Abstract
Coronary artery disease leads to injury and loss of myocardial tissue by deprivation of blood flow (ischemia) and is a major underlying cause of heart failure. Prolonged ischemia causes necrosis and apoptosis of cardiac myocytes and vascular cells; however, the mechanisms of ischemia-mediated cell death are poorly understood. Ischemia is associated with both hypoxia and acidosis due to increased glycolysis and lactic acid production. We recently reported that hypoxia does not induce cardiac myocyte apoptosis in the absence of acidosis. We now report that hypoxia-acidosis-associated cell death is mediated by BNIP3, a member of the Bcl-2 family of apoptosis-regulating proteins. Chronic hypoxia induced the expression and accumulation of BNIP3 mRNA and protein in cardiac myocytes, but acidosis was required to activate the death pathway. Acidosis stabilized BNIP3 protein and increased the association with mitochondria. Cell death by hypoxia-acidosis was blocked by pretreatment with antisense BNIP3 oligonucleotides. The pathway included extensive DNA fragmentation and opening of the mitochondrial permeability transition pore, but no apparent caspase activation. Overexpression of wild-type BNIP3, but not a translocation-defective mutant, activated cardiac myocyte death only when the myocytes were acidic. This pathway may figure significantly in muscle loss during myocardial ischemia.
Keywords: heart‖apoptosis‖ischemia‖mitochondria‖antisense
Atherosclerotic plaque restricts blood flow through coronary arteries and provides a substrate for occlusive thrombus formation. Reduced blood flow produces hypoxia in the tissues downstream of the lesion; complete occlusion leads to severe hypoxia that threatens the viability of the myocardium. Subsequent reperfusion by thrombolysis or removal of the plaque may subject cells to further damage through oxidative stress, and a region of permanent injury containing dead and dying cells develops (the “infarct”; ref. 1). Hypoxia may persist within the infarct and at its margins for days or weeks, exacerbating the injury (2–4). In response, the ischemic myocardium switches from respiration to glycolytic energy metabolism, with increased glucose consumption, lactic acid production, and lower intracellular pH (5–7). The extent of tissue loss to infarction is determined by the severity and duration of the ischemic period and is known to involve both necrotic and apoptotic cell death pathways (8–10). Oxidative stress caused by reperfusion may account for 50% of the tissue damage during early infarction (11–13). Multiple additional factors contribute to cell death as the infarcted area expands and more border cells die. These factors include collateral damage from necrosis and infiltrating macrophages (14), additional necrotic death resulting from energy depletion (15–17), and changes associated with hypoxia.
BNIP3 is a member of the so-called BH3-only subfamily of Bcl-2 family proteins that heterodimerizes and antagonizes the activity of prosurvival proteins (Bcl-2, Bcl-XL) and promotes apoptosis (18, 19). Proteins in this group do not possess the same Bcl-2 homology domains (BH1 and BH2) as the other Bcl-2 family members but may bind through a common BH3 domain. Bcl-2 proteins are usually associated with cell membranes, particularly the mitochondria, where they are anchored by a C-terminal domain. Individual family members may remain in the cytosol or be loosely membrane-bound and translocate into membranes only after a death signal is received (18–21). A major function of this class of proteins is to determine the on/off state of the mitochondrial permeability transition pore (MPTP; refs. 22–24). Although it contains a BH3 domain, the C-terminal transmembrane domain of BNIP3 is essential for membrane targeting and promotion of apoptosis. BNIP3 expression is normally undetectable in most organs, including the heart, but can be induced by hypoxia (18, 25). Overexpression of BNIP3 protein by transfection of the cDNA into some cultured cell lines results in membrane insertion and initiation of a cell death pathway with features similar to necrosis (18).
The role of hypoxia in ischemia-mediated death of cardiac cells is controversial. We recently demonstrated that hypoxia alone is not a major stimulus for apoptosis and that significant cell death requires the combination of hypoxia and acidosis (26). Acidosis regularly accompanies ischemia because of increased accumulation of lactic and phosphoric acid. Acidosis has been implicated in cardiac cell death; inhibition of the vacuolar ATPase, a proton pump involved in pH regulation, promotes apoptosis of cardiac myocytes (27). Here we present evidence that BNIP3 is the molecular effector of hypoxia-acidosis-mediated cardiac myocyte apoptosis.
Materials and Methods
Reagents.
Antibodies to Bax, Bcl-XL, Bad, Bcl-2, and actin were from Santa Cruz Biotechnology; anti-Bak was from LXR Biotechnology (Richmond, CA); and anti-BNIP3 was from R. K. Bruick (University of Texas Southwestern Medical Center, Dallas; ref. 25). Anti-vertebrate sarcomeric myosin antibody (MF-20) was from the Developmental Studies Hybridoma Bank, University of Iowa. Caspase inhibitors Boc-D and ZVAD, Hoechst 33342, propidium iodide, trypan blue, and the antisuccinate dehydrogenase antibody were obtained from Calbiochem. Plasmids T7-BNIP3 and T7-BNIP3 Δ-TM containing the wild-type and transmembrane-deleted BNIP3 cDNAs, respectively, were generous gifts from D. Dubik (University of Manitoba, Canada; ref. 19). The GFP expression plasmid was from CLONTECH. BNIP3 antisense phosphorothioate oligonucleotides containing fluorescein isothiocyanate tags were from Sigma-Genosys (The Woodlands, TX). MitoTracker Red CMXRos was obtained from Molecular Probes. All other reagents were from Sigma.
