Abstract
A total of 41 blood samples were collected from 40 Anaplasma phagocytophila-infected sheep in 11 sheep flocks from four different counties of southern Norway. The presence and nature of the Anaplasma species were identified by microscopic detection of morulae, PCR, reverse line blot hybridization, and 16S rRNA gene sequencing. A. phagocytophila was identified in all of the samples, and sequencing of the 16S rRNA gene revealed the presence of four variants of A. phagocytophila. Two of these variants have been described before, but two were newly identified 16S rRNA variants of this species. A. phagocytophila variant 1 was found in nine flocks, A. phagocytophila variant 2 was found in four flocks, the A. phagocytophila prototype was found in two flocks, and A. phagocytophila variant 5 was found in one flock. In two flocks, some sheep were infected with A. phagocytophila variant 1, whereas others were infected with A. phagocytophila variant 2, and in three animals a double infection with two variants was registered. Analyses of the blood samples revealed that blood from sheep infected with A. phagocytophila variant 2 contained nearly twice as many neutrophils and eight times as many Anaplasma-infected neutrophils as blood from sheep infected with the A. phagocytophila variant 1. Furthermore, only 43% of the A. phagocytophila variant 2-infected sheep displayed antibody responses in an immune fluorescence assay, whereas 93% of the sheep with the A. phagocytophila variant 1-infected sheep were seropositive.
Tick-borne fever (TBF) in sheep caused by Ehrlichia phagocytophila and transmitted by the tick Ixodes ricinus was the first granulocytic ehrlichial infection to be described and has for decades been a well-known disease in domestic ruminants in several countries in Europe (34). E. phagocytophila belongs to the same genogroup as Ehrlichia equi and human granulocytic ehrlichiosis (HGE) agent, and natural infection with granulocytic Ehrlichia has now been reported in a variety of animal species (9). Recently, Dumler et al. (7) reorganized the families Rickettsiaceae and Anaplasmataceae, and E. phagocytophila, E. equi, and the HGE agent were unified into the new species combination Anaplasma phagocytophila. For this reason we use A. phagocytophila as the emended name for this species throughout this study.
TBF is a common disease in domestic ruminants along the coast of southern Norway (26, 27). In 1995, more than 11,000 sheep flocks were treated prophylactically against TBF with tick repellent and/or insecticides, including ca. 40% of all flocks in Norway (29). In sheep, TBF is characterized by high fever, reduced milk yield, abortion, and reduced fertility in rams. The diagnosis was earlier based on the presence of inclusions (morulae) in circulating neutrophils in Giemsa-stained blood smears (35).
A. phagocytophila infection in sheep is known to produce profound effects on the immunological defense system, which increases susceptiblility to disease and mortality from intercurrent infections such as Staphylococcus aureus pyaemia and Pasteurella haemolytica/trehalosi septicemia (4, 25). Sheep flocks may suffer heavily on I. ricinus-infested pastures both due to direct mortality and to impairment of growth rate and production (4). In one flock investigated in Norway, almost one-third of the lambs died on Ixodes-infested pastures due to TBF and secondary infections (29). Lamb losses on I. ricinus-infested pastures may vary considerably between neighboring farms. The reasons for these variations are unknown but may be caused by differences in virulence between variants of Anaplasma. Such variations have earlier been found in both sheep and cattle (8, 31).
The identification of Anaplasma and Ehrlichia species is difficult because conventional bacteriological methods for cultivation and characterization cannot be used. Morphological and serological methods are also unreliable to differentiate Anaplasma and Ehrlichia species due to morphological similarities and antigen cross-reactivity between species (22). The purpose of the present study was therefore to identify and compare Anaplasma species from sheep with TBF from different areas of Norway by molecular methods. In addition, we wanted to study the number of neutrophils, their infection rate, and the antibody response in infected sheep.
MATERIALS AND METHODS
Animals, blood samples, and hematology.
Blood samples were collected from Norwegian sheep with TBF in different I. ricinus-infested areas in Norway. A number of collaborating sheep farmers were informed before the tick season, and they were instructed to contact the local veterinarian for blood sampling when a suspected case of TBF was found in their flocks. TBF had earlier caused high mortality in all of these flocks, except in one flock (flock D; see below and Table 3). The rectal temperatures of the actual sheep were measured, and whole blood and EDTA-blood samples were collected and sent to the Department of Sheep and Goat Research for further analyses. No further information of the animals was available after blood sampling.
TABLE 3.
