Abstract
Nonphotochemical quenching (NPQ) of excitation energy, which protects higher plant photosynthetic machinery from photodamage, is triggered by acidification of the thylakoid lumen as a result of light-induced proton pumping, which also drives the synthesis of ATP. It is clear that the sensitivity of NPQ is modulated in response to changing physiological conditions, but the mechanism for this modulation has remained unclear. Evidence is presented that, in intact tobacco or Arabidopsis leaves, NPQ modulation in response to changing CO2 levels occurs predominantly by alterations in the conductivity of the CFO-CF1 ATP synthase to protons (g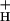 ). At a given proton flux, decreasing g
). At a given proton flux, decreasing g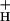 will increase transthylakoid proton motive force (pmf), thus lowering lumen pH and contributing to the activation of NPQ. It was found that an ≈5-fold decrease in g
will increase transthylakoid proton motive force (pmf), thus lowering lumen pH and contributing to the activation of NPQ. It was found that an ≈5-fold decrease in g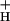 could account for the majority of NPQ modulation as atmospheric CO2 was decreased from 2,000 ppm to 0 ppm. Data are presented that g
could account for the majority of NPQ modulation as atmospheric CO2 was decreased from 2,000 ppm to 0 ppm. Data are presented that g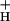 is kinetically controlled, rather than imposed thermodynamically by buildup of ΔGATP. Further results suggest that the redox state of the ATP synthase γ-subunit thiols is not responsible for altering g
is kinetically controlled, rather than imposed thermodynamically by buildup of ΔGATP. Further results suggest that the redox state of the ATP synthase γ-subunit thiols is not responsible for altering g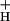 . A working model is proposed wherein g
. A working model is proposed wherein g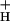 is modulated by stromal metabolite levels, possibly by inorganic phosphate.
is modulated by stromal metabolite levels, possibly by inorganic phosphate.
Keywords: violaxanthin deepoxidase‖photoinhibition‖xanthophyll cycle‖ proton motive force‖chemiosmotic coupling
Light-driven transthylakoid proton motive force (pmf) serves two essential roles in higher plant photosynthesis (1). First, it is the central intermediate in the chemiosmotic circuit for light-driven ATP synthesis. Light-driven electron transfer leads to the pumping of protons from the stroma to the thylakoid lumen, establishing pmf, which drives the endergonic synthesis of ATP from ADP and orthophosphate (Pi) at the CFO-CF1 ATP synthase (ATP synthase).
Second, the ΔpH component of pmf is the key regulatory signal for initiation of nonphotochemical quenching (NPQ) of excitation energy, which is important for photoprotection. Light absorption by the light-harvesting complexes (LHCs) in excess of that which can be processed can lead to harmful side reactions, collectively termed photoinhibition, that can occur at several levels, including the antenna complexes, the oxidizing and reducing sides of photosystem II (PS II), and the reducing side of photosystem I (PS I) (see reviews in refs. 2 and 3).
In higher plants, photoinhibition is avoided in part by activation of NPQ, which can dump a large fraction of excitation energy, preventing the accumulation of reactive intermediates (see reviews in refs. 4 and 5–7). It is now generally accepted that NPQ involves two processes activated by acidification of the lumen, the interconversion of xanthophyll cycle carotenoids by violaxanthin deepoxidase (VDE), and the protonation of residues on key LHC components, in particular the psbS subunit (reviewed in ref. 7). Arabidopsis mutants deficient in NPQ are light sensitive, confirming its role in photoprotection (e.g., refs. 8 and 9–12).
In the most basic working model for NPQ function (e.g., figure 2 of reference 12), where the kinetic and thermodynamic properties of each step in the process are consistent, NPQ should be a continuous function of linear electron flow (LEF). On the other hand, it has become clear that the relationship between LEF and NPQ is strongly modulated in response to rapid changes in physiological state, and we provide a direct demonstration of this below. It has been suggested that such NPQ modulation represents an important response to varying physiological states (e.g., 13–16). Heber and Walker (17) and Asada and coworkers (18) have pointed out that NPQ modulation would be particularly important to prevent photodamage under conditions where photosynthesis becomes limited by the availability of oxidized nicotinamide-adenine dinucleotide phosphate (NADP+). In the absence of NPQ modulation, buildup of reduced electron carriers would block LEF before the lumen could be significantly acidified. This overreduction could result in the formation of a stable, doubly-reduced (plastohydroquinone) QA species in PS II, allowing the formation of triplet chlorophyll species, which in turn can react with O2 to form singlet oxygen (1O2), a highly toxic species (19, 20). There is evidence that buildup of reduced FeS clusters under similar conditions can lead to destruction of PS I reaction centers (21).
There are strong indications that NPQ modulation plays a more general role in regulating photosynthesis (e.g., refs. 22 and 23). Under most conditions, high intensity illumination does not lead to complete reduction of QA in PS II, indicating that light input is decreased as electron transfer nears saturation (23). The ratios of [ATP]/[ADP] or [NADP]/[NADP+] change little even when photosynthetic rates are changed dramatically by either altering [CO2] (e.g., ref. 24), or light intensity (e.g., ref. 25), implying that output from the light reactions and its consumption by the Calvin cycle are tightly coregulated. Furthermore, the pH of the lumen appears to be tightly regulated to a narrow range, where the stabilities of luminal components and the effective rate constants for electron transfer are near optimal (1). Taken together, these observations indicate that a primary regulatory step governing light energy conversion must occur at light capture, and thus likely involves NPQ, rather than at downstream electron or proton transfer reactions. The fact that feedback regulation responds well to both light and CO2 limitations implies that the sensitivity of NPQ with respect to LEF is highly variable.
There are three competing models that could account for NPQ modulation. First, the pH responses of NPQ, at the levels of the VDE and the LHCs, could be altered. Such modulation could be accomplished, for example, by altering the pKas of protonatable groups on specific LHCs thought to control the dissipation of excitation energy. Alternatively, components in the membrane could be modulated, thereby affecting the propensity of LHCs to aggregate or associate with the xanthophyll components (as suggested in ref. 26–29). Variations of this model predict a variable relationship between NPQ and lumen pH on alteration of CO2 levels.