Cardiac Myocyte Culture and Hypoxia.
Our methods for the isolation and culture of primary neonatal rat cardiac myocytes and exposure to hypoxia have been described (26, 28, 29). Experiments were performed in defined serum-free DMEM/M-199 (4:1) medium. The oxygen levels in the hypoxia chamber were continuously monitored and maintained at <10 mmHg (1 mmHg = 133 Pa).
Quantitative Analysis of Apoptosis.
Cells were examined for morphologic evidence of apoptosis or necrosis by staining with antimyosin antibody and the fluorescent DNA-binding dyes Hoechst 33342 and propidium iodide, exactly as described (26, 30). Genomic DNA fragmentation analyses were also as described (30).
Northern and Western Blots.
Northern and Western blot procedures were exactly as described (26, 30, 31). Northern blots were probed with full-length rat BNIP3 and β-actin cDNAs. Proteins were separated on SDS-12% polyacrylamide gels.
Antisense.
Antisense oligonucleotides were complementary to bases 10–40 and 600–630 of the BNIP3 gene; sequences 5′-ACGGGGACGATGGAGAGCCACTGGCGGAGG and 5′-CCTAGATGTAACCTTCCGCAGACTGTTGAA, respectively. Random sequence oligonucleotides contained the same bases in a scrambled sequence. FITC-tagged oligonucleotides (2 μM final each) in DMEM were mixed with Lipofectamine (0.1 μg/ml, final concentration) and incubated with cardiac myocytes at 37°C for 8 h before treatments. Immediately before exposure to hypoxia, fresh medium with oligonucleotides was added. Fluorescence was visualized after 24, 36, and 48 h of exposure to hypoxia. Fresh oligonucleotides (400 nM) were added during the course of the experiment at 24 and 36 h.
Necrosis Assays.
Trypan blue exclusion assays were performed to identify compromised plasma membranes. Culture media were removed and replaced with 0.4% trypan blue in PBS for 15 min at 37°C. Positive cells were quantitated microscopically. Lactate dehydrogenase (LDH) in culture media was measured by using a colorimetric LDH assay kit (Sigma).
Subcellular Fractionation.
Cell fractionation and alkali treatments were performed as described (18). In brief, after treatments, cells were washed with PBS and lysed by homogenizing in buffer containing 100 mM mannitol, 10 mM Tris, 5 mM MgCl2, 1 mM EGTA, and 1 mM DTT. Samples were split in two, and the pH of one half was adjusted to 11.0 with 0.1 M NaHCO3 and incubated on ice for 20 min. Samples were fractionated by differential centrifugation. Intact cells and nuclei were separated by centrifugation at 120 × g for 5 min; supernatants were centrifuged at 10,000 × g for 10 min to collect the heavy (mitochondrial) membrane pellet. Cytoplasmic fractions were obtained by centrifuging supernatants at 100,000 × g for 30 min.
MitoTracker Red Labeling and Confocal Microscopy.
Cardiac myocytes plated on Nunc glass dishes were incubated with 0.2 μM MitoTracker Red in serum-free media for 20 min. Cells were rinsed and fixed for 30 min with 3.7% paraformaldehyde in PBS. To analyze BNIP3-transfected cardiac myocytes, cultures were cotransfected with pGFP to identify transfected cells and stained as stated above. Cells were analyzed by using an Odyssey XL laser-scanning confocal microscope (Noran Instruments, Middleton, WI) at excitation wavelength 579 nm and emission wavelength 599 nm; GFP was visualized at 520 nm.
Transfections.
Cardiac myocytes were transfected on day 1 after isolation with polycationic liposomes as described (30). Transfection efficiency was 11.5% ± 1.6% as estimated by GFP expression from a transfected GFP plasmid (32). The transfection procedure alone did not affect the level of apoptosis during treatments (data not shown). Apoptosis of transfected myocytes was quantitated by cotransfecting the β-galactosidase (β-Gal) gene, costaining with 5-bromo-4-chloro-3-indolyl β-D-galactoside and Hoechst 33342, and counting condensed Hoechst-positive nuclei as described (30). For acidosis, the pH of the medium was adjusted to 6.5 by adding lactic acid (16.5 mM) and phosphoric acid as described (26). The pH was maintained at 6.5 for the duration of the incubation by adding additional phosphoric acid as necessary. For MitoTracker Red analysis of transfected cells, myocytes were cotransfected with BNIP3 or BNIP3 Δ-TM and pGFP. After 3 days, transfected cultures were made acidic or remained at neutral pH for a further 8 h. Cultures were stained, fixed, and analyzed by confocal microscopy.
Statistics.
Error bars represent SEM; significance was calculated by using ANOVA software.
Results
BNIP3 Accumulation and Cell Death During Hypoxia-Acidosis.
