Abstract
In the Chapel Hill colony of factor VIII-deficient dogs, abnormal sequence (ch8, for canine hemophilia 8, GenBank no. AF361485) follows exons 1–22 in the factor VIII transcript in place of exons 23–26. The canine hemophilia 8 locus (ch8) sequence was found in a 140-kb normal dog genomic DNA bacterial artificial chromosome (BAC) clone that was completely outside the factor VIII gene, but not in BAC clones containing the factor VIII gene. The BAC clone that contained ch8 also contained a homologue of F8A (factor 8 associated) sequence, which participates in a common inversion that causes severe hemophilia A in humans. Fluorescence in situ hybridization analysis indicated that exons 1–26 normally proceed sequentially from telomere to centromere at Xq28, and ch8 is telomeric to the factor VIII gene. The appearance of an “upstream” genomic sequence element (ch8) at the end of the aberrant factor VIII transcript suggested that an inversion of genomic DNA replaced factor VIII exons 22–26 with ch8. The F8A sequence appeared also in overlapping normal BAC clones containing factor VIII sequence. We hypothesized that homologous recombination between copies of canine F8A inside and outside the factor VIII gene had occurred, as in human hemophilia A. High-resolution fluorescent in situ hybridization on hemophilia A dog DNA revealed a pattern consistent with this inversion mechanism. We also identified a HindIII restriction fragment length polymorphism of F8A fragments that distinguished hemophilia A, carrier, and normal dogs' DNA. The Chapel Hill hemophilia A dog colony therefore replicates the factor VIII gene inversion commonly seen in humans with severe hemophilia A.
Hemophilia A is a genetic bleeding disorder due to deficiency of coagulation factor VIII and is characterized by spontaneous hemorrhage (particularly into joints) and excessive bleeding after trauma or surgery. The clinical severity is inversely proportional to the circulating factor VIII level in plasma, and severe disease is associated with levels of factor VIII <1% of normal (1). About 40% of severe human hemophilia A is associated with recombination between a transcribed DNA sequence within intron 22 of the factor VIII gene and nontranscribed copies of homologous sequences that are telomeric to the factor VIII gene on Xq28 (2–6). The remaining cases are caused by hundreds of distinct point mutations, deletions, or insertions (7). Several experimental animal models of hemophilia A (spontaneous and artificially induced knockouts) have been characterized with regard to their phenotype and underlying genetic defect (8–11). The hemophilia A dog colony at the University of North Carolina at Chapel Hill was founded in 1947 from a purebred male Irish Setter with severe hemophilia A (8). The colony has been an important model for studies of hemostasis and preclinical testing of therapeutic factor VIII concentrates (12–15). Although the gene defects responsible for canine hemophilia B (coagulation factor IX deficiency) have been elucidated in at least two spontaneous hemophilia B dog colonies (16, 17), the molecular mechanism for spontaneous hemophilia A in animals has remained unknown. During analysis of the Chapel Hill hemophilia A dog colony, we discovered an abnormal transcript in which the normal canine factor VIII sequence changes to a novel sequence (designated ch8 for canine hemophilia 8: GenBank no. AF361485) immediately following exon 22. This finding suggested a mutation that permitted splicing of ch8 into the factor VIII transcript but did not indicate the mechanism for the mutation. In this report, we present evidence for an inversion in the Chapel Hill hemophilia A dog colony that is analogous to the common inversion seen in humans, indicating a recurring mechanism for hemophilia A due to instability of genomic DNA in the factor VIII gene in different species.
Materials and Methods
Chapel Hill Hemophilia A Dogs.
Experiments were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee. Hemizygous males and homozygous females from the Chapel Hill hemophilia A colony lack coagulation factor VIII activity, demonstrate prolonged in vitro laboratory clotting parameters, and exhibit a severe hemophilia A bleeding phenotype.
RACE.