Second, changes in the topology of electron or proton transfer could alter the ratio of proton pumping to LEF (H+/e−, i.e., the stoichiometry of protons translocated into thylakoid lumen per electron transferred through the electron transfer chain). It was previously proposed that this H+/e− modulation could occur by facultative engagement of the Q cycle at the cytochrome b6f complex (reviewed in ref. 30), but recent in vivo measurements indicated that H+/e− is likely constant (31). Alternatively, Heber and Walker (17) proposed that cyclic electron transfer around PS I (CEF1) should increase proton pumping without contributing to LEF. Although there is significant evidence for CEF1, its turnover rate in vivo in C3 plants appears low (reviewed in refs. 32 and 33).
Asada and coworkers (see review in ref. 18) have proposed that the so-called Mehler reaction, or water-water cycle (WWC), may function to increase NPQ when LEF is hindered. The WWC consists of the extraction of electrons from water at the PS II oxygen-evolving complex, their transfer through the linear electron transfer chain, followed by reduction of O2 to superoxide at PS I. Superoxide is then degraded by a robust detoxification system, producing water. The only net product of the WWC is pmf, which could drive ATP synthesis and activate NPQ. Whereas significant data exist supporting the involvement of the WWC in vitro (34), its degree of engagement in vivo remains a subject of debate (32, 35).
Both CEF1 and WWC versions of model 2 predict that NPQ modulation would change the relationship between LEF and total pmf, but not between pmf and NPQ.
Third, NPQ modulation could be achieved by altering the kinetic properties of the ATP synthase, as previously suggested (36–39). At a given proton flux, the pmf will be determined by the conductivity of the thylakoid membrane to protons, g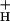 . The smaller the value of g
. The smaller the value of g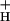 , the larger will be the steady-state pmf, and the more acidic the lumen. Modulating g
, the larger will be the steady-state pmf, and the more acidic the lumen. Modulating g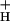 should thus affect the sensitivity of NPQ to LEF, CEF1, and the WWC. Like model 2, this model predicts that NPQ modulation should alter the relationship between LEF and pmf, but in addition predicts commensurate changes in g
should thus affect the sensitivity of NPQ to LEF, CEF1, and the WWC. Like model 2, this model predicts that NPQ modulation should alter the relationship between LEF and pmf, but in addition predicts commensurate changes in g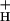 .
.
One of the main obstacles to defining the mechanism of NPQ modulation has been the lack of an independent probe for lumen pH in vivo. Recently we presented such a technique, based on the decay of the so-called electrochromic shift (ECS) signal, one that allows estimation of relative light-induced transthylakoid ΔpH and Δψ changes in vivo. In the present work, we extend this technique to also yield estimates of g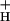 . We compare estimates of g
. We compare estimates of g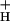 , NPQ, LEF, and pmf to assess the extent of, and proposed mechanisms for, NPQ modulation. A preliminary report of this work appeared in the Proceedings of the XIIth Congress on Photosynthesis (40).
, NPQ, LEF, and pmf to assess the extent of, and proposed mechanisms for, NPQ modulation. A preliminary report of this work appeared in the Proceedings of the XIIth Congress on Photosynthesis (40).
Materials and Methods
Plants and Growth Conditions.
Nicotiana tabacum (tobacco) plants were grown in a greenhouse with midday light intensity of about 900 μmol photons m−2⋅s−1, as described in ref. 41. Arabidopsis plants were grown under similar conditions, but with midday light intensity of about 500 μmol of photons m−2⋅s−1 (40). Plants were removed to the laboratory and dark-adapted overnight before experimentation. All experiments were performed at room temperature, ≈25°C.
Measurements of Absorbance Changes in the Steady State.
Steady-state light-driven pmf and g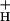 values were estimated by following the absorbance changes attributable to ECS, at 520 nm, on rapid light-to-dark transitions as described in refs. 31 and 42. The initial results were obtained by using a “diffused optics flash spectrophotometer” (ref. 43, and shown in ref. 40), but the data shown here were performed by using a nonfocusing optics flash spectrophotometer (NoFOSpec; ref. 42). Results from the two instruments were equivalent. These instruments were designed to observe light-induced absorbance changes in intact leaves, by specifically suppressing light-induced light-scattering signals. Pulsed measuring beams and frequency-selective amplification allow for high signal-to-noise ratios, while maintaining low cumulative measuring beam intensities. Young, fully expanded, intact leaves were gently clamped into a small (covering 1 cm2 leaf area) sealed leaf chamber in the NoFOSpec instrument, and premixed and analyzed gas mixtures from tanks were flowed through the chamber at approximately 40 ml/min. After steady-state conditions were established (see below), the actinic light-emitting diodes were rapidly and briefly switched off for 500-ms periods at 30-s intervals to allow decay of photoactivated processes, and the associated absorbance changes were measured. The temperature of the leaves, measured by a thermocouple, deviated from room temperature by less than 1°C during the experiments.
values were estimated by following the absorbance changes attributable to ECS, at 520 nm, on rapid light-to-dark transitions as described in refs. 31 and 42. The initial results were obtained by using a “diffused optics flash spectrophotometer” (ref. 43, and shown in ref. 40), but the data shown here were performed by using a nonfocusing optics flash spectrophotometer (NoFOSpec; ref. 42). Results from the two instruments were equivalent. These instruments were designed to observe light-induced absorbance changes in intact leaves, by specifically suppressing light-induced light-scattering signals. Pulsed measuring beams and frequency-selective amplification allow for high signal-to-noise ratios, while maintaining low cumulative measuring beam intensities. Young, fully expanded, intact leaves were gently clamped into a small (covering 1 cm2 leaf area) sealed leaf chamber in the NoFOSpec instrument, and premixed and analyzed gas mixtures from tanks were flowed through the chamber at approximately 40 ml/min. After steady-state conditions were established (see below), the actinic light-emitting diodes were rapidly and briefly switched off for 500-ms periods at 30-s intervals to allow decay of photoactivated processes, and the associated absorbance changes were measured. The temperature of the leaves, measured by a thermocouple, deviated from room temperature by less than 1°C during the experiments.