A. phagocytophila variants from 11 sheep flocks in Norway identified by reverse line blot hybridization and DNA sequencing
| Flock no. | No. of sheep | No. of sheep positive for:
|
|||
|---|---|---|---|---|---|
| Variant 1 | Variant 2 | Prototype | Variant 5 | ||
| A | 2 | 2 | |||
| B | 1 | 1 | |||
| C | 3a | 3 | 1 | ||
| D | 21 | 3 | 18 | ||
| E | 2 | 2 | |||
| F | 1 | 1 | |||
| G | 2 | 1 | 1 | ||
| H | 2 | 2 | |||
| I | 1a | 1 | 1 | ||
| J | 4a | 4 | 1 | ||
| K | 1 | 1 | |||
| Total | 40 | 19 | 21 | 2 | 1 |
One sheep infected with two variants of A. phagocytophila.
Hematological values, including total and differential leukocyte counts, were determined electronically from the EDTA-blood samples (Technicon H1; Miles, Inc.), and blood smears were prepared and stained with May-Grünwald Giemsa. A total of 400 neutrophils were examined on each smear by microscopy; the numbers of cells containing Anaplasma inclusions were recorded, and the percentages of infected neutrophilic granulocytes were calculated. The rest of the EDTA-blood was frozen at −20°C until further analyses could be performed.
Serology.
Serum samples were analyzed for the presence of antibodies to Anaplasma by an indirect immunofluorescence antibody assay (2). Briefly, twofold dilutions of sera were added to slides precoated with E. equi antigen (Protatek, St. Paul, Minn.). Bound antibodies were visualized by fluorescein-isothiocyanate-conjugated rabbit anti-sheep immunoglobulin (Cappel; Organon Teknika, West Chester, Pa.). Sera were screened for antibodies at a dilution of 1:40. If positive, the serum was further diluted and retested. A titer of 1.6 (log10 reciprocal of 1:40) or more was regarded positive.
DNA extraction and PCR amplification.
DNA extraction on blood samples was performed according to Olsson Engvall et al. (16), with some modifications. Briefly, 400 μl of thawed EDTA-blood was treated with 220 μl of cold lysis buffer (10 mM Tris-HCl [pH 7.4], 100 mM EDTA), 0.5% sodium dodecyl sulfate, and 10 μl of proteinase K (20 mg/ml) and then mixed gently and incubated at 50°C for 2 h. The mixture was mingled every 15 min, and after 1 h another 6 μl of proteinase K (20 mg/ml) was added. The mixture was then extracted twice with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) and once with an equal volume of chloroform-isoamyl alcohol (24:1). DNA was precipitated by the addition of a 1/10 volume of 2 M sodium acetate (pH 6.5) and 2.5 volumes of cold ethanol (99%) and was then collected by centrifugation. The pellet was washed once in cold ethanol (70%), dried, and resuspended in 50 μl of sterile water, and the DNA concentration was then measured with a spectrophotometer (GeneQuant II; Pharmacia Biotech, Uppsala, Sweden).
PCR amplifications were performed in a GeneAmp PCR system 9700 thermal cycler (Applied Biosystems, Nieuwerkerk a/d Ijssel, The Netherlands). The 5′ part of the 16S rRNA gene of the Anaplasma species in the sheep blood samples were amplified in 50-μl volumes consisting of 25 μl of HotStarTaq mix (Qiagen, Hilden, Germany), 4 μl of primer 16S8FE (80 pmol), 4 μl of primer B-GA1B (80 pmol), 2 μl of tmpB spike DNA (10 fg) (1), 10 μl of water, and 5 μl of DNA sample. To minimize nonspecific amplification, a touchdown-up PCR program was used: 15 min at 94°C, followed by two cycles of 20 s at 94°C, 30 s at 65°C, and 30 s at 72°C; followed by two cycles under conditions identical to the previous cycles but with an annealing temperature of 63°C. During subsequent two cycle sets, the annealing temperature was lowered by 2°C until it reached 55°C. We then carried out an additional 20 cycles of 20 s at 94°C, 30 s at 55°C, and 30 s at 72°C, followed by 20 cycles of 20 s at 94°C, 30 s at 63°C, and 30 s at 72°C, followed again by the touchdown program. The PCR was ended by an extra incubation for 7 min at 72°C.
Each time that the PCR was performed, negative (no sample added) and positive (Anaplasma or Ehrlichia DNA) control samples were included. Each sample was spiked with a critical amount (150 copies) of the tmpB spike control DNA to detect any inhibition of the PCR that might lead to false-negative results. When a relatively high concentration of Anaplasma DNA was available, the spike was weak or absent. In order to minimize contamination, the reagent setup, the sample addition, and the PCR and sample analysis were performed in three separate rooms, of which the first two rooms were kept at a positive pressure and had airlocks.
Reverse line blot hybridization.
