarizations occur repetitively has not been elucidated. We found that the transient depolarizations were significantly stimulated by the net translocation of protons from the matrix to the intermembrane space. The observed transient depolarizations should be due to the brief opening of channels or pores in the inner membrane. Based on these observations, we propose a model that can explain why the depolarizations are transient and occur repetitively (Fig. 7). According to our model, at first, the pH beneath the inner membrane increases due to the translocation of protons when the channels are closed. Next, openings of the channels are caused by an increase in the pH. Then, protons reenter the matrix though the channels and the pH beneath the inner membrane decreases, resulting in depolarization of the inner membrane. Finally, the channels close due to the decrease in the pH, and the membrane is polarized again by proton pumping. These cycles, however, will be observed infrequently under the physiological conditions. In this study, to analyze the repeated cycles of depolarizations and repolarizations, we rapidly increased the concentration of substrates around mitochondria. If the influx of protons into the matrix is suppressed, the rapid increase in the substrate concentration leads to alkalinization of the matrix, which will induce the depolarization of mitochondria. Under the intracellular physiological conditions, however, such a rapid increase in the substrate concentration does not occur. In addition, ADP and Mg2+ that suppress the oscillations are present in the cytosol. Therefore, the oscillations will not occur frequently under the physiological conditions. Nevertheless, we should mention that it has been reported that individual mitochondria show fluctuations of the membrane potential in cells (Jacobson and Duchen, 2002; Loew et al., 1993) and that the mechanism of the fluctuations remain elusive. Although the physiological role of the alkalinization-induced depolarization is unclear, depolarization is reported to reduce the generation of ROS (Arsenijevic et al., 2000; Nishikawa et al., 2000). Since the conditions for the alkalinization of the matrix by proton pumping is closely associated to the conditions for the enhancement of ROS generation from the electron transport chain, it might be speculated that the observed transient depolarization suppresses the generation of ROS.
FIGURE 7.
Schematic illustration of the proposed model for transient and repetitive depolarizations. (a) Proton pumps in the electron transfer chain translocate protons from the matrix to the intermembrane space. (b) The pH beneath the inner membrane is increased by proton pumping. (c) Opening of a pH sensitive and proton conductive channel is induced by the increase in the pH. (d) Reentry of protons through the channel decreases the pH, resulting in closing of the channel.
Since some rhodamine-based ΔΨm probes produce ROS upon illumination, it was suggested that the fluctuation of ΔΨm observed with these probes at a high concentration was caused by oxidative damage to the mitochondria (Hüser et al., 1998; Jacobson and Duchen, 2002). Under the conditions described here, however, this appears not to be the case, for the following two reasons: 1), the rapid depolarizations did not occur when mitochondria were polarized with the efflux of K+ in the presence of 2 nM TMRE. This concentration was equal to or <1% of the concentration used in previous experiments (Hüser et al., 1998; Hüser and Blatter, 1999; Jacobson and Duchen, 2002); and 2), catalase, GSH, H2O2, and TMPyP did not affect the frequency of the oscillations when they were exogenously added. Recently, Vergun and colleagues reported that fluctuations of ΔΨm in the presence of rhodamine 123 were not suppressed by antioxidants (Vergun et al., 2003). Their observation is also consistent with our results showing that the fluctuation of ΔΨm is not due to oxidative damage of mitochondria upon light exposure.
The oscillations of ΔΨm were enhanced under the conditions in which Mg2+ was absent and matrix pH was alkaline. Since the IMAC is activated under the precise conditions (Beavis and Powers, 1989) and is proposed to mediate oscillations of ΔΨm (Aon et al., 2003), IMAC might be involved in the observed oscillations. To examine the involvement of IMAC, we measured the oscillations in the presence of 4,4′-diisothiocyannostilbene-2,2′-disulfonate (DIDS), an inhibitor of IMAC (Beavis and Davatol-Hag, 1996). DIDS did not suppress the oscillations at 75 μM although IC50 is 25.7 μM. In addition, 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide (PK11195), another inhibitor of IMAC, did not affect the oscillations at 50 μM. Therefore, IMAC is not likely to be involved in the observed oscillations of ΔΨm.