Chlorophyll a Fluorescence Yield Measurements and Analysis.
Chlorophyll a fluorescence yield was measured as in ref. 42. Saturation pulses of about 20,000 μmol photons m−2⋅s−1 white light lasting 1 s were provided by a 1,000-W xenon arc lamp, passed through a heat-reflecting filter, and focused on the entrance compound parabolic concentrator of the nonfocusing optics flash spectrophotometer. Decreasing the flash intensity by 50% had no effect on the results, indicating full saturation. The quantum yield of photon capture by PS II was determined from the model of Genty et al. (44) and used to estimate the rates of LEF under steady-state conditions by using the parameters described in Krall and Edwards (45). The extent of NPQ was calculated from chlorophyll a fluorescence parameters as previously described (see review in ref. 46).
Results
Variable Relationship Between NPQ and LEF.
Steady-state conditions, defined in our case as those where LEF did not change significantly during the course of the experiment, were chosen so that essentially all NPQ could be attributable to the rapidly reversible, pH-dependent quenching process (qE). At 50–2,000 ppm CO2, up to 40 min illumination resulted in negligible slowly reversible quenching (qI). Shorter illumination times, of between 10–20 min, were used at 0 ppm CO2 to avoid significant contributions from qI.
Steady-state LEF in wild-type tobacco plants responded to light and CO2 levels (with O2 levels held constant at 20%) as has been generally reported for C3 plants (44, 45); i.e., decreasing CO2 lowered light-saturated LEF as well as the half-saturating light intensity. At ambient CO2 (350 ppm), LEF reached half-saturation at about 275 μmol of photons m−2⋅s−1, and at full light-saturation reached a rate of about 120 μmol of electrons m−2⋅s−1. At 0 ppm CO2, light saturated LEF was 25–30% that at ambient CO2; it was assumed that this flow was consumed by photorespiration (45). Increasing CO2 from ambient to 2,000 ppm increased the half-saturating light intensity and the light-saturated rate by about 35%.
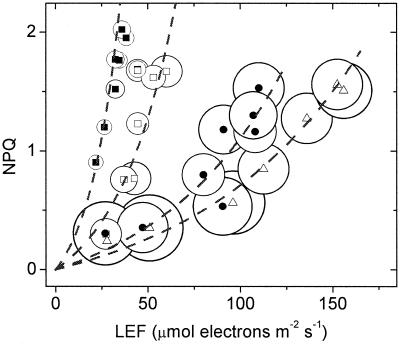
Fig. 1 shows down-regulation of excitation delivery to PS II, estimated by the NPQ parameter, as a function of LEF at different CO2 levels in wild-type tobacco plants. (The open circles of variable diameter in Fig. 1 will be discussed below). A series of discontinuous relationships between LEF and NPQ resulted from the interplay of two tendencies. (i) When light intensity was changed at constant CO2, NPQ tended to increase with increasing LEF. (ii) NPQ was substantially more sensitive to LEF at lower CO2 levels, and confirms earlier observations of NPQ modulation.
Figure 1.
Demonstration of a variable relationship between LEF and NPQ of excitation energy. Experiments were performed on intact wild-type tobacco leaves, under steady-state photosynthetic conditions with light intensities from 45 to 2,000 μmol of photons m−2⋅s−1 and CO2 levels of 2,000 ppm CO2 (open triangles), 350 ppm CO2 (ambient, filled circles), 50 ppm CO2 (open squares), and 0 CO2 (filled squares). The O2 level was held constant at 20%. The dashed lines represent a global fit of the data points by using the equation y = A(10(x/t) − 1). The value of A was held constant at 0.152 and that of t was fit at 35, 66, 140, and 180 at 0, 50, 350 and 2,000 ppm CO2, respectively. The diameters of the open circles surrounding each data point were set proportional to relative values for g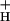 , as described in the text. The largest and smallest diameter symbols represented ≈15- and 80-ms decay times.
, as described in the text. The largest and smallest diameter symbols represented ≈15- and 80-ms decay times.
We introduce the term SNPQ to define the relative “sensitivity” of NPQ to LEF, and define it as:
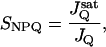 |
1 |
where JQ is the LEF at which NPQ reaches 1, and J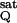 is a reference JQ, measured at saturating CO2 levels (in our case 2,000 ppm). By interpolation of the results in Fig. 1, we obtained SNPQ values of about 5, 2.8, 1.5, and 1, at 0, 50, 350, and 2,000 ppm, respectively, i.e., a change in sensitivity of NPQ to LEF of 5-fold, from zero to saturating CO2 levels.
is a reference JQ, measured at saturating CO2 levels (in our case 2,000 ppm). By interpolation of the results in Fig. 1, we obtained SNPQ values of about 5, 2.8, 1.5, and 1, at 0, 50, 350, and 2,000 ppm, respectively, i.e., a change in sensitivity of NPQ to LEF of 5-fold, from zero to saturating CO2 levels.
Estimation of the Transthylakoid pmf.
The decay kinetics of the ECS were used to estimate relative light-induced pmf as described in detail in refs. 47 and 48. During the dark intervals that punctuated steady-state illumination, the ECS decayed to a quasi-stable level, with a relaxation time (τECS) of between about 15 and 80 ms (see below). It was shown previously (47) that this decay process reflects both relaxation of the light-driven transthylakoid Δψ, as well as the establishment of a “reverse” Δψ (positive on the stromal side of the thylakoid membrane) as a result of the transthylakoid proton diffusion potential, i.e., the ΔpH component of pmf. Under a broad range of conditions, the total ECS change from steady-state to stable dark level, termed ECSt (the full extent of change in ECS on a rapid light-dark transition), was found to be essentially linear, with the light-dark difference in transthylakoid pmf (i.e., both Δψ and ΔpH components).