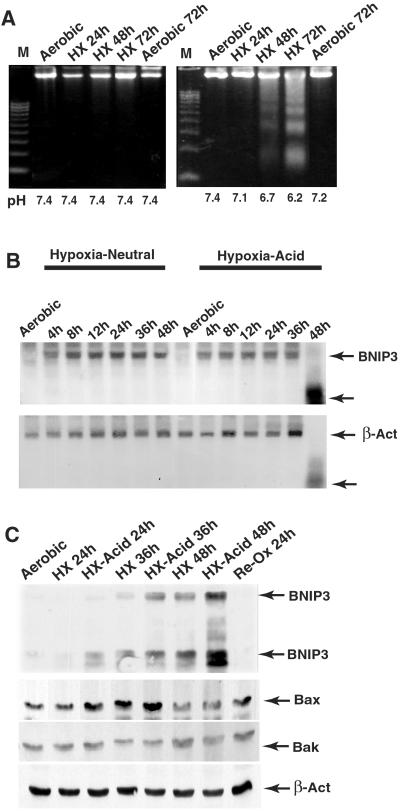
DNA fragmentation and nuclear condensation were measured in cardiac myocytes subjected to hypoxia in neutral or acidic pH media. Extensive fragmentation of DNA was observed in hypoxic-acidotic cells, but not in hypoxic cells maintained at neutral pH (Fig. 1A). After 72 h of hypoxia without neutralization, [pH]o fell to 6.4, and 63% ± 8% of myocytes contained condensed Hoechst-stained nuclei, compared with 7.1% of cells in pH-neutral cultures (data not shown). No significant changes occurred in propidium iodide staining under either condition (not shown). BNIP3 accumulation is shown in Fig. 1 B and C. BNIP3 mRNA levels increased progressively during hypoxia and peaked after 8 h at similar levels in both neutralized and acidic conditions. BNIP3 mRNA was degraded after 48 h of hypoxia in acidic, but not in neutral, pH media, probably as a consequence of cell death. BNIP3 protein accumulated more rapidly under acidic pH and peaked at a significantly higher level than the pH-neutral samples (3.3 ± 0.7-fold; n = 3; P < 0.01). No corresponding changes occurred in Bax, Bak, or β-actin proteins; the apparent small increase of Bax at 24 and 36 h was not reproducible (see ref. 27). These results demonstrate that hypoxia activates BNIP3 transcription and protein accumulation is stabilized by acidosis.
Figure 1.
BNIP3 induction correlates with increased apoptosis. (A) Cardiac myocytes were subjected to hypoxia with (Left) or without (Right) medium change, harvested at the indicated times, and processed for genomic fragmentation assays. The pH of the media at the time of harvesting is shown at the bottom. (B) Northern blots of cardiac myocyte RNA extracted from hypoxic cultures. (C) Western blot analysis of proteins from hypoxic cardiac myocytes as in A. Anti-BNIP3 recognizes two bands at approximately 60 and 30 kDa, corresponding to SDS-resistant homodimers and monomers, respectively. Lower gels show the same blot probed with anti-Bax, Bak, and β-actin. Results are representative of at least three experiments.
Cell Death Is Blocked by BNIP3 Antisense Oligonucleotides.
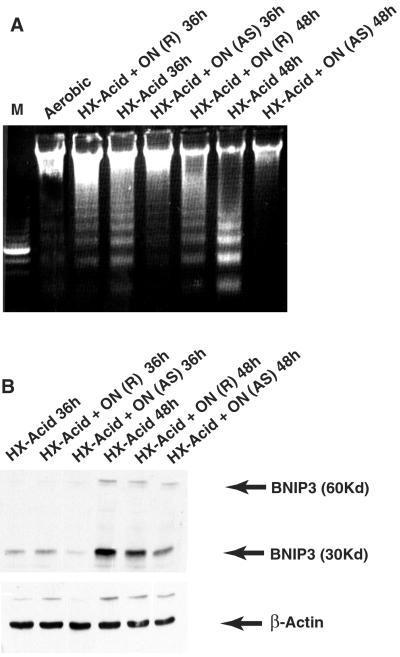
To determine whether a relationship existed between BNIP3 accumulation and cell death, cardiac myocytes were treated with antisense BNIP3 oligonucleotides before and during exposure to hypoxia as described in Materials and Methods. As shown in Fig. 2A, treatment with antisense BNIP3 reduced DNA fragmentation markedly at both time points. Random sequence oligonucleotides also delayed DNA fragmentation slightly, reflecting a small nonspecific side effect of oligonucleotide treatment. Uptake of oligonucleotides was >95%, estimated microscopically by visualizing the fluorescent tag (not shown). Antisense oligonucleotides reduced BNIP3 protein by 78% ± 8% (n = 3) during the incubations (Fig. 2B). Oligonucleotide treatment had no significant effect on levels of β-actin. These results show that even incomplete suppression of BNIP3 protein production by antisense treatment causes a dramatic reduction of cell death.
Figure 2.
BNIP3 antisense inhibits programmed death of cardiac myocytes. (A) Cultures were incubated with BNIP3 (AS) or random sequence (R) oligonucleotides (ON) as described in Materials and Methods, subjected to hypoxia-acidosis as indicated, and analyzed for DNA fragmentation. (B) Cardiac myocytes were treated with oligonucleotides as in A, and extracted proteins were analyzed by Western blots with anti-BNIP3 and β-actin. Results are representative of three experiments.