The reverse line blotting technique has been described before (11, 12, 21, 23). Briefly, solutions with 5′ amino-linked oligonucleotide probes were coupled covalently to an activated Biodyne C membrane in a line pattern by using a miniblotter (Immunetics, Cambridge, Mass.). After binding of the oligonucleotide probes, the membrane was taken from the miniblotter, washed, and again placed in the miniblotter with the oligonucleotide lines perpendicular to the slots. The slots of the miniblotter were filled with the biotin-labeled denatured PCR product, and hybridization was performed. The membrane was removed from the miniblotter, washed, and subsequently incubated with streptavidin-peroxidase to detect bound biotin-labeled PCR product. After a washing step, hybridization was visualized by incubating the membrane with enhanced chemiluminescence detection liquid (Amersham International, plc., Den Bosch, The Netherlands) and exposing the membrane to X-ray film. For species identification, the biotinylated PCR product was hybridized with 10 different Anaplasma- and Ehrlichia-specific oligonucleotide probes in the reverse line blot assay. All primers and probes are described in Table 1.
TABLE 1.
Oligonucleotide primers and probes used in PCRs and hybridization assays
| Oligonucleotide primer or probe | Sequence | Target organism | Target gene | Nucleotide position (range) | Reference or source |
|---|---|---|---|---|---|
| Primers | |||||
| 16S8FE | GGAATTCAGAGTTGGATCMTGGYTCAG | Eubacteria | 16S rRNA gene | 8-27 | L. M. Schouls |
| B-GA1B | 5′biotin-CGGGATCCCGAGTTTGCCGGGACTTCTTCT | Anaplasma/Ehrlichia genus | 16S rRNA gene | 476-456 | L. M. Schouls |
| 44k-start | ATGATGTCTATGGCTATAGTCATG | A. phagocytophila group | 44-kDa genea | 49-72 | This study |
| 44k-end | ATCTCCCACAACACGATG | A. phagocytophila group | 44-kDa genea | 1180-1197 | This study |
| 16SEhrSeq | GGAATTCAGAGTTTGATCMTGGYTCAGAAC | Eubacteria | 16S rRNA gene | 8-30 | This study |
| GA1BSeq | CGGGATCCCGAGTTTGCCGGGACTTCTTCTGTA | Anaplasma/Ehrlichia genus | 16S rRNA gene | 476-459 | This study |
| Probes | |||||
| A-EhrA11 | 5′ amino-TTATCGCTATTAGATGAGCC | Anaplasma/Ehrlichia genus | 16S rRNA gene | 203-222 | L. M. Schouls |
| A-HGE | 5′ amino-GCTATAAAGAATAGTTAGTGG | A. phagocytophila prototype | 16S rRNA gene | 87-107 | L. M. Schouls |
| A-Phago | 5′ amino-TTGCTATAAAGAATAATTAGTGG | A. phagocytophila variant 1 | 16S rRNA gene | 85-107 | L. M. Schouls |
| A-Phagovar2 | 5′ amino-GAACGGATTATTCTTTGTAGC | A. phagocytophila variant 2 | 16S rRNA gene | 87-106 | This study |
| A-D-Phago | 5′ amino-TTGCTATGAAGAATAATTAGTG | A. phagocytophila variant 3 | 16S rRNA gene | 87-106 | L. M. Schouls |
| A-D-HGE | 5′ amino-GCTATGAAGAATAGTTAGTG | A. phagocytophila variant 4 | 16S rRNA gene | 87-106 | L. M. Schouls |
| A-Eschot | 5′ amino-GCTGTAGTTTACTATGGGTA | Ehrlichia sp., Schotti variant | 16S rRNA gene | 76-95 | L. M. Schouls |
| A-Ecan | 5′ amino-TCTGGCTATAGGAAATTGTTA | E. canis | 16S rRNA gene | 85-105 | L. M. Schouls |
| A-Echaf | 5′ amino-ACCTTTTGGTTATAAATAATTGTTA | E. chaffeensis | 16S rRNA gene | 83-107 | L. M. Schouls |
| A-EmurisC | 5′ amino-GCTATAGGTTCGCTATTAG | E. muris | 16S rRNA gene | 85-103 | A. N. Alekseev |
| A-TmpBEhr | 5′ amino-GAAAACTCGAAGAACAAAGAA | T. pallidumb | tmpB | 502-522 | A. N. Alekseev |
DNA sequencing and data analysis.