Hüser and colleagues reported the involvement of MPT in the rapid depolarizations in ventricular mitochondria, based on their observation that fluorescence of calcein entrapped in mitochondria suddenly decreased upon the initiation of rapid depolarizations (Hüser et al., 1998). On the other hand, Vergun and colleagues concluded, based on studies with brain mitochondria, that the transient depolarizations were not due to MPT. Their conclusion was based on the observation that CsA did not suppress the rapid depolarizations and that mitochondria did not show a stochastic loss of calcein fluorescence (Vergun et al., 2003). When we made simultaneous imaging of TMRE fluorescence and calcein fluorescence in single mitochondria, significant release of calcein from mitochondria was not observed upon the rapid depolarizations. Since MPT is the increase in the permeability of the inner membrane to solutes up to 1.5 kDa, the release of calcein (MW 623) from mitochondria should be observed if MPT occurred. Based on the above observation, we consider that the rapid and transient depolarizations might occur by a mechanism different from MPT pore opening. The fact that the potent inhibitors of MPT (CsA and BA) did not suppress the depolarizations also supports that MPT might not be involved in the depolarizations. Although we did not observe the calcein release upon the depolarizations, we observed the increase in calcein fluorescence in the supernatant of mitochondria and the significant decrease in calcein fluorescence in individual mitochondria. These results indicate that calcein leaked from mitochondria during incubation with malate. The leakage of calcein from the matrix through the lipid bilayer might have been enhanced when mitochondria were polarized since calcein has negative charges. This might explain why ADP suppressed the release of calcein from mitochondria and why Mg2+ did not suppress the calcein release although it suppressed the oscillations of ΔΨm. It should be noted that calcein was released from almost all mitochondria during incubation with malate (Fig. 6 C, 1 and 2), indicating that calcein in the supernatant derived from intact mitochondria because mitochondria used in this study showed a high activity of ATP synthesis with 415 ± 17 μmol ATP/g of protein/min and because ∼80% of mitochondria were highly polarized upon addition of malate.
On the other hand, the possibility of the involvement of MPT in the rapid and transient depolarizations can not be excluded. The amount of calcein released from mitochondria during incubation with malate in the absence of ADP was small, being ∼⅓ the amount of calcein released from mitochondria in which long-lasting MPT was induced by 50 μM Ca2+ (Fig. 6 B). This may be because the duration of the transiently opened state of MPT pores is not long enough for mitochondria to release large amounts of calcein. The amount of calcein in the buffer correlated with the frequency of the rapid depolarizations, also suggesting that the rapid and transient depolarizations might be due to transient MPT. Neither CsA nor BA inhibited the transient depolarizations induced by proton pumping. Also, the transient depolarizations occurred independently of Ca2+. These results indicate that the characteristics of the observed transient depolarizations are different from the well-known characteristics of MPT. On the other hand, there have been several reports showing that CsA or BA does not always block the permeability transition (Brustovetsky and Dubinsky, 2000; Chinopoulos et al., 2003; Gudz et al., 1997; Sultan and Sokolove, 2001). The detailed mechanisms of MPT remain elusive. It should be noted that MPT can occur even in the absence of ANT (Kokoszka et al., 2004) although CsA, Ca2+, and BA are considered to affect MPT through the action on ANT (Halestrap et al., 2002).
In summary, the main conclusion of this study is that the rapid depolarizations of inner mitochondrial membranes probably result from transient openings of a pH sensitive and proton conductive channel. This occurs when the pH beneath the inner membrane is rapidly increased by the combination of accelerated proton pumping and slow proton influx. The measurements with ROS scavengers show that these transient depolarizations are not due to the oxidative damage induced by ROS. The involvement of MPT in the pH sensitive and proton conductive channel remains elusive. These results represent significant progress in our understanding of mitochondrial behavior. Further details about the roles of particular proteins and the specific mechanisms involved in the pH-dependent transient depolarizations remain to be elucidated.
Acknowledgments
We thank Prof. Shigeki Mitaku (Nagoya University) for helpful discussions.
This work was funded by grants from the Japan Ministry of Education, Science, and Culture.
Abbreviations used: ΔΨm, mitochondrial membrane potential; ANT, adenine nucleotide translocator; BA, bongkrekic acid; BCA, bicinchoninic acid; BSA, bovine serum albumin; calcein-AM, calcein acetoxymethyl ester; CsA, cyclosporin A; DTT, dithiothreitol; GSH, reduced glutathione; IMAC, mitochondrial inner membrane anion channel; MPT, mitochondrial permeability transition; ROS, reactive oxygen species; TMPD, tetramethyl-p-phenylenediamine; TMPyP, Mn(III)tetrakis(1-methyl-4-pyridil)porphyrin pentachloride.