Acidosis Increases BNIP3 Binding to Mitochondrial Membranes.
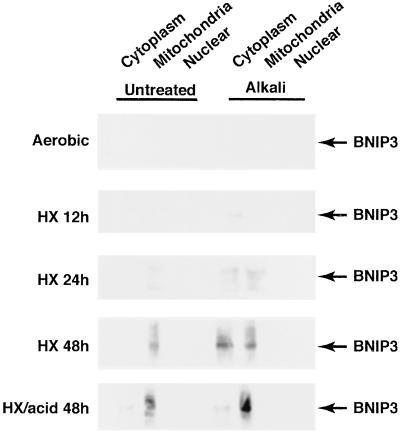
BNIP3 accumulates under hypoxia at neutral and acidic pH, but cell death occurs only with coincident acidosis. To test the possibility that low pH activates the proapoptotic functions of BNIP3 by stimulating its intracellular translocation, cells were separated into subcellular fractions after hypoxia exposure. After cell lysis, samples were treated with alkaline buffer to dislodge loosely membrane-associated protein, as described (18, 20). Results from Western blots of untreated and alkali-solubilized fractions are shown in Fig. 3. BNIP3 levels were initially detected in the alkalinized cytoplasmic fraction at 12 h of hypoxia and increased progressively at 24 and 48 h. BNIP3 was present exclusively in the mitochondrial fraction of hypoxic samples without alkali treatment and was primarily mitochondrial in hypoxia-acidosis samples. Alkaline treatment caused a significant shift of BNIP3 into the cytoplasmic fraction from hypoxic-neutral but not hypoxia-acidic treatments. In 48-h hypoxia-neutral samples, 73% of BNIP3 was in the cytoplasm compared with <10% of the hypoxia-acidosis sample (mean of two determinations). Alkali treatment did not affect the distribution of succinate dehydrogenase that was localized in the mitochondrial and nuclear fractions in all samples (data not shown). These results show that acidic pH promotes a stronger alkali-resistant association of BNIP3 with mitochondrial membranes.
Figure 3.
Association of BNIP3 with subcellular fractions. Cardiac myocytes were subjected to hypoxia as described in Fig. 1. At the indicated times cells were harvested, rinsed, lysed, and subjected to alkaline solubilization (right-hand gels) as described in Materials and Methods. After treatments, samples were separated into subcellular fractions and analyzed by Western blots. Blots were reprobed with antisuccinate dehydrogenase probes to define the purity of fractions (not shown). Results are representative of three separate experiments.
Characteristics of Hypoxia-Acidosis-Mediated Cell Death Pathway.
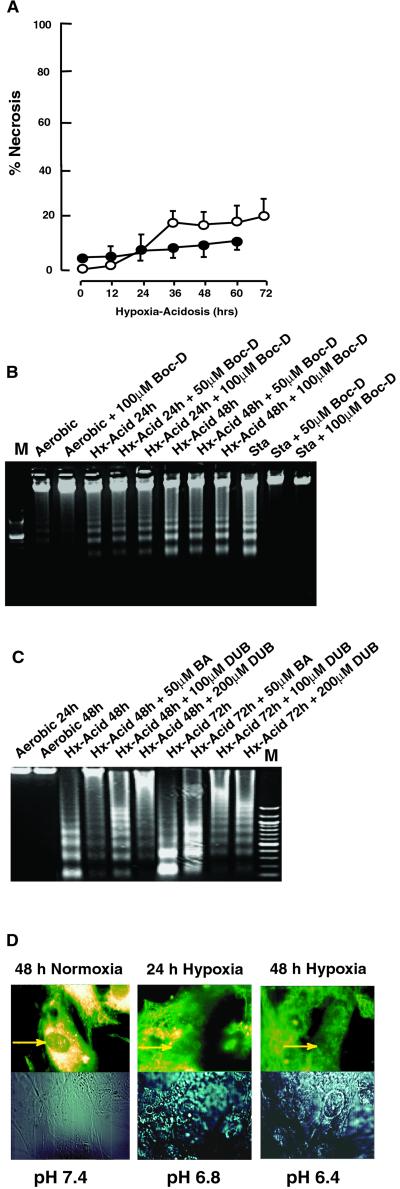
Overexpression of BNIP3 by transient transfection of cell lines was reported to activate a necrosis-like pathway that included early loss of plasma membrane integrity (18). To determine whether a similar pathway was activated by hypoxia-acidosis we analyzed membrane permeability changes, caspase activity, and MPTP function. Progressive hypoxia-acidosis with >70% cell death caused <20% loss of membrane integrity even at the late time points as determined by trypan blue exclusion and LDH release (Fig. 4A). These results agree with our previous report that propidium iodide staining did not change significantly under these treatments (26). Fig. 4B shows the effects of the broad-range caspase inhibitor Boc-D on DNA fragmentation. DNA fragmentation was unaffected by Boc-D and the same result was obtained by using another broad-range caspase inhibitor, ZVAD (not shown). Also, in results not shown here, we detected no cleavage of the caspase-3 substrate poly[ADP-ribose]polymerase 1 (PARP) in extracts of hypoxia-acidosis-treated cells.
Figure 4.