Most PCR products were used directly for sequencing, but some were cloned into a TA-TOPO vector (Invitrogen, Groningen, The Netherlands). The plasmids were isolated and purified by using the Qiagen plasmid minikit and used for sequencing. The PCR products used for DNA sequencing were purified with QiaQuick PCR purification kits (Qiagen). Since PCR products were obtained in a PCR that included a spike control, the PCR yielded a mixture of Anaplasma PCR product and the tmpB spike. Therefore, we used sequence primers that were specific for the Anaplasma PCR product only (16SEhrSeq and GA1BSeq). For DNA sequencing reactions, the fluorescence-labeled dideoxynucleotide technology was used (Perkin-Elmer, Applied Biosystems Division). The sequenced fragments were separated, and data were collected with an ABI 3700 automated DNA sequencer (ABI, Applied Biosystems Division). The collected sequences were assembled, edited, and analyzed with the DNAStar package (DNAStar, Inc., Madison, Wis.).
Statistics.
Statistical calculations on seroprevalences were performed by using the chi-square contingency test, and a two-sample t test was used for the hematological variables and the antibody titers (Statistix, version 4.0; Analytical Software). A P value of <0.05 was considered significant.
Nucleotide sequence accession number.
The 16S rRNA gene sequences of the new variants of A. phagocytophila found in the present study are available in the GenBank database under the accession numbers AF336220 and AY035312.
RESULTS
Blood samples.
Altogether 41 blood samples from 40 different sheep were collected. Two samples originated from the same sheep and were drawn 1 month apart. All sampled sheep revealed clinical signs of TBF, such as fever, increased respiration, dullness, and inappetence. Concurrent diseases were not observed. The samples were from 11 sheep flocks in four different counties of southern Norway and were collected from April to October in two consecutive years. The age of the sheep varied from 1 month to 2 years; however, most of the animals (68%) were less than 4 months old.
Reverse blot line hybridization and DNA sequence analysis.
In order to confirm the results observed by the reverse line blot assay, the PCR products used in hybridizations were also sequenced. Although the sequence analysis largely confirmed the reverse line blot results, additional sequence variation was found. The blood samples carried A. phagocytophila that displayed minor sequence variation of the 16S rRNA gene and were designated variants. Two samples carried 16S rRNA gene sequences identical to the A. phagocytophila prototype (GenBank accession no. U02521) and a second group carried 16S sequences identical to the sequence with the accession number M73220 that differed at nucleotide position 80 from the prototype sequence and was designated variant 1. The largest group of sheep carried A. phagocytophila that differed at positions 80 and 100 of the 16S rRNA gene, and this type was designated A. phagocytophila variant 2 (accession no. AF336220). One sample contained the new A. phagocytophila variant 5, which differed at position 93 of the 16S gene (accession no. AY035312). The prototype and all variant signature sequences, including some other published sequences, are displayed in Table 2.
TABLE 2.
The 5′ end of the 16S rRNA gene sequences (bp 81 to 126) of different Anaplasma and Ehrlichia strains were determined and compared with similar sequences from GenBank
| Sequencea | Accession no. | Species | Nucleotide change |
|---|---|---|---|
| TTATTCTTTATAGCTTGCTATAAAGAATAGTTAGTGGCAGACGGGTGAGTAATGCA | U02521 | A. phagocytophila prototype | Prototype |
| .............................A.......................... | M73220 | A. phagocytophila variant 1 | 100A |
| .........G...................A.......................... | AF336220 | A. phagocytophila variant 2 | 80G, 100A |
| .....................G.......A.......................... | AJ242784 | A. phagocytophila variant 3 | 92G, 100A |
| .....................G.................................. | AJ242783 | A. phagocytophila variant 4 | 92G |
| ......................G................................. | AY035312 | A. phagocytophila variant 5 | 93G |
| .............................A....................G..... | AF227954 | E. phagocytophila | 100A, 121G |
| ...................G.................................... | AF036647 | E. equi | 90G |
| .......................G................................ | AF036646 | E. equi | 94G |
| .....................GG..G.............................. | AF205140 | HGE agent | 92G 93G 96G |
Nucleotides at positions 80, 92, and 100, respectively, are indicated in boldface for each sequence.
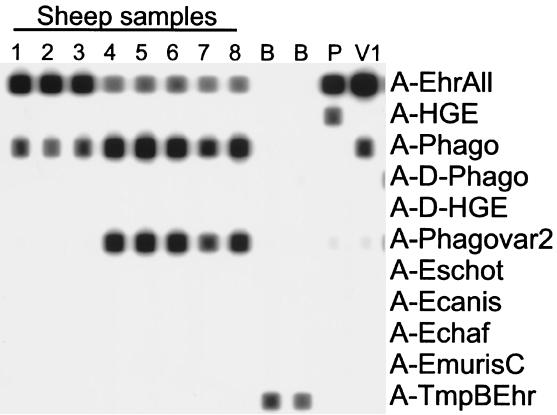
A probe was designed to detect the A. phagocytophila variant 2, and all samples were retested on a reverse line blot that included this probe (Fig. 1). This analysis was in complete concordance with the sequence analysis and confirmed that the observed sequence variation was not an artifact introduced by the sequencing procedure.