Because there is reasonable consensus that the pH of the stroma in the light remains in a narrow range between 7.5 and 8 (e.g., refs. 49–53), the lumen pH should be roughly proportional to ΔpH. We have recently found that the fractions of ECS decay attributable to Δψ and ΔpH remained roughly 1:1 over the experimental conditions used here (47), and conclude that ΔpH should be approximately proportional to ECSt. Transthylakoid pmf will not decay to zero on an abrupt light-dark transition, but rather to a dark (or basal) level, pmfd, set by equilibration with ΔGATP (1, 47). In other words, ECSt reflects light-driven increase in pmf, above pmfd. The magnitude of pmfd will be a function of ΔGATP and the ratio of H+/ATP at the ATP synthase (n). Previous results under conditions similar to those used here showed that ΔGATP was nearly constant over a wide range of conditions (54, 55). Furthermore, recent structural analysis of the ATP synthase indicates that variation of n is highly unlikely (56). Thus, the offset in pmf imposed by equilibration with ΔGATP is probably nearly constant over our experimental conditions. We conclude that ECSt should, at least qualitatively, reflect changes in lumen pH.
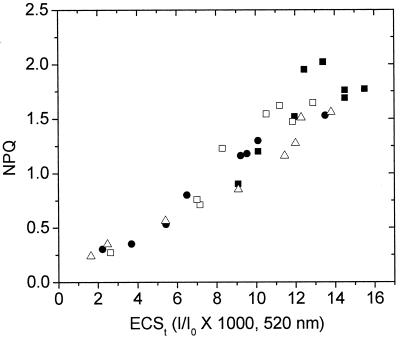
Fig. 2 shows a continuous, nearly linear relationship between NPQ and pmf estimated by the ECSt parameter. Importantly, within the noise level, increases in pmf were consistently accompanied by increases in NPQ, regardless of whether light intensity or CO2 levels were altered. The continuity of the relationship strongly supports our conclusion that ECSt reflects lumen pH.
Figure 2.
The relationship between light-induced pmf and NPQ. The y axis data were taken from Fig. 1, and plotted against the steady-state pmf, estimated by the decay of the ECS, as described in the text. The symbols and conditions are as in Fig. 1.
The Effects of Light and CO2 Levels on the Conductivity of the ATP Synthase to Protons.
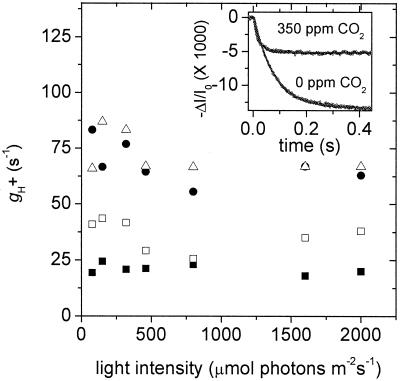
The Inset to Fig. 3 shows ECS decay kinetics on a light-dark transition at constant light intensity (320 μmol of photons m−2⋅s−1) but at 350 or 0 ppm CO2. Both the decay time constant for ECS (τECS) and the amplitude of the change (ECSt) increased dramatically on decreasing CO2 levels.
Figure 3.
Relationships among light intensity, CO2 levels, and the conductivity of the ATP synthase to protons (g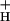 ). Values of g
). Values of g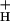 were estimated as described in the text and plotted as a function of light intensity. Conditions and symbols were as in Fig. 1. (Inset) Decay kinetics of the ECS on abrupt light-dark transitions from steady-state photosynthetic conditions. The light intensity was 320 μmol of photons m−2⋅s−1, and the CO2 levels were 350 ppm (top curve) and 0 ppm (bottom curve). The curves were fit to single first order decays as described in the text.
were estimated as described in the text and plotted as a function of light intensity. Conditions and symbols were as in Fig. 1. (Inset) Decay kinetics of the ECS on abrupt light-dark transitions from steady-state photosynthetic conditions. The light intensity was 320 μmol of photons m−2⋅s−1, and the CO2 levels were 350 ppm (top curve) and 0 ppm (bottom curve). The curves were fit to single first order decays as described in the text.
Because the vast majority of proton efflux from the lumen is coupled to ATP synthase under steady state conditions in vivo (see ref. 57), g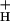 should be primarily determined by the catalytic properties of the ATP synthase (content, activation state, the availability of ADP and Pi, the concentrations of any inhibitors, etc.). If these factors are reasonably constant over the course of an experiment, proton flux through the ATP synthase should be proportional to pmf, and the resulting decay kinetics should be pseudofirst order. In support of this analysis, ECS decay kinetics were reasonably well fit to single exponential decay curves (see Fig. 3 Inset). These data are also consistent with earlier measurements showing that, in vivo under steady-state photosynthetic conditions, the γ-subunit thiol groups are reduced (58) and pmf is well above the activation threshold for ATP synthesis (31, 47), where proton conduction through the ATP synthase is roughly proportional to pmf (e.g., ref. 59). We thus estimate relative g
should be primarily determined by the catalytic properties of the ATP synthase (content, activation state, the availability of ADP and Pi, the concentrations of any inhibitors, etc.). If these factors are reasonably constant over the course of an experiment, proton flux through the ATP synthase should be proportional to pmf, and the resulting decay kinetics should be pseudofirst order. In support of this analysis, ECS decay kinetics were reasonably well fit to single exponential decay curves (see Fig. 3 Inset). These data are also consistent with earlier measurements showing that, in vivo under steady-state photosynthetic conditions, the γ-subunit thiol groups are reduced (58) and pmf is well above the activation threshold for ATP synthesis (31, 47), where proton conduction through the ATP synthase is roughly proportional to pmf (e.g., ref. 59). We thus estimate relative g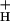 as the reciprocal of the ECS decay time constant, (1/τECS), as described in ref. 60.
as the reciprocal of the ECS decay time constant, (1/τECS), as described in ref. 60.
Fig. 3 shows the relationships between relative g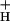 and light intensity at various CO2 levels. Decreasing CO2 levels from 2,000 to 0 ppm lowered g
and light intensity at various CO2 levels. Decreasing CO2 levels from 2,000 to 0 ppm lowered g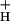 by about 5-fold. In contrast, increasing light intensity from 45 to 2,000 μmol of photons m−2⋅s−1 had remarkably little effect on g
by about 5-fold. In contrast, increasing light intensity from 45 to 2,000 μmol of photons m−2⋅s−1 had remarkably little effect on g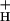 .