Total RNA was prepared from euthanized hemophilia A dog liver or spleen by extraction with Trizol (Life Sciences, Bethesda, MD) and used to make polyA RNA with the PolyAttract mRNA isolation kit (Promega, Madison, WI). RACE (5′ and 3′) was performed by using the CLONTECH SMART RACE kit (CLONTECH). Gene-specific primers were designed by using published normal canine factor VIII sequence (18). Synthetic primers were incorporated into the initial first- and second-strand DNA synthesis steps, and nested primers were also used in subsequent amplification steps. Initial PCR was performed by using a Perkin–Elmer 480 PCR cycler and “touchdown” PCR conditions described for the CLONTECH SMART RACE kit. Nested PCR was performed by using a Perkin–Elmer 2400 cycler. See Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org, for PCR amplification strategy.
TA Cloning of PCR Fragments.
PCR fragments were cloned into pCR3.1 (Invitrogen) and sequenced with universal and/or gene-specific primers from normal canine factor VIII and ch8 sequence.
DNA Sequence Determination and Analysis.
PCR products were sequenced by Bioserve (Laurel, MD). Analysis of sequence data was performed with dnastar (DNAstar, Madison, WI) by using the sequence editing, mapping, comparison, and assembly modules. Comparison of canine factor VIII, ch8, and factor 8-associated locus (F8A) sequence with GenBank submissions was performed with the Entrez Genomes blastn/blastp programs (19).
Bacterial Artificial Chromosome (BAC) Library Screening.
The RPCI-81 BAC library (Children's Hospital of Oakland Research Institute, Oakland, CA), which contains normal male Doberman Pinscher genomic DNA, was screened with 32P-labeled DNA probes from normal canine factor VIII exon 14, exon 22, or ch8 from the abnormal hemophilia A transcript. The exon 14 probe was prepared from the 1.2-kb EcoRI digest fragment from the pCR3.1 TA clone containing part of the B-domain amplified by using reverse transcription–PCR. The 170-bp exon 22 probe was obtained by PCR amplification of exon 22 from normal genomic DNA with exon 22 primers. The ch8 probe was obtained by digestion of the TA clone containing the exon 22/ch8 fusion fragment with RcaI and EcoO109I restriction enzymes (Fig. 1) and gel purification of the 423-bp fragment. Positive clones identified by autoradiography of robotically spotted filters were grown in chloramphenicol–LB medium and BAC DNA purified after alkaline lysis and ultracentrifugation in cesium chloride/trifluoroacetic acid (Amersham Pharmacia Biotech). DNA for fluorescent in situ hybridization was prepared in the absence of ethidium bromide. BAC clones were analyzed for the presence of canine factor VIII exons 2, 14, 22, 23, 23–25, and 26 by PCR by using the TaKaRa LA PCR kit, Ver. 2.1 (Intergen, Purchase, NY) and the primers from the canine factor VIII gene (all primer sequences available on request).
Figure 1.
Chapel Hill canine factor VIII exon 22/ch8 junction. In the Chapel Hill hemophilia A canine factor VIII transcript, the novel sequence ch8 follows exon 22. The predicted amino acid sequence is shown in three-letter code below the nucleic acid sequence data. The ORF of exon 22 continues 22 amino acids after canine factor VIII Met 2116 before the first of several stop codons (depicted as periods) in ch8. Sites of cleavage by various restriction sites used to characterize the genomic DNA or generate DNA probes are shown above the DNA sequence. pA, the polyadenylation signal sequence, AATAAA.
Fluorescence in Situ Hybridization (FISH) Analysis of Normal and Hemophilia A Dog Chromosomes/DNA Fibers.
Metaphase spreads were prepared by standard air-drying methods as described previously (20) by using peripheral blood leukocytes or skin fibroblasts. DNA fibers were obtained by lysis of cells in 2 M MgCl2/25 mM Tris⋅HCl/1%Triton X-100 for 30 min at room temperature, followed by air drying on slides in an upright position for 5 min. Slides were fixed in methanol/acetic acid (3:1) for 20 min. FISH of metaphases and fibers was performed with Spectrum orange, Spectrum green (Vysis, Downers Grove, IL), and Cy5- (Life Science, Boston) labeled DNA by nick translation (20). On each slide, 200 ng of each labeled DNA probe was applied. Repetitive sequences were blocked with normal canine genomic DNA. Ten microliters of a hybridization mixture containing the labeled DNA in 50% formamide, 2× SSC, and 10% dextran sulfate (pH 7.0) were denatured at 75°C for 10 min, then incubated at 37°C for 30 min for preannealing. Slides containing chromosomes or DNA fibers were incubated for at least 1 h at 37°C in 2× SSC and dehydrated in a series of 70, 80, and 90% ethanol solutions for 2 min each. Slides were then denatured in 70% formamide/2× SSC for 2 min and sequentially in 70, 80, 90, and 100% ethanol at −20°C before hybridization. Posthybridization washes were performed at 45°C sequentially in 50% formamide, 2× SSC for 15 min, then 0.1 × SSC for 10 min. Slides were counterstained with 250 ng/μl 4′,6-diamidino-2-phenylindole-antifade (Vector, Burlingame, CA).