FIG. 1.
Reverse blot analysis of PCR products obtained from blood samples of A. phagocytophila-infected sheep. The oligonucleotide probes are shown as lines in the horizontal direction, and the biotin-labeled PCR products are perpendicular in the vertical direction. Samples 1 to 3, samples from A. phagocytophila variant 1-infected sheep; samples 4 to 8, samples from A. phagocytophila variant 2-infected sheep; B, blank controls (no DNA added); P, A. phagocytophila prototype-positive PCR control; V1, A. phagocytophila variant 1-positive PCR control.
When blood samples from a total of 11 sheep flocks were examined, A. phagocytophila variant 1 was found in nine flocks, A. phagocytophila variant 2 was found in four flocks, A. phagocytophila prototype was found in two flocks, and A. phagocytophila variant 5 was found in one flock. In two flocks, some sheep were infected with A. phagocytophila variant 1, whereas others were infected with A. phagocytophila variant 2. In three animals the PCR product reacted with two different Anaplasma probes in the reverse line blot, which might indicate a double infection with two different variants. DNA sequencing of these PCR products revealed ambiguous bases at a few positions in the sequence, supporting the supposition that double infection with two variants had occurred. For this reason the PCR products were cloned into a plasmid, and the inserts of 10 clones of each cloned PCR product were sequenced. This indeed revealed the simultaneous presence of two different variants in these three animals. Both samples of the one animal that was sampled twice carried the same variant of A. phagocytophila. In one flock, where 21 animals were examined, 3 (14%) were infected with A. phagocytophila variant 1, and 18 (86%) were infected with A. phagocytophila variant 2 (Table 3).
To exclude the possibility that A. phagocytophila carried two different copies of the 16S rRNA gene, we performed a Southern blot hybridization with a biotin-labeled 16S rRNA oligonucleotide probe on XbaI- and PstI-digested genomic A. phagocytophila DNA. This revealed the presence of a single 16S rRNA gene in the genome (data not shown). This result was not completely unexpected since BLAST searches in the E. chaffeensis genome sequence had also shown that this Ehrlichia species contains a single 16S rRNA gene.
Clinical parameters, hematology, and serology.
Clinical variables at the time of blood sampling were obtained from 37 sheep. A marked and significant difference was found in the number of neutrophils. Blood samples from sheep infected with A. phagocytophila variant 2 contained nearly twice as many neutrophils as blood samples from sheep infected with A. phagocytophila variant 1. In addition, blood from A. phagocytophila variant 2-infected sheep carried eight times as many neutrophils with Anaplasma inclusions as blood from sheep infected with A. phagocytophila variant 1. The clinical parameters and hematology in sheep infected with different variants of granulocytic Anaplasma are shown in Table 4.
TABLE 4.
Clinical variables (geometric mean ± SD) and antibody titer to E. equi antigen in 38 sheep infected with different variants of A. phagocytophila
| A. phagocytophila variant(s) | n | Result (mean ± SD)
|
||||
|---|---|---|---|---|---|---|
| Rectal temp (°C) | No. of neutrophils (109 cells/liter)a | Absolute no. of infected neutrophils (109 cells/liter)a | No. of seropositive sheepb (%) | Mean antibody titer in seropositive sheep | ||
| A. phagocytophila variant 1 | 14c | 40.74 ± 1.015 | 0.80 ± 0.182 | 0.024 ± 0.004 (3.1 ± 2.91) | 13 (93) | 1:386 |
| A. phagocytophila variant 2 | 21 | 40.64 ± 1.014 | 1.48 ± 0.227 | 0.193 ± 0.006 (13.1 ± 3.07) | 9 (43) | 1:320 |
| A. phagocytophila prototype and variant 1 | 2 | 41.54 ± 1.005 | 1.38 ± 1.154 | 0.067 ± 0.013 (4.6 ± 1.53) | 2 (67) | 1:452 |
| A. phagocytophila variant 1 and variant 5 | 1 | 40.40 | 1.62 | 0.008 (0.5) | 0 | Negative |
The number of neutrophils and infected neutrophils in sheep infected with A. phagocytophila variant 1 and those infected with A. phagocytophila variant 2 were significantly different: P < 0.03 and P < 0.004, respectively. Percent values are given in parentheses.
Comparison of A. phagocytophila variant 1 and A. phagocytophila variant 2: Yates corrected χ2 = 6.98 and P < 0.01.
Clinical data from one of the A. phagocytophila variant 1-infected sheep were not available.