.
Discussion
A Demonstration of NPQ Modulation.
As shown in Fig. 1, the relationship between LEF and NPQ became significantly steeper as CO2 was lowered. Quantification of this effect by using the SNPQ parameter suggests that the sensitivity of NPQ to LEF was increased 5-fold on decreasing CO2 from 2,000 to 0 ppm. Because the most basic model for NPQ (see introduction) would predict a continuous relationship between NPQ and LEF, these data confirm the existence of NPQ modulation induced by altering CO2 levels. The effect of this modulation appears consistent with its proposed function, to afford greater photoprotection under substrate-limiting conditions (see above).
The Short-Term Relationship Between NPQ and pmf Is Constant.
In contrast, we observed a continuous, nearly linear dependence of NPQ on ECSt despite the large modulation of NPQ sensitivity to LEF on changing CO2 (Fig. 2). Because we consider ECSt to be an indicator of lumen pH (see above), we conclude that the pH response of NPQ (i.e., via VDE and psbS protonation) was essentially constant over the conditions and time scale of our experiments. This result strongly argues against model 1, and instead supports models 2 and 3, where NPQ modulation occurs by altering the relationship between lumen pH and LEF.
At first glance, the nearly linear relationship between NPQ and ECSt (Fig. 2) is somewhat surprising, considering the expected large Hill coefficient of about 5 for both VDE (e.g., ref. 61) and LHC (reviewed in ref. 23) protonation. However, this behavior is consistent with a series of arguments that lumen pH is normally restricted to a fairly narrow range from about 5.5 to 6.5 where it modulates NPQ, but does not hinder the stabilities or activities of luminal enzymes (1). It is also consistent with the expected dual role of lumen acidification to act as the key signal intermediate in feedback regulation of photosynthesis, because exceeding the pH range over which NPQ is activated would lead to an uncontrolled flux, and eventual photoinhibition.
Changes in g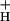 Can Account for the Majority of NPQ Modulation.
Can Account for the Majority of NPQ Modulation.
Model 3 predicts that modulation of NPQ will be initiated by changes in g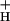 . Indeed, we observed changes in g
. Indeed, we observed changes in g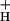 consistent with this role (Fig. 3 and Inset). Low g
consistent with this role (Fig. 3 and Inset). Low g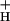 was seen at low CO2, where SNPQ was highest. A close correlation between g
was seen at low CO2, where SNPQ was highest. A close correlation between g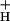 and the relationship between NPQ and LEF is revealed in Fig. 1, where the diameters of the open circles were set proportional to g
and the relationship between NPQ and LEF is revealed in Fig. 1, where the diameters of the open circles were set proportional to g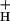 . As CO2 was lowered, the increase in SNPQ was accompanied by concomitant decreases in g
. As CO2 was lowered, the increase in SNPQ was accompanied by concomitant decreases in g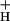 . We hypothesize that the relationship between g
. We hypothesize that the relationship between g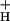 and SNPQ is causal, i.e., that, as g
and SNPQ is causal, i.e., that, as g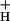 is decreased, proton efflux is restricted, leading to buildup in ΔpH, which triggered NPQ at relatively low LEF.
is decreased, proton efflux is restricted, leading to buildup in ΔpH, which triggered NPQ at relatively low LEF.
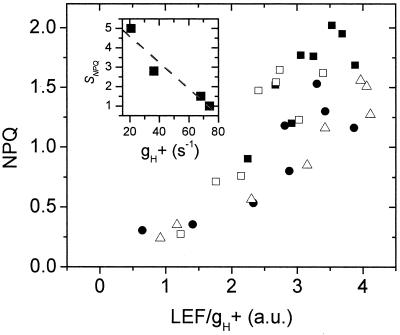
If model 3, but not model 2, is correct, lumen pH (i.e., proton accumulation) should be a continuous function of proton flux and g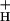 , regardless of whether flux was modulated by light intensity or CO2 level (see ref. 60). Because proton flux is expected to be proportional to LEF (31), we plotted NPQ against LEF/g
, regardless of whether flux was modulated by light intensity or CO2 level (see ref. 60). Because proton flux is expected to be proportional to LEF (31), we plotted NPQ against LEF/g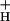 (Fig. 4). Within the noise level, a continuous relationship with curvature similar to that between NPQ and ECSt (Fig. 2) was observed, lending significant support to model 3. Lastly, as shown in the Inset to Fig. 4, a linear relationship was observed between SNPQ and the average g
(Fig. 4). Within the noise level, a continuous relationship with curvature similar to that between NPQ and ECSt (Fig. 2) was observed, lending significant support to model 3. Lastly, as shown in the Inset to Fig. 4, a linear relationship was observed between SNPQ and the average g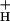 for each CO2 level, as predicted if g
for each CO2 level, as predicted if g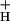 was the controlling factor in determining SNPQ.
was the controlling factor in determining SNPQ.
Figure 4.
The relationship between pmf, estimated by LEF/g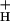 , and NPQ. The data from Figs. 1 and 4 were used to estimate pmf based on model 3 (see text). Conditions and symbols were as in Fig. 1. (Inset) The relationship between the sensitivity of NPQ to LEF, SNPQ, and the average value of g
, and NPQ. The data from Figs. 1 and 4 were used to estimate pmf based on model 3 (see text). Conditions and symbols were as in Fig. 1. (Inset) The relationship between the sensitivity of NPQ to LEF, SNPQ, and the average value of g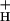 at each CO2 level. The dashed line represents the least-squares linear fit to the data, with slope of −0.068, y intercept of 5.9 and r value of −0.96.
at each CO2 level. The dashed line represents the least-squares linear fit to the data, with slope of −0.068, y intercept of 5.9 and r value of −0.96.