Characteristics of programmed cell death by BNIP3. (A) Cardiac myocytes were subjected to hypoxia-acidosis as described in Fig. 1. At the indicated times, samples of media were taken for analysis of LDH activity (open circles) or plates were stained with trypan blue (closed circles). Data are expressed as percent of cells stained with trypan blue or percent LDH released relative to total LDH in homogenates. (B) Cardiac myocytes were subjected to hypoxia-acidosis in the absence or presence of the broad-range caspase inhibitor Boc-D, as indicated. Staurosporine (Sta; 1.0 μM for 8 h) is shown as a positive control. (C) Cardiac myocytes were subjected to hypoxia-acidosis in the absence or presence of the MPTP inhibitors BA or DUB, as indicated. (D) Cardiac myocytes were exposed to normoxic or hypoxia-acidosis conditions. At the times indicated, cells were loaded with MitoTracker Red dye and analyzed by confocal microscopy. Arrows indicate intense staining around nuclei in aerobic myocytes and reduced/diffuse staining under hypoxia. Results are representative of three experiments.
To test for a contribution of MPTP activity in this pathway, cardiac myocytes were exposed to hypoxia-acidosis in the presence and absence of the specific MPTP inhibitors bongkrekic acid (BA) and decylubiquinone (DUB). Fragmentation of cardiac myocyte DNA by hypoxia-acidosis was blocked at the 48-h time point by either 50 μM BA or 200 μM DUB (Fig. 4C, lanes 4 and 6). Even after 72 h when the control DNA was fully cleaved into small fragments, both BA and DUB treatment mediated protection. As an additional test for MPTP opening, cardiac myocytes were loaded under identical conditions with MitoTracker Red dye and analyzed as described in Materials and Methods. Aerobic cultures displayed bright punctate patterns of intense staining around the nuclei, characteristic of mitochondrial staining (Fig. 4D Left). Fluorescence was weaker in cells exposed to 24 h of hypoxia-acidosis and absent after 48 h (Fig. 4D Right). These results are consistent with hypoxia-acidosis-induced MPTP opening as part of the cell death pathway. In results not shown here, we did not detect significant leakage of cytochrome c from the mitochondria during hypoxia-acidosis, under conditions where significant leakage occurred from S-nitrosoglutathione-treated myocytes (32). This result seems anomalous with MPTP opening but is consistent with a report documenting a similar effect on BNIP3-transfected cells (18).
Acid and Hypoxia-Dependent Cell Death Induced by Wild-Type BNIP3 Transfection.
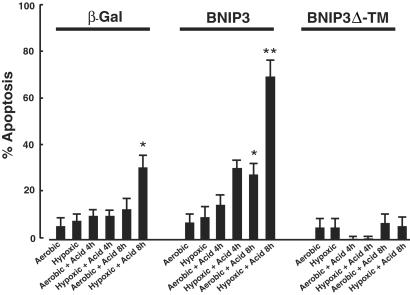
Our results indicate that the accumulation of endogenous BNIP3 protein during hypoxia is not sufficient to activate cardiac myocyte death at neutral pH. To determine whether transfected BNIP3 is also regulated by pH, cardiac myocytes were transfected with wild-type BNIP3, BNIP3 with a deletion in the transmembrane domain (BNIP3 Δ-TM), or empty vector. Cells were cotransfected with β-Gal to identify transfected cells, and costained with sarcomeric myosin to identify myocytes. Apoptotic nuclei were quantitated as described (26, 30). After transfection, cultures were exposed to aerobic or hypoxic conditions at low or neutral pH for the times indicated in Fig. 5. Acidic media, with or without hypoxia, caused a small increase of the apoptotic index in control cells at 4 and 8 h, but the increase was only significant after 8 h of hypoxia-acidosis. Apoptotic indices of BNIP3-transfected cells were not significantly different from controls under aerobic, hypoxic, or aerobic-acidotic 4-h treatments. However, the indices were significantly increased in 4-h hypoxic-acidotic treatments and both 8-h treatments compared with aerobic or hypoxia-only treatments and controls. The significant increase of apoptosis by acid treatment under aerobic incubation confirms the regulation of BNIP3 activity by pH. The synergistic effect of combined acid and hypoxia probably reflects a lower intracellular pH mediated by this condition. BNIP3 Δ-TM-transfected myocytes exhibited lower rates of apoptosis than both BNIP3 and control. The lower incidence of apoptosis was significantly different from the control under the conditions of 8-h hypoxia with acidosis. This finding may reflect a dominant negative effect of BNIP3 Δ-TM.
Figure 5.
Programmed death of BNIP3-transfected cardiac myocytes. Cardiac myocytes were transfected with expression plasmids containing β-Gal plus empty vector, β-Gal with BNIP3, or β-Gal with BNIP3 Δ-TM, as indicated. After 48 h, transfected cultures were exposed to continued normoxic culture or to hypoxia, acidosis, or hypoxia + acidosis as described in Materials and Methods. At the indicated times, plates were rinsed, costained with 5-bromo-4-chloro-3-indolyl β-D-galactoside and Hoechst 33342, and visualized by microscopy. Bars indicate SEM from at least 200 5-bromo-4-chloro-3-indolyl β-D-galactoside-positive cells per condition. *, P < 0.02, and **, P < 0.01, refer to the respective condition compared with aerobic controls. BNIP3 Δ-TM transfection with 8-h hypoxia + acid was significantly different from either β-Gal (P < 0.05) or BNIP3 8-h hypoxia + acid (P < 0.01).