Southern Blot Analysis of Genomic and BAC DNA.
Genomic DNA (10–20 μg) was digested overnight with various restriction enzymes and subjected to 0.7% agarose gel electrophoresis. After UV photography, the gel was soaked for 30 min in denaturing solution, then subjected to downward capillary transfer of DNA fragments onto BrightStar-Plus nylon membrane per the manufacturer's directions (Ambion, Austin, TX). After UV crosslinking, the membrane was prehybridized in Hybrisol II (Intergen) at 68° for at least 1 h, then the appropriate 32P-labeled probe was added, and hybridization was performed overnight at 68°. The membrane was subsequently washed twice in 2 × SSC at 45°, once in 0.5 × SSC/0.4% SDS at 55°, and once in 0.5 × SSC/0.4% SDS at 68° before autoradiography at –80°. BAC clone DNA was analyzed similarly by using 30–300 ng of DNA for the restriction digest.
Results
Determination of cDNA Sequence in Chapel Hill Hemophilia A Dog.
Seven thousand fifty-five nucleotides of factor VIII cDNA sequence were elucidated from analysis of RACE PCR fragments derived from spleen and/or liver mRNA. Normal canine factor VIII sequence starting 187 nucleotides 5′ to the ATG start site proceeded through exon 22. The sequence was in complete agreement with normal canine factor VIII sequence (18), including all arginine cleavage sites for protein C and S and all tyrosines predicted to be phosphorylated after translation. The 5′ sequence upstream of published normal canine factor VIII sequence was in agreement with unpublished normal canine factor VIII data (Christine Hough, personal communication). Immediately after exon 22, there was a novel sequence, ch8, which diverged from the normal canine factor VIII cDNA (Fig. 1). Four hundred twenty-one nucleotides after the exon 22/ch8 junction, there was a polyadenylation signal sequence (AATAAA) followed by a polyA tail. This abnormal transcript was consistently amplified with various primer combinations anchored at the 5′ end by canine factor VIII-specific oligonucleotides and at the 3′ end by either the CLONTECH nested universal primer for the 3′ RACE reaction or by a primer specific to ch8 sequence. Northern blot analysis of the factor VIII transcript was unsuccessful in both normal and hemophilia A dogs because of the extremely low abundance of the factor VIII transcript.
Analysis of the Novel Fusion Transcript Sequence.
A search of all known nucleic acid sequence data for homology with the 421 bp of ch8 sequence was performed with the blastn, Ver. 2.1.2, program (19). The search revealed homology with human BAC clone RP13–228I21 (GenBank accession no. AL356738) that is found telomeric to the human factor VIII gene on the X chromosome (data not shown). A direct comparison of ch8 with the human factor VIII-associated gene (F8A) that is involved in the common inversion that causes severe hemophilia A in humans (2–6) revealed no similarity. The normal canine factor VIII ORF ends 21 amino acids after Met 2116 of the C1 domain of factor VIII. After the first stop codon, all other reading frames in register with the factor VIII reading frame are <25 aa in length. Analysis of all potential ch8 ORFs revealed no significant homology to any known protein sequence by using the blastp program (19).
Exon 22 and ch8 Are Not Contiguous in Hemophilia A Genomic DNA.
Southern blot analysis showed no difference in length between hemophilia A and normal canine DNA fragments that hybridized with the ch8 probe after digestion with BanII, RcaI, AflIII, and BglI, or EcoO109I and BglI. Furthermore, exon 22 and ch8 were not contiguous in normal and hemophilia A dog genomic DNA, because the fragments were larger than predicted if exon 22 were contiguous with ch8 in genomic DNA (see Fig. 6, which is published as supporting information on the PNAS web site).