References
- Aon, M. A., S. Cortassa, E. Marban, and B. O'Rourke. 2003. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 278:44735–44744. [DOI] [PubMed] [Google Scholar]
- Arsenijevic, D., H. Onuma, C. Pecqueur, S. Raimbault, B. S. Manning, B. Miroux, E. Couplan, M.-C. Alves-Guerra, M. Goubern, R. Surwit, F. Bouillaud, D. Richard, and others. 2000. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 26:435–439. [DOI] [PubMed] [Google Scholar]
- Baracca, A., G. Sgarbi, G. Solaini, and G. Lenaz. 2003. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F(0) during ATP synthesis. Biochim. Biophys. Acta. 1606:137–146. [DOI] [PubMed] [Google Scholar]
- Beavis, A. D., and H. Davatol-Hag. 1996. The mitochondrial inner membrane anion channel is inhibited by DIDS. J. Bioenerg. Biomembr. 28:207–214. [DOI] [PubMed] [Google Scholar]
- Beavis, A. D., and M. F. Powers. 1989. On the regulation of the mitochondrial inner membrane anion channel by magnesium and protons. J. Biol. Chem. 264:17148–17155. [PubMed] [Google Scholar]
- Bernardi, P. 1992. Modulation of the mitochondrial cyclosporin A-sensitive permeability transition pore by the proton electrochemical gradient. Evidence that the pore can be opened by membrane depolarization. J. Biol. Chem. 267:8834–8839. [PubMed] [Google Scholar]
- Bernardi, P., S. Vassanelli, P. Veronese, R. Colonna, I. Szabó, and M. Zoratt. 1992. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J. Biol. Chem. 267:2934–2939. [PubMed] [Google Scholar]
- Boitier, E., R. Rea, and M. R. Duchen. 1999. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J. Cell Biol. 145:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky, N., and J. M. Dubinsky. 2000. Dual responses of CNS mitochondria to elevated calcium. J. Neurosci. 20:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon, E., J. M. Reece, A.-L. Nieminen, G. Zahrebelski, B. Herman, and J. J. Lemaster. 1994. Distribution of electrical potential, pH, free Ca2+, and volume inside cultured adult rabbit cardiac myocytes during chemical hypoxia: a multiparameter digitized confocal microscopic study. Biophys. J. 66:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance, B., and T. Yoshioka. 1966. Sustained oscillations of ionic constituents of mitochondria. Arch. Biochem. Biophys. 117:451–465. [DOI] [PubMed] [Google Scholar]
- Chinopoulos, C., A. A. Starkov, and G. Fiskum. 2003. Cyclosporin A-insensitive permeability transition in brain mitochondria inhibition by 2-aminoethoxydiphenyl borate. J. Biol. Chem. 278:27382–27389. [DOI] [PubMed] [Google Scholar]
- Collins, T. J., M. J. Berridge, P. Lipp, and M. D. Bootman. 2002. Mitochondria are morphologically and functionally heterogeneous within cells. EMBO J. 21:1616–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgi, F., L. Lartigue, and F. Ichas. 2000. Electrical coupling and plasticity of the mitochondrial network. Cell Calcium. 28:365–370. [DOI] [PubMed] [Google Scholar]
- Duchen, M. R., A. Leyssens, and M. Crompton. 1998. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J. Cell Biol. 142:975–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner, K. M., S. I. Liochev, and I. Fridovich. 1994. Stable Mn(III) porphyrins mimic superoxide dismutase in vitro and substitute for it in vivo. J. Biol. Chem. 269:23471–23476. [PubMed] [Google Scholar]
- Gardner, P. R., D. D. Nguyen, and C. W. White. 1996. Superoxide scavenging by Mn(II/III) tetrakis (1-methyl-4-pyridyl) porphyrin in mammalian cells. Arch. Biochem. Biophys. 325:20–28. [DOI] [PubMed] [Google Scholar]
- Gooch, V. D., and L. Packer. 1974. Oscillatory systems in mitochondria. Biochim. Biophys. Acta. 346:245–260. [DOI] [PubMed] [Google Scholar]
- Gudz, T., O. Eriksson, Y. Kushnareva, N. E. Saris, and S. Novgorodov. 1997. Effect of butylhydroxytoluene and related compounds on permeability of the inner mitochondrial membrane. Arch. Biochem. Biophys. 342:143–156. [DOI] [PubMed] [Google Scholar]
- Halestrap, A. P., G. P. McStay, and S. J. Clarke. 2002. The permeability transition pore complex: another view. Biochimie. 84:153–166. [DOI] [PubMed] [Google Scholar]
- Hunter, D. R., and R. A. Haworth. 1979. The Ca2+-induced membrane transition in mitochondria. III. Transitional Ca2+ release. Arch. Biochem. Biophys. 195:468–477. [DOI] [PubMed] [Google Scholar]
- Hüser, J., and L. A. Blatter. 1999. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem. J. 343:311–317. [PMC free article] [PubMed] [Google Scholar]