Wild-Type BNIP3 Transfection Stimulates MPTP Opening.
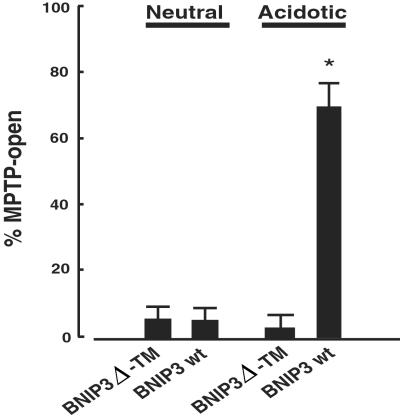
To determine whether MPTP opening was associated with BNIP3 activity, cardiac myocytes were cotransfected with pGFP and either wild-type BNIP3 or BNIP3 Δ-TM and exposed to neutral or acidic pH for 8 h. Cultures were stained with MitoTracker Red, fixed, and analyzed by confocal microscopy. GFP-positive cells were scored as MPTP-closed if the staining was sharp and punctate or MPTP-open if staining was diffuse (as shown in Fig. 4). In untreated-transfected cultures, 95% of GFP-positive cells were scored as closed-MPTP; this fraction did not change significantly in the BNIP3 Δ-TM-acid treatment group. In contrast, open-MPTP myocytes significantly increased in cultures transfected with wild-type BNIP3 and subjected to acidic conditions (Fig. 6). These results confirm that MPTP opening is an integral part of this pathway of cardiac myocyte death mediated by BNIP3 and acidosis.
Figure 6.
Acid-mediated opening of MPTP in BNIP3-transfected myocytes. Cardiac myocytes were cotransfected with pGFP and BNIP3 or BNIP3 Δ-TM, as indicated. After 48 h, transfected cultures were exposed to continued culture at neutral pH or to acidosis for an additional 8 h as described in Materials and Methods. Cultures were stained with MitoTracker Red dye, fixed, and analyzed by microscopy. At least 50 GFP-positive myocytes per condition were scored from four separate dishes.
Discussion
We present evidence that BNIP3 mediates cardiac myocyte death under conditions of hypoxia and acidosis. BNIP3 mRNA and protein were almost undetectable in aerobic myocytes but accumulated significantly during hypoxia. Acidosis enhanced BNIP3 protein, but not mRNA accumulation, by almost 3-fold, indicating increased protein translation or stability at low pH. Antisense-mediated depletion of BNIP3 dramatically reduced cardiac myocyte death by hypoxia-acidosis. Despite significant accumulation of BNIP3 under hypoxia at neutral pH, cell death did not occur without coincident acidosis, which suggests that, at neutral pH, BNIP3 exists in an inactive state, possibly similar to other Bcl-2 family proteins that require a death signal for activation (21). Acidosis promoted a tighter association between BNIP3 and the mitochondria, because BNIP3 could be dislodged from hypoxia-neutral myocyte mitochondria by alkali treatment, but not from hypoxia-acidotic mitochondria. Therefore, integration of BNIP3 into mitochondrial membranes, promoted by acidosis, may constitute the activation step. Acid-mediated stabilization of BNIP3 may be a consequence of this sequestration by protecting against cellular proteases. Alternatively, the increase in myocyte death during acidosis may simply reflect enhanced levels of BNIP3 tilting the balance between pro- and antiapoptotic Bcl-2 proteins present in the cells.
Previous work implicated a necrosis-like pathway of death in BNIP3-transfected nonmuscle cells (18). Our results with cardiac myocytes indicate a different pathway for hypoxia-acidosis that is more similar to apoptosis. Necrosis typically involves a loss of plasma membrane integrity and ATP as early events in cell death (8, 18). We did not observe these effects in cardiac myocytes subjected to hypoxia-acidosis; the plasma membrane remained intact (Fig. 4A) and ATP levels were maintained in >70% of control cultures until late time points (data not shown; see ref. 26). However, the cell death pathway mediated by BNIP3 is unusual in that it does not seem to involve caspase activation. Cell death was not blocked by either of two broad-range caspase inhibitors, and PARP, a caspase-3 substrate, did not undergo detectable cleavage (26). MPTP opening, however, seems to be part of the BNIP3-mediated program in our system. MPTP inhibitors effectively prevented cell death, and MitoTracker Red dye was not retained in cells subjected to hypoxia-acidosis or in BNIP3-transfected cells subjected to acidosis. The loss of MitoTracker dye suggests that BNIP3 integration mediates increased mitochondrial permeability and probably loss of membrane potential.
Analyses of BNIP3-transfected cardiac myocytes confirmed that pH regulates the function of this protein. Exposure of wild-type BNIP3-transfected myocytes to acid caused a 4- to 6-fold increase in the apoptotic index compared with pH-neutral aerobic cells; the combination of acidosis and hypoxia caused a 12-fold increase. Both values were significantly different from nonacidic aerobic or hypoxic BNIP3-transfected cells, or from control, empty vector-transfected cells. This finding provides strong supporting evidence that both endogenous and transfected BNIP3 are subject to pH regulation. The apoptotic index of cardiac myocytes transfected with BNIP3 Δ-TM was significantly lower than that in cells transfected with either wild-type BNIP3 or empty vector after treatments, possibly because BNIP3 Δ-TM dimerizes with endogenously generated BNIP3 and the heterodimers are unable to integrate into the mitochondrial membranes and activate programmed death. Therefore, BNIP3 Δ-TM may behave like a dominant negative.