Exons 23–26 Are Intact in Hemophilia A Dog Genomic DNA.
Exons 23–25 were amplified from normal or hemophilia A dog DNA as a single PCR product. Likewise, exon 26 (which is separated from exons 23–25 by a large intron) could be amplified separately as a 209-bp PCR product (see Fig. 7, which is published as supporting information on the PNAS web site). All amplified PCR products from normal and hemophilia A dogs were subjected to sequence analysis, and hemophilia A and normal dog factor VIII sequences were identical for all PCR products.
ch8 Hybridizes to Dog Chromosome Xq28.
BAC clones that contained either ch8 (291 M9) or factor VIII exons 1–22 (292 C4) were isolated from the normal male canine genomic DNA BAC library RPCI-81 and used in FISH analysis of normal and hemophilia A dogs. Two-color FISH analysis of metaphase spreads from hemophilia A, and normal dog lymphocytes showed localization of factor VIII exons 1–22 to Xq28 as expected in normal and hemophilia A metaphases (20). The ch8 probe localizes to Xq28 in a position immediately telomeric to exons 1–22 in both hemophilia A and normal metaphases (Fig. 2).
Figure 2.
Metaphase FISH Analysis of hemophilia A and normal dogs with ch8 and factor VIII BAC clone probes. FISH of normal and hemophilia A dog chromosomes with BAC clones specific for canine factor VIII exons 1–22 (292 C4) or the novel ch8 sequence (291 M9) localizes both sequences to Xq28. 292 C4 (containing canine factor VIII exons 1–22) is red and 291 M9 (containing the novel ch8 sequence) is green.
Evidence for an Inversion in Hemophilia A Dogs from FISH Analysis of Individual DNA Fibers.
Analysis of individual DNA fibers from normal and hemophilia A dog fibroblasts, with the normal BAC probe containing ch8 and F8A (291 M9) and two overlapping probes (292 C4 and 314 O16) representing portions of dog factor VIII, is shown in Fig. 3. BAC probe 291 M9 is approximately 140 kb in length, and shotgun sequence analysis shows that it contains the novel sequence ch8 as well as F8A but contains no factor VIII sequence (see Fig. 8, which is published as supporting information on the PNAS web site). It was shown by two-color FISH analysis of normal dog metaphase chromosomes (Fig. 2 and Fig. 9, which is published as supporting information on the PNAS web site) to lie in a position telomeric to 292 C4 (which contains factor VIII exons 1–22 of the dog factor VIII gene). In other analyses, we also demonstrated that 291 M9 localized to a position telomeric to probe 314 O16, which contained factor VIII exons 22–26 (Fig. 3 and metaphase FISH data not shown). Thus, the normal dog DNA fiber FISH analysis with these three probes shown in Fig. 3 indicated that 291 M9 is extragenic to and separate from the factor VIII gene, whose direction of transcription must proceed from exons 1–22 (292 C4) toward exons 23–26 (314 O16). The appearance of ch8 at the 3′ end of the hemophilia A factor VIII transcript (despite its position upstream to factor VIII in genomic DNA) makes sense only in the context of an inversion of genomic DNA. Fiber FISH analysis of hemophilia A dog DNA reveals that the extragenic 291 M9 probe signal has been split, and factor VIII sequence (292 C4) has been brought next to the 291 M9 signal where a gap existed in normal dog DNA (Fig. 3). These data confirmed an inversion of genomic DNA in the Chapel Hill hemophilia A dogs.
Figure 3.
DNA fiber FISH analysis of hemophilia A and normal dogs with ch8 and factor VIII BAC clone probes. FISH of normal and hemophilia A dog genomic DNA with factor VIII and related BAC probes is shown. BAC clone 291 M9 (which is outside of the factor VIII gene and contains both ch8 (the 3′ end of the abnormal hemophilia A factor VIII transcript) and F8A (the factor VIII-associated gene sequence) is green. BAC clone 292 C4 (which contains factor VIII exons 1–22 as well as a copy of F8A) is blue. BAC clone 314 016, which contains factor VIII exons 23–26 and one copy of F8A, is red. BAC clones 292 C4 and 314 O16 presumably overlap in the vicinity of intron 22. BAC clones 292 C4 and 314 O16 establish the orientation of factor VIII exons 1–22 and 23–26. Rearrangement of DNA is evident from inversion of part of the 291 M9 probe with part of that for 292 C4 as well as the shift in the normal gap between extragenic probe 291 M9 and factor VIII probes 292 C4 and 314 O16.