Antibody titers to E. equi measured at the day of blood sampling are shown in Table 4. Only 24 of 39 (62%) of the acute Anaplasma-infected animals were found to be seropositive at the time of sampling. Remarkably, 93% of the A. phagocytophila variant 1-infected animals carried antibodies reacting with the E. equi antigen, whereas only 43% of the sheep infected with A. phagocytophila variant 2 were seropositive (P < 0.02). However, the mean antibody titer (log10) was not significantly different between sheep in these two variant groups.
DISCUSSION
We found four 16S rRNA gene sequence variants of A. phagocytophila in blood from sheep suffering from TBF. To our knowledge, three of these variants have not earlier been identified in sheep, and two of them have not been identified in any other study before. Nucleotide differences at 16S rRNA level in A. phagocytophila have been found in isolates from rodents, deer, and Ixodes ticks (3, 5, 6, 15, 17, 20, 23, 32, 33). However, whether all variants can cause disease in humans and animals remains to be determined. Therefore, the importance of these sequence differences remains to be elucidated. In the study presented here, at least two of the variants found seem to cause classical A. phagocytophila infection in sheep.
The sampled sheep were more than 1 month old. Age resistance in lambs older than 1 month and variation in clinical symptoms among Norwegian sheep breeds have not been found in experimentally A. phagocytophila-infected lambs (24). In the present study, the number of neutrophils, the number of infected neutrophils, and the serological response differed significantly between sheep infected with either A. phagocytophila variant 1 or 2. In the flock with few disease problems, 86% of the variants were of the A. phagocytophila variant 2 type. One possible reason for this difference could be that the A. phagocytophila variant 2 is better equipped to elude the immune system by inhibiting antibody response, resulting in more proliferation within granulocytes. This theory is supported by the observation in mice that pathology due to host immunity seems to play a more important role than pathogenicity of Anaplasma itself during infection with HGE (14). However, the role of host immunity in the pathogenesis of TBF in sheep has to be elucidated. Alternatively, the differences in morbidity and antibody response may be explained by sampling in the later phase of the infection in case of A. phagocytophila variant 1-infected sheep. However, later sampling may also have been caused by less-apparent acute disease manifestations in the A. phagocytophila variant 2-infected animals. The time point of sampling is important since earlier studies have shown that, in the later phase of the acute infection, both the number of neutrophils and the rate of infected neutrophils decrease (35).
Different clinical and serological responses between variants of Anaplasma have earlier been observed in experimental infections in cattle, horses, and sheep (8, 19, 28, 31). In the present study it was difficult to compare different clinical and serological values since only single point measurements were available. However, a recent experimental inoculation study in lambs of a single breed revealed a significant difference in the clinical, hematological, and serological responses between these two variants of A. phagocytophila (S. Stuen, K. Bergström, M. Petrovec, I. Van De Pol, and L. M. Schouls, unpublished data).
The present serology results indicate that only 61% of the acute Anaplasma-infected animals were seropositive at the time of sampling. This result is in accordance with an earlier study in which 22 of 30 (73%) of Anaplasma-positive animals were found to be seropositive (16).
An earlier experimental needle inoculation trial in sheep with A. phagocytophila-infected blood indicated that infected neutrophils may be found by Giemsa-stained blood smears examination several days before seroconversion appears (18). In addition, some Anaplasma-infected lambs have been found to remain seronegative for up to 6 weeks after the primary inoculation with a species similar to the HGE agent (28). Serologic investigation is therefore not reliable as the only diagnostic tool to detect acute Anaplasma infection in sheep.
There has been much debate about the species definition and nomenclature of the group of granulocytic Ehrlichia. Recently, Dumler et al. (7) clarified this by unifying E. phagocytophila, E. equi, and the HGE agent into a single species: A. phagocytophila. However, there are minor differences in the 16S rRNA gene of the latter species, and these differences can be used to differentiate particular groups within the species A. phagocytophila. Typing within species will require more polymorphism than the limited variation found in the 16S rRNA gene. Therefore, analysis of particular highly polymorphic sequences or of a number of housekeeping genes, such as those used in the multilocus sequence typing, are required for reliable discrimination of strain types. Variations in other genes, especially those coding for surface proteins, are more likely to affect properties such as virulence, host range, and interaction with arthropod vectors. Recently, it has been shown that sera from mice with high concentrations of antibodies that bind to the P44 proteins of the HGE agent or monoclonal antibodies specific to these proteins partially protect mice from the infection (13, 30) and that this protein may be located in the outer membrane of the HGE agent (36). This suggests that the P44-protein specific antibodies may play a role in the immunity against this infection and that the genes encoding the P44 outer membrane proteins may have a role in pathogenesis and immunresponse in A. phagocytophila infection in mice (10, 13, 37).