We reported that hypoxia alone was not a stimulus for apoptosis (26). The present results confirm this report and implicate a pH-sensitive pathway of ischemic cell death with a central role for BNIP3. BNIP3 may also mediate low-level apoptosis during chronic hypoxia, even when the extracellular pH is neutralized, because [pH]i is likely to fluctuate more than [pH]o when the intracellular acid production increases (28, 33). This decrease of [pH]i may be sufficient to activate BNIP3 and the death pathway in some cells. Consistent with this model, proton pumps have been shown to play a role in apoptosis signaling (10, 27), and acidosis has been shown to correlate with apoptosis in many other systems (34, 35).
Loss of cardiac myocytes is a central feature of heart disease of both ischemic and nonischemic origin (reviewed in refs. 10, 36, and 37). It has been described in multiple regions of the myocardium during infarction, hibernation (38), and both ischemia and subsequent reperfusion (13, 39). Death pathways involving necrosis, apoptosis, and oncosis have been described (reviewed in refs. 8 and 40). The probability that hypoxia and acidosis coexist in diseased and/or infarcted myocardial tissue is high because of the disrupted vasculature and elevated lactic acid production in ischemic tissue (39, 41). Therefore, BNIP3 may be expected to play a significant role in cell loss during ischemic heart disease. Although the experiments described in the current report were implemented by using neonatal cardiac myocytes, we have detected BNIP3 mRNA and protein in intact adult hearts (data not shown). It seems probable that a similar death pathway occurs in other cell types including those in ischemic heart tissue. Combined hypoxia and acidosis reflects a greater hemodynamic disruption than acidosis or hypoxia alone, and the dual signal may provide a selective advantage by activating the death pathway only as a last resort. Acidosis may also activate BNIP3 in skeletal muscle to allow myocyte removal under conditions of severe ischemia or hypoxia in which the probability of irreversible damage is high. Finally, antisense BNIP3 or the dominant negative action of BNIP3 Δ-TM may have a therapeutic role in the treatment of ischemic heart disease or myocardial infarction.
Acknowledgments
We thank Dr. John Barrett for his patience and assistance with the confocal microscope. This work was supported by Grants HL44578 and HL69812 from the National Institutes of Health and Grant BM034 from the Chiles Endowment Biomedical Research Program of the Florida Department of Health (to K.A.W.). The opinions, findings, and conclusions expressed in this publication are those of the author(s) and do not necessarily reflect the views of the Biomedical Research Program or the Florida Department of Health.
Abbreviations
- MPTP
mitochondrial permeability transition pore
- LDH
lactate dehydrogenase
- β-Gal
β-galactosidase
- BA
bongkrekic acid
- DUB
decylubiquinone
References
- 1.Jennings R B, Steebergen C, Reimer K A. Monogr Pathol. 1995;37:47–80. [PubMed] [Google Scholar]
- 2.Vanoverschelde J-L J, Wijns W, Borgers M, Heyndrickx G, Depre C, Flambeng W, Melin J A. Circulation. 1997;95:1961–1971. doi: 10.1161/01.cir.95.7.1961. [DOI] [PubMed] [Google Scholar]
- 3.Zurbier C J, van Iterson M, Ince C. Cardiovasc Res. 1999;44(3):488–497. doi: 10.1016/s0008-6363(99)00231-x. [DOI] [PubMed] [Google Scholar]
- 4.Narula J, Hajjar R J, Dec G W. Cardiol Clin. 1998;16(4):691–710. doi: 10.1016/s0733-8651(05)70045-x. [DOI] [PubMed] [Google Scholar]
- 5.Dennis S C, Gevers W, Opie L H. J Mol Cell Cardiol. 1991;23:1077–1086. doi: 10.1016/0022-2828(91)91642-5. [DOI] [PubMed] [Google Scholar]
- 6.Neely J R, Grotyohann L W. Circ Res. 1984;55:816–824. doi: 10.1161/01.res.55.6.816. [DOI] [PubMed] [Google Scholar]
- 7.Webster K A, Discher D J, Hernandez O M, Yamashita K, Bishopric N H. Adv Exp Med Biol. 2000;475:161–175. doi: 10.1007/0-306-46825-5_16. [DOI] [PubMed] [Google Scholar]
- 8.Kajstura J, Cheng W, Reiss K, Clark W A, Sonnenblick E H, Krajewski S, Reed J C, Olivetti G, Anversa P. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 9.Gottlieb R A, Burleson K O, Kloner R A, Babior B M, Engler R L. J Clin Invest. 1994;94:1612–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anversa P, Kajstura J. Circ Res. 1998;82:1231–1233. doi: 10.1161/01.res.82.11.1231. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz L D, Fennessey P V, Shikes R H, Kong Y. Circulation. 1994;89:1792–1801. doi: 10.1161/01.cir.89.4.1792. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Watanabe M, Engelman D T, Engelman R M, Schley J A, Maulik N, Ho Y S, Oberley T D, Das D K. J Mol Cell Cardiol. 1996;28:1759–1767. doi: 10.1006/jmcc.1996.0165. [DOI] [PubMed] [Google Scholar]