BAC Clones 291 M9, 292 C4, and 314 O16 Contain F8A Sequence.
F8A sequence was amplified from the three normal dog DNA BAC clones 291 M9, 292 C4, and 314 O16 by PCR (see Fig. 10, which is published as supporting information on the PNAS web site). All three F8A PCR product sequences were identical. Hence, identical F8A sequence is found within the normal dog factor VIII gene (in clones 292 C4 and 314 O16) and outside the factor VIII gene (in clone 291 M9).
A HindIII Restriction Fragment Length Polymorphism (RFLP) of F8A Sequence That Differs Among Hemophilia A, Carrier, and Normal Dog Genomic DNA.
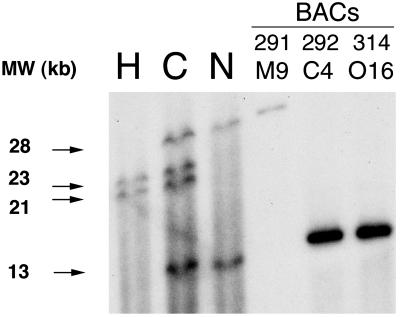
We predicted that a factor VIII gene rearrangement involving multiple genomic copies of the canine F8A sequences would result in an RFLP that could distinguish hemophilia A, carrier, and normal dogs' genomic DNA. Fig. 4 shows a Southern blot of dog genomic DNA digested with HindIII and probed with canine F8A. There were 28- and 13-kb fragments present in normal dog genomic DNA that were replaced by two bands of 21 and 23 kb in the hemophilia A dog. As expected in an X-linked disease, the carrier female had bands from both the normal and hemophilia A patterns.
Figure 4.
A HindIII RFLP of F8A fragments distinguishes hemophilia A, carrier female, and normal dog genomic DNA. The 28- and 13-kb HindIII bands found in normal dog genomic DNA (N) are not found in hemophilia A dog DNA (H), which demonstrates bands at 21 and 23 kb. The carrier female (C) has all four bands seen in hemophilia A or normal dog DNA. The normal dog BAC clone 291 M9 has the 28-kb band, suggesting the 28-kb fragment is extragenic to factor VIII. Normal dog BAC clones 292 C4 and 314 O16 contain the 13-kb band, suggesting the 13-kb fragment is within factor VIII, presumably in intron 22 where these BACs overlap. Not shown are bands at ≈5 and ≈3.5 kb that are present in all three genomic DNA samples (see Fig. 11).
Additional HindIII fragments of equal intensity could be seen in normal, carrier, and hemophilia A dog genomic DNA at molecular weights of ≈3.5 and 5 kb (see Fig. 11, which is published as supporting information on the PNAS web site), suggesting additional copies of F8A not involved in the hemophilia A gene rearrangement exist (as is true in humans). The smaller of these two HindIII fragments corresponded to a discrete band seen by ethidium bromide staining after electrophoresis of digested DNA and may represent nonspecific binding to a particularly abundant HindIII fragment in genomic DNA. BACS 291 M9, 292 C4, and 314 O16 were also digested with HindIII and probed with canine F8A after electrophoresis. In 291 M9 (the extragenic clone) a 28-kb band was seen, whereas BAC clones 292 C4 and 314 O16 (which overlap in the factor VIII gene) both contained the 13-kb band seen in normal dog genomic DNA (Fig. 4).
Discussion
The Hemophilia A Inversion in Dogs Recapitulates the Human Inversion.