In conclusion, the present study shows the existence of different A. phagocytophila variants in sheep: within different flocks, within each flock, and also within a single animal. Variants of A. phagocytophila causing TBF in sheep may accordingly exist simultaneously on the same pasture and may cause differences in both clinical and immunosuppressive reactions within each flock. However, the clinical and epidemiological consequences of these findings have to be further elucidated.
Acknowledgments
We thank all of the farmers and veterinarians who participated in this study.
REFERENCES
- 1.Alekseev, A. N., H. V. Dubinina, I. Van De Pol, and L. M. Schouls. 2001. Identification of Ehrlichia and Borrelia burgdorferi species in Ixodes ticks in the Baltic regions of Russia. J. Clin. Microbiol. 39:2237-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artursson, K., A. Gunnarsson, U.-B. Wikstrøm, and E. Olsson Engvall. 1999. A serological and clinical follow-up in horses with confirmed equine granulocyctic ehrlichiosis. Equine Vet. J. 31:473-477. [DOI] [PubMed] [Google Scholar]
- 3.Baumgarten, B. U., M. Rollinghoff, and C. Bogdan. 1999. Prevalence of Borrelia burgdorferi and granulocytic and monocytic Ehrlichiae in Ixodes ricinus ticks from southern Germany. J. Clin. Microbiol. 37:3448-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodie, T. A., P. H. Holmes, and G. M. Urquhart. 1986. Some aspects of tick-borne diseases of British sheep. Vet. Rec. 118:415-418. [DOI] [PubMed] [Google Scholar]
- 5.Cao, W. C., Q. M. Zhao, P. H. Zhang, J. S. Dumler, X. T. Zhang, L. Q. Fang, and H. Yang. 2000. Granulocytic Ehrlichiae in Ixodes persulcatus ticks from an area in China where Lyme disease is endemic. J. Clin. Microbiol. 38:4208-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson, J. E., C. K. Warner, V. Baker, S. A. Ewing, D. E. Stallknecht, W. R. Davidson, A. A. Kocan, J. M. Lockhart, and J. G. Olson. 1996. Ehrlichia-like 16S rDNA sequence from wild white-tailed deer (Odocoileus virginianus). J. Parasitol. 82:52-58. [PubMed] [Google Scholar]
- 7.Dumler, J. S., A. F. Barbet, C. P. J. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales; unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia, and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 8.Foggie, A. 1951. Studies on the infectious agent of tick-borne fever in sheep. J. Pathol. Bacteriol. 63:1-15. [DOI] [PubMed] [Google Scholar]
- 9.Foley, J. E., P. Foley, M. Jecker, P. K. Swift, and J. E. Madigan. 1999. Granulocytic ehrlichiosis and tick infestation in mountain lions in California. J. Wildl. Dis. 35:703-709. [DOI] [PubMed] [Google Scholar]
- 10.Ijdo, J. W., W. Sun, Y. Zhang, L. A. Magnarelli, and E. Fikrig. 1998. Cloning of the gene encoding the 44-kilodalton antigen of the agent of human granulocytic ehrlichiosis and characterization of the humoral response. Infect. Immun. 66:3264-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamerbeek, J., L. Schouls, A. Kolk, M. Van Agterveld, D. Van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. Van Emdben. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufhold, A., A. Podbielski, G. Baumgarten, M. Bolkpoel, J. Top, and L. Schouls. 1994. Rapid typing of group A streptococci by the use of DNA amplification and non-radioactive allele-specific oligonucleotide probes. FEMS Microbiol. Lett. 119:19-25. [DOI] [PubMed] [Google Scholar]
- 13.Kim, H. Y., and Y. Rikihisa. 1998. Characterization of monoclonal antibodies to the 44-kilodalton major outer membrane protein of the human granulocytic ehrlichiosis agent. J. Clin. Microbiol. 36:3278-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin, M. E., J. E. Bunnell, and J. S. Dumler. 2000. Pathology, immunohistology, and cytokine responses in early phases of human granulocytic ehrlichiosis in a murine model. J. Infect. Dis. 181:374-378. [DOI] [PubMed] [Google Scholar]
- 15.Massung, R. F., K. Slater, J. H. Owens, W. L. Nicholson, T. N. Mather, V. B. Solberg, and J. G. Olson. 1998. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 36:1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olsson Engvall, E., B. Pettersson, M. Persson, K. Artursson, and K.-E. Johansson. 1996. A 16S rRNA-based PCR assay for detection and identification of granulocytic Ehrlichia species in dogs, horses, and cattle. J. Clin. Microbiol. 34:2170-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parola, P., L. Beati, M. Cambon, P. Brouqui, and D. Raoult. 1998. Ehrlichial DNA amplified from Ixodes ricinus (Acari: Ixodidae) in France. J. Med. Entomol. 35:180-183. [DOI] [PubMed] [Google Scholar]