We conclude that only model 3 can account for all of our data, and propose it as a working model. At this point, we cannot completely exclude the participation of alternate electron transfer cycles (model 2), especially at low CO2 and high light, where the scatter in the data are highest (see Fig. 4). We note, however, that the impact of such alternate cycles on lumen pH would also be accentuated by lowering of g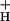 . In fact, the existence of a modulated g
. In fact, the existence of a modulated g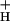 might resolve the apparent contradiction between the proposed function in down-regulation of CEF1 and WWC, with their very low turnover rates. In addition, because NPQ selectively decreases the quantum efficiency of PS II, which is involved in LEF, while not significantly affecting that of PS I, which is involved in both LEF and CEF1, it is possible that decreasing g
might resolve the apparent contradiction between the proposed function in down-regulation of CEF1 and WWC, with their very low turnover rates. In addition, because NPQ selectively decreases the quantum efficiency of PS II, which is involved in LEF, while not significantly affecting that of PS I, which is involved in both LEF and CEF1, it is possible that decreasing g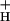 would increase the ratio of CEF1 to LEF and consequently affect the output ratio of ATP/reduced nicotinamide-adenine dinucleotide phosphate (NADPH).
would increase the ratio of CEF1 to LEF and consequently affect the output ratio of ATP/reduced nicotinamide-adenine dinucleotide phosphate (NADPH).
What Controls g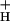 ?
?
Although at this point we cannot fully answer this question, we can likely eliminate some of the more obvious candidates. At each CO2 level, g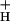 remained nearly constant, even as LEF and pmf (ECSt) increased with light intensity (Fig. 3). As discussed in Sacksteder et al. (31), such behavior would be expected only if the ATP synthase reaction remained far from equilibrium under our conditions, i.e., n⋅pmf ≫ ΔGATP, as is also implied from previous observations that ΔGATP is essentially constant over these conditions (see above). It further implies that factors responsible for altering g
remained nearly constant, even as LEF and pmf (ECSt) increased with light intensity (Fig. 3). As discussed in Sacksteder et al. (31), such behavior would be expected only if the ATP synthase reaction remained far from equilibrium under our conditions, i.e., n⋅pmf ≫ ΔGATP, as is also implied from previous observations that ΔGATP is essentially constant over these conditions (see above). It further implies that factors responsible for altering g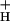 were constant over light intensity, but altered by CO2 levels. Because, [ATP]/[ADP] ratios change by little on altering [CO2] (24, 62) or light intensity (25), we conclude that neither [ATP] nor [ADP] determines g
were constant over light intensity, but altered by CO2 levels. Because, [ATP]/[ADP] ratios change by little on altering [CO2] (24, 62) or light intensity (25), we conclude that neither [ATP] nor [ADP] determines g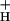 .
.
The ATP synthase is allosterically regulated by pmf and by reduction of γ-subunit thiols via thioredoxin (e.g., refs. 57 and 63–65). The lack of effect of light intensity on τECS strongly suggests that pmf remained above that needed to activate the ATP synthase under all our conditions. Further, results similar to those shown above were obtained with the cfg1 mutant of Arabidopsis, which is deficient in redox control (66), as well as with wild-type Arabidopsis and tobacco leaves infiltrated with dithiothrietol, which should maintain the thiols in their reduced state (data not shown). This result is not surprising because thiol modulation in vivo is complete at very low light levels and is thought to act more as a light-dark switch rather than a fine-tuning mechanism (58).
The situation is less clear in the case of substrate Pi, which is difficult to measure in organelles. Whereas some have reported that light and CO2 levels had little effect on chloroplast Pi (24), other results have implied substantial effects (e.g., refs. 62, 67, and 68). The measured KD for Pi at the ATP synthase is 0.6 mM, fairly close to its expected concentration, about 10 mM (reviewed in ref. 68). It is thus possible that sequestration of Pi in metabolic pools during CO2 limitation could limit g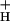 , either at the level of substrate binding or allosterically. Indeed, a number of researchers have championed Pi as an important player in controlling photosynthesis, and proposed mechanisms by which photosynthesis-related processes (including restriction of transport at the chloroplast inner envelope, and sequestration in various metabolic pools or in vacuoles) could account for substantial changes in soluble Pi (see refs. 62, 67, and 68–71). On the other hand, the apparent lack of change in pmfd (see Fig. 2 and above) would seem to rule out large changes in Pi chemical activity.
, either at the level of substrate binding or allosterically. Indeed, a number of researchers have championed Pi as an important player in controlling photosynthesis, and proposed mechanisms by which photosynthesis-related processes (including restriction of transport at the chloroplast inner envelope, and sequestration in various metabolic pools or in vacuoles) could account for substantial changes in soluble Pi (see refs. 62, 67, and 68–71). On the other hand, the apparent lack of change in pmfd (see Fig. 2 and above) would seem to rule out large changes in Pi chemical activity.
Conclusions
We have presented evidence that the sensitivity of NPQ to LEF (SNPQ) is primarily regulated by changing the catalytic properties of the ATP synthase. Although we cannot exclude the participation of alternative electron transfer cycles, we argue that their impact will be greatly enhanced under conditions where g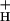 is lowered. It is yet unclear what processes regulate g
is lowered. It is yet unclear what processes regulate g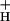 , but our data and analysis likely exclude redox regulation of the γ-subunit thiols, or the interaction of ATP or ADP with the ATP synthase. One possibility is that short-term modulation is accomplished by altering Pi levels, whereas longer-term adjustments could be made by altering expression levels of the ATP synthase. These adjustments may complement changes in antenna composition (4). Finally, the spectroscopic tools presented here should be useful in discriminating the influence of different mechanisms of NPQ modulation in vivo in mutant plants and under environmental stress.
, but our data and analysis likely exclude redox regulation of the γ-subunit thiols, or the interaction of ATP or ADP with the ATP synthase. One possibility is that short-term modulation is accomplished by altering Pi levels, whereas longer-term adjustments could be made by altering expression levels of the ATP synthase. These adjustments may complement changes in antenna composition (4). Finally, the spectroscopic tools presented here should be useful in discriminating the influence of different mechanisms of NPQ modulation in vivo in mutant plants and under environmental stress.
Acknowledgments
We dedicate this work to Drs. Anne and Pierre Joliot for their support and contributions to this field. We thank Drs. G. E. Edwards, J. A. Cruz, A. Portis, K. Niyogi, and J. Berry for important discussions. This work was supported by U.S. Department of Energy Grant DE-FG03-98ER20299 and National Science Foundation Grant IBN-9817980.