A novel sequence, ch8, replaces the last four exons of the normal factor VIII transcript from Chapel Hill hemophilia A dogs. The last four exons of the factor VIII are not deleted from genomic DNA. Isolation of a normal BAC clone containing ch8 permitted sequence and cytogenetic analysis of this region. Large-scale sequence analysis of this BAC (AF523316) revealed no factor VIII sequences, but FISH analysis of normal and hemophilia A dogs showed that ch8 and a copy of F8A are located together near the factor VIII gene on the X chromosome. This finding focused our attention on the Xq28 region. We sought and found in the BAC clone containing ch8 an 87% identical canine homologue of F8A (see Fig. 12, which is published as supporting information on the PNAS web site). We also found copies of this sequence in overlapping BAC clones that contained the factor VIII gene. FISH analysis of normal dog metaphase chromosomes and DNA fibers, by using the ch8 BAC clone and the two factor VIII BAC clones, revealed the order of sequences on the long arm of the X chromosome to be: telomere →ch8→ factor VIII exons 1–22, factor VIII exons 23–26,→ centromere. The presence at the 3′ end of the hemophilia A factor VIII transcript of a sequence normally found in genomic DNA at a position 5′ (“upstream”) of the factor VIII gene indicated an inversion of DNA. We have shown direct evidence of an inversion by FISH analysis of hemophilia A dog DNA fibers (Fig. 3). Finally, we have found a HindIII RFLP involving F8A-containing DNA fragments that discriminated among normal, hemophilia A, and carrier females in the Chapel Hill dog colony (Fig. 4).
The Abnormal Factor VIII Transcript Occurs in Different Hemophilia A Dog Colonies.
The canine X-chromosome in this region is shown to be syntenic with the human factor VIII locus at Xq28 (20). Independent cases of abnormal mRNA splicing after exon 22 in dogs and humans suggest that this is an intrinsically unstable region of DNA across species. Other investigators have found a nearly identical transcript in a different hemophilia A dog colony at Queen's University, Ontario (21). The independent origin of the Queen's University colony (in 1980) from the Chapel Hill dog colony (in 1947) is assured by the separate time and place of origin, as well as the different purebred dog strains (miniature schnauzer and Irish Setter, respectively) in which they appeared (8–10). The transcript from the Queen's University colony also differs from the Chapel Hill colony at five polymorphic sites in the normal coding sequence in exons 1–22, after which the same aberrant sequence is encountered in each (21). Finding the same abnormal transcript in separate spontaneous hemophilia A dog colonies was also reminiscent of the common F8A-mediated, factor VIII intron 22 inversions in separate de novo cases of severe hemophilia A in humans (2–6). Southern blot analysis of KpnI-digested Chapel Hill hemophilia A dog DNA by using an F8A probe shows a pattern similar to that shown in the Queen's University colony (data not shown). These findings predict that inversions involving the factor VIII gene should occur spontaneously in other animal species that have duplicated F8A sequence in and near the factor VIII gene. In this regard, it is notable that no spontaneous factor VIII mutation or inversion has been observed in mice, which lack the multiple copies of F8A that would be required for homologous recombination but do have a single extragenic copy of F8A (22).
Role of ch8 and F8A.
Because we were unable to find any RFLP that distinguished normal and hemophilia or carrier dog genomic DNA by using ch8 sequence as a probe (Fig. 6 and other data not shown), it seems likely that ch8 is not at the immediate site of recombination but is the best alternative sequence for splicing near the rearranged factor VIII gene. Part of the ch8 sequence bears modest homology to human X-chromosome working draft sequence (with which it is syntenic); however, its role is not obvious. The relatively short potential ORFs that are implied by this sequence just before the polyadenylation signal (Fig. 1) suggest that it does not encode a functional protein and may reside in an untranslated portion of another gene. There is modest homology between ch8 and various repetitive DNA elements, particularly short, interspersed, nuclear elements that are found throughout most eukaryotic genomes, including the dog (23–26).
The function of F8A (which is ubiquitously expressed in mammalian tissues) is unclear at this time, although it may have a role in localization of the huntingtin gene product to the cell nucleus (27). The multiple canine F8A bands on the Southern blot of genomic DNA and normal dog BAC DNA suggest that two copies are involved in the recombination and at least one more copy that is not involved in the hemophilia A inversion (Figs. 4 and 11). This is analogous to the common human factor VIII inversion mediated by F8A.
The Chapel Hill Hemophilia A Dog as a Model for Preclinical Testing of Novel Hemophilia A Therapies.