- 13.Fliss H, Gattinger D. Circ Res. 1996;79:949–956. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 14.Williams F M, Kus M, Tanda K, Williams T J. Br J Pharmacol. 1994;111:1123–1128. doi: 10.1111/j.1476-5381.1994.tb14861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochachka P W, Buck L T, Doll C J, Land S C. Proc Natl Acad Sci USA. 1996;93:9493–9498. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buja L M, Eigenbrodt M L, Eigenbrodt E H. Arch Pathol Lab Med. 1993;117:1208–1214. [PubMed] [Google Scholar]
- 17.Majno G, Joris I. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg A H. Mol Cell Biol. 2000;20:5454–5468. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray R, Chen G, Vande Velde C, Cizeau J, Park J H, Reed J C, Gietz R D, Greenberg A H. J Biol Chem. 2000;275:1439–1448. doi: 10.1074/jbc.275.2.1439. [DOI] [PubMed] [Google Scholar]
- 20.Goping I S, Gross A, Lavoie J N, Nguyen M, Jemmerson R, Roth K, Korsemeyer S J, Shore G C. J Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams J M, Cory S. Trends Biochem Sci. 2001;26:61–66. doi: 10.1016/s0968-0004(00)01740-0. [DOI] [PubMed] [Google Scholar]
- 22.Green D, Reed J. Science. 1998;281:1309–1314. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 23.Earshaw W, Martins L, Kaufmann S. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 24.Crompton M. Curr Opin Cell Biol. 2000;12:414–419. doi: 10.1016/s0955-0674(00)00110-1. [DOI] [PubMed] [Google Scholar]
- 25.Bruick R K. Proc Natl Acad Sci USA. 2000;97:9082–9087. doi: 10.1073/pnas.97.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webster K A, Discher D J, Kaiser S, Hernandez O M, Sato B, Bishopric N H. J Clin Invest. 1999;104:239–252. doi: 10.1172/JCI5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karwatowska-Prokopczuk E, Nordberg J A, Li H L, Engler R L, Gottlieb R A. Circ Res. 1998;82:1139–1144. doi: 10.1161/01.res.82.11.1139. [DOI] [PubMed] [Google Scholar]
- 28.Webster K A, Bishopric N H. J Mol Cell Cardiol. 1992;24:741–751. doi: 10.1016/0022-2828(92)93388-z. [DOI] [PubMed] [Google Scholar]
- 29.Webster K A, Discher D, Bishopric N H. J Biol Chem. 1993;268:16852–16859. [PubMed] [Google Scholar]
- 30.Dougherty C J, Kubasiak L, Prentice H, Andreka P, Bishopric N H, Webster K A. Biochem J. 2002;362:561–571. doi: 10.1042/0264-6021:3620561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webster K A, Muscat G E O, Kedes L. Nature (London) 1988;332:553–561. doi: 10.1038/332553a0. [DOI] [PubMed] [Google Scholar]
- 32.Andreka P, Zang J, Dougherty C, Slepak T I, Webster K A, Bishopric N H. Circ Res. 2001;88:305–312. doi: 10.1161/01.res.88.3.305. [DOI] [PubMed] [Google Scholar]
- 33.Webster K A, Discher D J, Bishopric N H. Circ Res. 1994;75:361–371. doi: 10.1161/01.res.74.4.679. [DOI] [PubMed] [Google Scholar]
- 34.Gottlieb R A, Giesing H A, Zhu J Y, Engler R L, Babior B M. Proc Natl Acad Sci USA. 1995;92:5965–5968. doi: 10.1073/pnas.92.13.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Sala D, Collado-Escobar D, Mollinedo F. J Biol Chem. 1998;270:6235–6242. doi: 10.1074/jbc.270.11.6235. [DOI] [PubMed] [Google Scholar]
- 36.Kajstura J, Leri A, Finato N, Loreto C, Beltrami C A, Anversa P. Proc Natl Acad Sci USA. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narula J, Haider N, Virmani R, DiSalvo T G, Kolodgie F D, Hajjar R J, Schmidt Y, Semigran M J, Dec G W, Khaw B A. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 38.Heusch G, Schulz R. J Mol Cell Cardiol. 1996;28:2359–2372. doi: 10.1006/jmcc.1996.0229. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, Ma L, Linfert D R, Lai T, Fallon J T, Gillam L D, Waters D D, Tsongalis G J. J Am Coll Cardiol. 1997;30:1407–1412. doi: 10.1016/s0735-1097(97)00309-4. [DOI] [PubMed] [Google Scholar]
- 40.Ohno M, Takemura G, Ohno A, Misao J, Hayakawa Y, Minatoguchi S, Fujiwara T, Fujiwara H. Circulation. 1998;98:1422–1430. doi: 10.1161/01.cir.98.14.1422. [DOI] [PubMed] [Google Scholar]
- 41.Krayenbuehl H P, Hess O M. J Myocard Ischemia. 1992;4:49–58. [Google Scholar]