Abbreviations
- CEF1
cyclic electron flow involving PS I
- ECS
absorbance changes because of the electrochromic shift
- ECSt
the full extent of change in ECS on a rapid light-dark transition
-
g
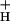
the conductivity of the thylakoid membrane to protons, predominantly attributable to the activity of the ATP synthase
- LEF
linear electron flow
- LHC
light harvesting complex
- NPQ
nonphotochemical quenching
- Pi
orthophosphate
- pmf
proton motive force
- PS I
photosystem I
- PS II
photosystem II
- SNPQ
the sensitivity of NPQ to LEF
- VDE
violaxanthin deepoxidase
- WWC
water-water cycle or Mehler peroxidase reaction
Footnotes
See commentary on page 12518.
References
- 1.Kramer D M, Sacksteder C A, Cruz J A. Photosynth Res. 1999;60:151–163. [Google Scholar]
- 2.Anderson B, Aro E-M. In: Regulation of Photosynthesis. Anderson B, Aro E-M, editors. Dordrecht, The Netherlands: Kluwer; 2001. pp. 377–393. [Google Scholar]
- 3.Hirada Y, Sonoike K. In: Regulation of Photosynthesis. Anderson B, Aro E-M, editors. Dordrecht, The Netherlands: Kluwer; 2001. pp. 507–531. [Google Scholar]
- 4.Demmig-Adams B, Adams W W., III Plant Physiol. 1992;43:599–626. [Google Scholar]
- 5.Horton P, Ruban A, Walters R. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 6.Wentworth M, Ruban A V, Horton P. FEBS Lett. 2000;471:71–74. doi: 10.1016/s0014-5793(00)01369-7. [DOI] [PubMed] [Google Scholar]
- 7.Müller P, Li X-P, Niyogi K K. Plant Physiol. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niyogi K K, Björkman O, Grossman A R. Proc Natl Acad Sci USA. 1997;94:14162–14167. doi: 10.1073/pnas.94.25.14162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niyogi K K, Björkman O, Grossman A R. Plant Cell. 1997;9:1369–1380. doi: 10.1105/tpc.9.8.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havaux M, Niyogi K K. Proc Natl Acad Sci USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pogson B J, Niyogi K K, Bjorkman O, DellaPenna D. Proc Natl Acad Sci USA. 1998;95:13324–13329. doi: 10.1073/pnas.95.22.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nixon P J, Mullilneaux C W. In: Regulation of Photosynthesis. Anderson B, Aro E-M, editors. Dordrecht, The Netherlands: Kluwer; 2001. pp. 533–555. [Google Scholar]
- 13.Genty B, Harbinson J, Briantais J-M, Baker N R. Photosynth Res. 1990;25:249–257. doi: 10.1007/BF00033166. [DOI] [PubMed] [Google Scholar]
- 14.Genty B, Harbinson J, Baker N R. Plant Physiol Biochem. 1990;28:1–10. [Google Scholar]
- 15.Genty B, Harbinson J. In: Photosynthesis and the Environment. Baker N R, editor. Dordrecht, The Netherlands: Kluwer; 1996. pp. 67–99. [Google Scholar]
- 16.Harbinson J, Foyer C H. Plant Physiol. 1991;97:41–49. doi: 10.1104/pp.97.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heber U, Walker D. Plant Physiol. 1992;100:1621–1626. doi: 10.1104/pp.100.4.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asada K. In: Photosynthesis and the Environment. Baker N R, editor. Dordrecht, The Netherlands: Kluwer; 1996. pp. 123–150. [Google Scholar]
- 19.Vass I, Styring S. Biochemistry. 1993;32:3334–3341. doi: 10.1021/bi00064a016. [DOI] [PubMed] [Google Scholar]
- 20.Hideg E, Kalai T, Hideg K, Vass I. Biochemistry. 1998;37:11405–11411. doi: 10.1021/bi972890+. [DOI] [PubMed] [Google Scholar]
- 21.Sonoike K. Plant Cell Physiol. 1996;37:239–247. [Google Scholar]
- 22.Horton P. In: Photosynthesis, Plant Biology. Briggs W, editor. Vol. 9. New York: Liss; 1989. pp. 393–406. [Google Scholar]
- 23.Horton P, Ruban A V, Young A J. In: The Photochemistry of Carotenoids. Frank H A, Young A J, Britton G, Cogdell R J, editors. Dordrecht, The Netherlands: Kluwer; 1999. pp. 271–291. [Google Scholar]
- 24.Dietz K-J, Heber U. Biochim Biophys Acta. 1984;767:432–443. [Google Scholar]
- 25.Siebke K, Laisk A, Oja V, Kiirats O, Raschke K, Heber U. Planta. 1990;182:513–522. doi: 10.1007/BF02341026. [DOI] [PubMed] [Google Scholar]
- 26.Horton P, Ruban A V. Photosynth Res. 1992;34:375–385. doi: 10.1007/BF00029812. [DOI] [PubMed] [Google Scholar]
- 27.Noctor G, Ruban A V, Horton P. Biochim Biophys Acta. 1993;1183:339–344. [Google Scholar]
- 28.Ruban A V, Young A, Horton P. Biochim Biophys Acta. 1994;1186:123–127. [Google Scholar]
- 29.Oxborough K, Horton P. Biochim Biophys Acta. 1988;934:135–143. [Google Scholar]
- 30.Berry S, Rumberg B. Biochim Biophys Acta. 1999;1410:248–261. doi: 10.1016/s0005-2728(99)00003-1. [DOI] [PubMed] [Google Scholar]
- 31.Sacksteder C A, Kanazawa A, Jacoby M E, Kramer D M. Proc Natl Acad Sci USA. 2000;97:14283–14288. doi: 10.1073/pnas.97.26.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badger M R, von Caemmerer S, Ruuska S, Nakano H. Philos Trans R Soc London B Biol Sci. 2000;355:1433–1446. doi: 10.1098/rstb.2000.0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ott T, Clarke J, Birks K, Johnson G. Planta. 1999;209:250–258. doi: 10.1007/s004250050629. [DOI] [PubMed] [Google Scholar]