The Chapel Hill hemophilia A dogs have been useful for preclinical testing of human factor VIII concentrates, because they have a severe bleeding phenotype and no activity or antigen to interfere with measurements of factor VIII levels. These features are also useful for the testing of factor VIII gene transfer vectors that might be used for gene therapy of hemophilia A. Most current approaches contemplate replacement of factor VIII by expression of factor VIII after transduction of various tissues with a gene transfer vector. The finding of a gene inversion in the Chapel Hill hemophilia A dog colony similar to the human hemophilia A inversion suggests a strategy whereby the missing exons could be replaced by transsplicing of mRNA. Transsplicing of mRNA takes advantage of the capacity of certain group I or II intron RNA sequences to catalyze splicing of one mRNA with another (28–30). This strategy has been used in vitro to repair sickle β globin mRNA in erythrocytes (31). In principle, this approach should be applicable to human hemophilia A and testable in hemophilia A dogs. Notably, the Chapel Hill hemophilia A dogs would require the replacement of only the final 627 nucleotides of the transcript (exons 23–26) for correction. Furthermore, the exon 22 sequence proximal to the inversion site is identical in humans and dogs. Thus, potentially useful mRNA transsplicing constructs for humans with the common factor VIII inversion could be tested in the Chapel Hill hemophilia A dogs with the same mutation and identical exon 22 target sequence.
Supplementary Material
Acknowledgments
We dedicate this manuscript to the memory of a pioneer in blood coagulation research, the late Dr. Kenneth M. Brinkhous, who initially identified the Chapel Hill strain of hemophilia A dogs and used them for over 50 years to improve the lives of human hemophilia A patients. We thank Ms. Robin Raymer for outstanding management and care of the hemophilic dogs at the Francis Owen Blood Research Laboratory at University of North Carolina, Chapel Hill, and Jeff Touchman of the National Institutes of Health Intramural Sequencing Core facility for help with sequence analysis of BAC 291 M9 and deposition of sequence data in GenBank. This work was supported in part by National Institutes of Health Grant HL63098.
Abbreviations
- BAC
bacterial artificial chromosome
- FISH
fluorescence in situ hybridization
- ch8
canine hemophilia 8 locus
- F8A
factor VIII-associated locus
- RFLP
restriction fragment length polymorphism
Footnotes
References
- 1.Lozier J N, Kessler C M. In: Hematology, Principles and Practice. 3rd Ed. Hoffman R, Benz E, Shattil S, Furie B, Cohen P, editors. New York: Churchill-Livingstone; 2000. pp. 1883–1904. [Google Scholar]
- 2.Lakich D, Kazazian H H, Antonarakis S E, Gitschier J. Nat Genet. 1993;5:236–241. doi: 10.1038/ng1193-236. [DOI] [PubMed] [Google Scholar]
- 3.Naylor J, Brinke A, Hassock S, Green P M, Giannelli F. Hum Mol Genet. 1993;2:1773–1778. doi: 10.1093/hmg/2.11.1773. [DOI] [PubMed] [Google Scholar]
- 4.Antonarakis S E, Rossiter J P, Young M, Horst J, de Moerloose P, Sommer S S, Ketterling R P, Kazazian H H, Negrier C, Vinciguerra C, et al. Blood. 1995;86:2206–2212. [PubMed] [Google Scholar]
- 5.Naylor J A, Nicholson P, Goodeve A, Hassock S, Peake I, Giannelli F. Blood. 1996;87:3255–3261. [PubMed] [Google Scholar]
- 6.Naylor J A, Buck D, Green P, Williamson H, Bentley D, Giannelli F. Hum Mol Genet. 1995;4:1217–1224. doi: 10.1093/hmg/4.7.1217. [DOI] [PubMed] [Google Scholar]