- Hüser, J., C. E. Rechenmacher, and L. A. Blatter. 1998. Imaging the permeability pore transition in single mitochondria. Biophys. J. 74:2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichas, F., L. S. Jouaville, and J.-P. Mazat. 1997. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 89:1145–1153. [DOI] [PubMed] [Google Scholar]
- Ichas, F., and J.-P. Mazat. 1998. From calcium signaling to cell death: two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim. Biophys. Acta. 1366:33–50. [DOI] [PubMed] [Google Scholar]
- Jacobson, J., and M. R. Duchen. 2002. Mitochondrial oxidative stress and cell death in astrocytes—requirement for stored Ca2+ and sustained opening of the permeability transition pore. J. Cell Sci. 115:1175–1188. [DOI] [PubMed] [Google Scholar]
- Kokoszka, J. E., K. G. Waymire, S. E. Levy, J. E. Sligh, J. Cai, D. P. Jones, G. R. MacGregor, and D. C. Wallace. 2004. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 427:461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss, S., C. Y. Zhang, and B. B. Lowell. 2002. A significant portion of mitochondrial proton leak in intact thymocytes depends on expression of UCP2. Proc. Natl. Acad. Sci. USA. 99:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew, L. M., W. Carrington, R. A. Tuft, and F. S. Fay. 1994. Physiological cytosolic Ca2+ transients evoke concurrent mitochondrial depolarizations. Proc. Natl. Acad. Sci. USA. 91:12579–12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew, L. M., R. A. Tuft, W. Carrington, and F. S. Fay. 1993. Imaging in five dimensions: time-dependent membrane potentials in individual mitochondria. Biophys. J. 65:2396–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, S., T. Sakuyama, S. Mitaku, and Y. Ohta. 2002. Fluorescence imaging of metabolic responses in single mitochondria. Biochem. Biophys. Res. Commun. 290:23–28. [DOI] [PubMed] [Google Scholar]
- Nishikawa, T., D. Edelstein, X. L. Du, S. Yamagishi, T. Matsumura, Y. Kaneda, M. A. Yorek, D. Beebe, P. J. Oates, H.-P. Hammes, I. Giardino, and M. Brownlee. 2000. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 404:787–790. [DOI] [PubMed] [Google Scholar]
- Palmer, J. W., B. Tander, and C. L. Hoppel. 1977. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 252:8731–8739. [PubMed] [Google Scholar]
- Petronilli, V., G. Miotto, M. Canton, M. Brini, R. Colonna, P. Bernardi, and F. Di Lisa. 1999. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys. J. 76:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricquier, D., and F. Bouillaud. 2000. Mitochondrial uncoupling proteins: from mitochondria to the regulation of energy balance. J. Physiol. 529:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto, R., P. Pinton, W. Carrington, F. S. Fay, K. E. Fogarty, L. M. Lifshitz, R. A. Tuft, and T. Pozzan. 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 280:1763–1766. [DOI] [PubMed] [Google Scholar]
- Robb-Gaspers, L. D., P. Burnett, G. A. Rutter, R. M. Denton, R. Rizzuto, and A. P. Thomas. 1998. Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 17:4987–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selivanov, V. A., F. Ichas, E. L. Holmuhamedov, L. S. Jouaville, Y. V. Evtodienko, and J. P. Mazat. 1998. A model of mitochondrial Ca2+-induced Ca2+ release simulating the Ca2+ oscillations and spikes generated by mitochondria. Biophys. Chem. 72:111–121. [DOI] [PubMed] [Google Scholar]
- Smiley, S. T., M. Reers, C. Mottola-Hartshorn, M. Lin, A. Chen, T. W. Smith, G. D. Steele Jr., and L. B. Chen. 1991. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. USA. 88:3671–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan, A., and P. M. Sokolove. 2001. Palmitic acid opens a novel cyclosporin A-insensitive pore in the inner mitochondrial membrane. Arch. Biochem. Biophys. 386:37–51. [DOI] [PubMed] [Google Scholar]
- Vergun, O., T. V. Votyakova, and I. J. Reynolds. 2003. Spontaneous changes in mitochondrial membrane potential in single isolated brain mitochondria. Biophys. J. 85:3358–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibom, R., and E. Hultman. 1990. ATP production rate in mitochondria isolated from microsamples of human muscle. Am. J. Physiol. 259:E204–E209. [DOI] [PubMed] [Google Scholar]
- Wibom, R., A. Lundin, and E. Hultman. 1990. A sensitive method for measuring ATP-formation in rat muscle mitochondria. Scand. J. Lab. Invest. 50:143–152. [DOI] [PubMed] [Google Scholar]
- Zoratti, M., and I. Szabó. 1994. Electrophysiology of the inner mitochondrial membrane. J. Bioenerg. Biomembr. 26:543–553. [DOI] [PubMed] [Google Scholar]
- Zorov, D. B., C. R. Filburn, L.-O. Klotz, J. L. Zweier, and S. J. Sollott. 2000. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 192:1001–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]