- 34.Asada K. Philos Trans R Soc London B Biol Sci. 1999;355:1419–1431. doi: 10.1098/rstb.2000.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiese C. Physiol Plant. 1998;102:437–446. [Google Scholar]
- 36.Braun G, Evron Y, Malkin S, Avron M. FEBS Lett. 1991;280:57–60. doi: 10.1016/0014-5793(91)80203-f. [DOI] [PubMed] [Google Scholar]
- 37.Ort D. FEBS Lett. 1976;69:81–85. doi: 10.1016/0014-5793(76)80658-8. [DOI] [PubMed] [Google Scholar]
- 38.Graan T, Ort D R. J Biol Chem. 1983;258:2831–2836. [PubMed] [Google Scholar]
- 39.Ortiz-Lopez A, Ort D R, Boyer J S. In: Photosynth. Res. Biggens J, editor. IV. Dordrecht, The Netherlands: Martinus Nijhoff; 1987. pp. 153–156. [Google Scholar]
- 40.Kanazawa A, Kiirats O, Edwards G, Cruz J, Kramer D M. Proceedings of the XIIth International Congress on Photosynthesis. Collingwood, Australia: CSIRO Publishing; 2001. , S3–047 (CD-ROM). [Google Scholar]
- 41.Sacksteder C A, Kramer D M. Photosynth Res. 2000;66:145–158. doi: 10.1023/A:1010785912271. [DOI] [PubMed] [Google Scholar]
- 42.Sacksteder C A, Jacoby M E, Kramer D M. Photosynth Res. 2001;70:231–240. doi: 10.1023/A:1017906626288. [DOI] [PubMed] [Google Scholar]
- 43.Kramer D M, Sacksteder C A. Photosynth Res. 1998;56:103–112. [Google Scholar]
- 44.Genty B, Briantais J-M, Baker N R. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- 45.Krall J P, Edwards G E. Physiol Plant. 1992;86:180–187. [Google Scholar]
- 46.Maxwell K, Johnson G N. J Exp Bot. 2000;51:659–668. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- 47.Cruz J A, Sacksteder C A, Kanazawa A, Kramer D M. Biochemistry. 2001;40:1226–1237. doi: 10.1021/bi0018741. [DOI] [PubMed] [Google Scholar]
- 48.Cruz J A, Kanazawa A, Kramer D M. Proceedings of the XIIth International Congress on Photosynthesis. S12. Collingwood, Australia: CSIRO Publishing; 2001. –007. [Google Scholar]
- 49.Heldt H W, Werdan K, Milovancev M, Geller G. Biochim Biophys Acta. 1973;314:224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- 50.Robinson S P. Biochim Biophys Acta. 1985;806:187–194. [Google Scholar]
- 51.Wu W, Berkowitz G A. Plant Physiol. 1992;98:666–672. doi: 10.1104/pp.98.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hauser M, Eichelmann H, Heber U, Laisk A. Planta. 1995;196:199–204. [Google Scholar]
- 53.Hauser M, Eichelmann H, Oja V, Heber U, Laisk A. Plant Physiol. 1995;108:1059–1066. doi: 10.1104/pp.108.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerst U, Schönknecht G, Heber U. Planta. 1994;193:421–429. [Google Scholar]
- 55.Heber U, Neimanis S, Dietz K, Viil J. Biochim Biophys Acta. 1986;852:114–155. [Google Scholar]
- 56.Muller D J, Dencher N A, Meier T, Dimroth P, Suda K, Stahlberg H, Engel A, Seelert H, Matthey U. FEBS Lett. 2001;504:219–222. doi: 10.1016/s0014-5793(01)02708-9. [DOI] [PubMed] [Google Scholar]
- 57.Kramer D M, Crofts A R. Biochim Biophys Acta. 1989;976:28–41. [Google Scholar]
- 58.Kramer D M, Wise R R, Frederick J R, Alm D M, Hesketh J D, Ort D R, Crofts A R. Photosynth Res. 1990;26:213–222. doi: 10.1007/BF00033134. [DOI] [PubMed] [Google Scholar]
- 59.Gräber P, Witt H T. Biochim Biophys Acta. 1976;423:141–163. doi: 10.1016/0005-2728(76)90174-2. [DOI] [PubMed] [Google Scholar]
- 60.Schönknecht G, Neimanis S, Katona E, Gerst U, Heber U. Proc Natl Acad Sci USA. 1995;92:12185–12189. doi: 10.1073/pnas.92.26.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pfündel E E, Dilley R A. Plant Physiol. 1993;101:65–71. doi: 10.1104/pp.101.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noctor G, Foyer C H. J Exp Bot. 2000;51:347–356. doi: 10.1093/jexbot/51.suppl_1.347. [DOI] [PubMed] [Google Scholar]
- 63.Ort D R, Oxborough K. Plant Physiol. 1992;43:269–291. [Google Scholar]
- 64.Gräber P, Fromme P, Junesch U, Schmidt G, Thulke G. Ber Bunsenges Phys Chem. 1986;90:1034–1040. [Google Scholar]
- 65.McCarty R E. In: Oxygenic Photosynthesis: The Light Reactions. Ort D R, Yocum C F, editors. Dordrecht, The Netherlands: Kluwer; 1996. pp. 439–451. [Google Scholar]
- 66.Gabrys H, Kramer D M, Croft A R, Ort D R. Plant Physiol. 1994;104:769–776. doi: 10.1104/pp.104.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharkey T D. Bot Mag (Tokyo) 1990;2:87–105. [Google Scholar]
- 68.Woodrow I E, Berry J A. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:533–594. [Google Scholar]
- 69.Sharkey T D, Vassey T L. Plant Physiol. 1988;90:385–387. doi: 10.1104/pp.90.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sivak M N, Walker D A. New Phytol. 1986;102:499–512. [Google Scholar]
- 71.Leegood R C, Furbank R T. Planta. 1986;168:84–93. doi: 10.1007/BF00407013. [DOI] [PubMed] [Google Scholar]