- 7.Kemball-Cook G, Tuddenham E G, Wacey A I. Nucleic Acids Res. 1998;26:216–219. doi: 10.1093/nar/26.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham J B, Buckwalter J A, Hartley L J, Brinkhous K M. J Exp Med. 1949;90:97–111. doi: 10.1084/jem.90.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkhous K M, Graham J B. Science. 1950;111:723. doi: 10.1126/science.111.2896.723. [DOI] [PubMed] [Google Scholar]
- 10.Giles A R, Tinlin S, Greenwood R. Blood. 1982;60:727–730. [PubMed] [Google Scholar]
- 11.Bi L, Lawler A M, Antonarakis S E, High K A, Gearhart J D, Kazazian H H. Nat Genet. 1995;10:119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 12.Nichols T C, Bellinger D A, Reddick R L, Smith S V, Koch G G, Davis K, Sigman J, Brinkhous K M, Griggs T R, Read M S. Blood. 1993;81:2644–2651. [PubMed] [Google Scholar]
- 13.Brinkhous K M, Davis P D, Graham J B, Dodds W J. Blood. 1973;41:577–585. [PubMed] [Google Scholar]
- 14.Brinkhous K M, Sandberg H, Garris J B, Mattsson C, Palm M, Griggs T, Read M S. Proc Natl Acad Sci USA. 1985;82:8752–8756. doi: 10.1073/pnas.82.24.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brinkhous K M, Hedner U, Garris J B, Diness V, Read M S. Proc Natl Acad Sci USA. 1989;86:1382–1386. doi: 10.1073/pnas.86.4.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans J P, Brinkhous K M, Brayer G D, Reisner H M, High K A. Proc Natl Acad Sci USA. 1989;86:10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauser A E, Whitlark J, Whitney K M, Lothrop C D., Jr Blood. 1996;88:3451–3455. [PubMed] [Google Scholar]
- 18.Cameron C, Notley C, Hoyle S, McGlynn L, Hough C, Kamisue S, Giles A, Lillicrap D. Thromb Haemostasis. 1998;79:317–322. [PubMed] [Google Scholar]
- 19.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutra A S, Mignot E, Puck J M. Cytogenetic Cell Genet. 1996;74:113–117. doi: 10.1159/000134395. [DOI] [PubMed] [Google Scholar]
- 21.Hough C, Kamisue S, Cameron C, Notley C, Tinlin S, Giles A, Lillicrap D. Thromb Haemostasis. 2002;87:659–665. [PubMed] [Google Scholar]
- 22.Levinson B, Bermingham J R, Jr, Metzenberg A, Kenwrick S, Chapman V, Gitschier J. Genomics. 1992;5:236–241. doi: 10.1016/0888-7543(92)90170-w. [DOI] [PubMed] [Google Scholar]
- 23.Bentolila S, Bach J M, Kessler J L, Bordelais I, Cruaud C, Weissenbach J, Panthier J J. Mamm Genome. 1999;10:699–705. doi: 10.1007/s003359901074. [DOI] [PubMed] [Google Scholar]
- 24.Brouillette J A, Andrew J R, Venta P J. Mamm Genome. 2000;11:1079–1086. doi: 10.1007/s003350010220. [DOI] [PubMed] [Google Scholar]
- 25.Das M, Sakul H, Kong J, Acland G M, Pelletier J. Physiol Genomics. 2000;4:13–24. doi: 10.1152/physiolgenomics.2000.4.1.13. [DOI] [PubMed] [Google Scholar]
- 26.Das M, Chu L L, Ghahremani M, Abrams-Ogg T, Roy M S, Housman D, Pelletier J. Mamm Genome. 1998;9:64–69. doi: 10.1007/s003359900681. [DOI] [PubMed] [Google Scholar]
- 27.Peters M F, Ross C A. J Biol Chem. 2001;276:3188–3194. doi: 10.1074/jbc.M008099200. [DOI] [PubMed] [Google Scholar]
- 28.Sullenger B A. Gene Ther. 1999;6:461–462. doi: 10.1038/sj.gt.3300903. [DOI] [PubMed] [Google Scholar]
- 29.Long M B, Sullenger B A. Mol Cell Biol. 1999;19:6479–6487. doi: 10.1128/mcb.19.10.6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe T, Sullenger B A. Adv Drug Delivery Rev. 2000;44:109–118. doi: 10.1016/s0169-409x(00)00089-2. [DOI] [PubMed] [Google Scholar]
- 31.Lan N, Rooney B L, Lee S-W, Howrey R P, Smith C A, Sullenger B A. Mol Ther. 2000;2:245–255. doi: 10.1006/mthe.2000.0125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.