- 18.Paxton, E. A., and G. R. Scott. 1989. Detection of antibodies to the agent of tick-borne fever by indirect immunofluorescence. Vet. Microbiol. 21:133-138. [DOI] [PubMed] [Google Scholar]
- 19.Pusterla, N., J. B. Pusterla, U. Braun, and H. Lutz. 1999. Experimental cross-infections with Ehrlichia phagocytophila and human granulocytic ehrlichia-like agent in cows and horses. Vet. Rec. 145:311-314. [DOI] [PubMed] [Google Scholar]
- 20.Reubel, G., R. Kimsey, J. Barlough, and J. Madigan. 1998. Experimental transmission of Ehrlichia equi to horses through naturally infected ticks (Ixodes pacificus) from northern California. J. Clin. Microbiol. 36:2131-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rijpkema, S. G., M. J. Molkenboer, L. M. Schouls, F. Jongejan, and J. F. Schellekens. 1995. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J. Clin. Microbiol. 33:3091-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rikihisa, Y. 1991. The tribe Ehrlichieae and ehrlichial diseases. Clin. Microbiol. Rev. 4:286-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schouls, L. M., I. Van De Pol, S. G. T. Rijpkema, and C. S. Schot. 1999. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J. Clin. Microbiol. 37:2215-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuen, S. 1993. Tick-borne fever in lambs of different ages. Acta Vet. Scand. 34:45-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stuen, S. 1996. Tick-borne fever (TBF) and secondary infections in sheep, p. 347-349. In J. Kazar and R. Toman (ed.), Rickettsiae and rickettsial diseases. Veda, Bratislava, Czechoslovakia.
- 26.Stuen, S. 1997. The distribution of tick-borne fever (TBF) in Norway. Nor. Vet. Tidsskr. 109:83-87. (In Norwegian.)
- 27.Stuen, S. 1998. Sjodogg (tick-borne fever): a historical review. Nor. Vet. Tidsskr. 110:703-706. (In Norwegian.)
- 28.Stuen, S., K. Artursson, and E. Olsson Engvall. 1998. Experimental infection of lambs with an equine granulocytic Ehrlichia species resembling the agent that causes human granulocytic ehrlichiosis. Acta Vet. Scand. 39:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuen, S., and K. Kjølleberg. 2000. An investigation of lamb deaths on tick pastures in Norway, p. 111-115. In M. Kazimirová, M. Labuda, and P. A. Nuttall (ed.), Ticks and tick-borne pathogens: into the 21st century. Proceedings of the 3rd International Conference. Slovak Academy of Sciences, Bratislava, Czechoslovakia.
- 30.Sun, W., J. W. Ijdo, S. R. Telford III, E. Hodzic, Y. Zhang, S. W. Barthold, and E. Fikrig. 1997. Immunization against the agent of human granulocytic ehrlichiosis in a murine model. J. Clin. Investig. 100:3014-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuomi, J. 1967. Experimental studies on bovine tick-borne fever. III. Immunological strain differences. Acta Pathol. Microbiol. Scand. 71:89-100. [PubMed] [Google Scholar]
- 32.von Stedingk, L. V., M. Gürtelschmid, H. S. Hanson, R. Gustafson, L. Dotevall, E. Olsson Engvall, and M. Granström. 1997. The human granulocytic ehrlichiosis (HGE) agent in Swedish ticks. Clin. Microbiol. Infect. 3:573-574. [DOI] [PubMed] [Google Scholar]
- 33.Walls, J. J., B. Grieg, D. F. Neitzel, and J. S. Dumler. 1997. Natural infection of small mammal species in Minnesota with the agent of human granulocytic ehrlichiosis. J. Clin. Microbiol. 35:853-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woldehiwet, Z. 1983. Tick-borne fever: a review. Vet. Res. Commun. 6:163-175. [DOI] [PubMed] [Google Scholar]
- 35.Woldehiwet, Z., and G. R. Scott. 1993. Tick-borne (pasture) fever, p. 233-254. In Z. Woldehiwet and M. Ristic (ed.), Rickettsial and chlamydial diseases of domestic animals. Pergamon Press, Oxford, England.
- 36.Zhi, N., Y. Rikihisa, H. Y. Kim, G. P. Wormser, and H. W. Horowitz. 1997. Comparison of major antigenic proteins of six strains of the human granulocytic ehrlichiosis agent by Western immunoblot analysis. J. Clin. Microbiol. 35:2606-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhi, N., N. Ohashi, and Y. Rikihisa. 1999. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J. Biol. Chem. 274:17828-17836. [DOI] [PubMed] [Google Scholar]