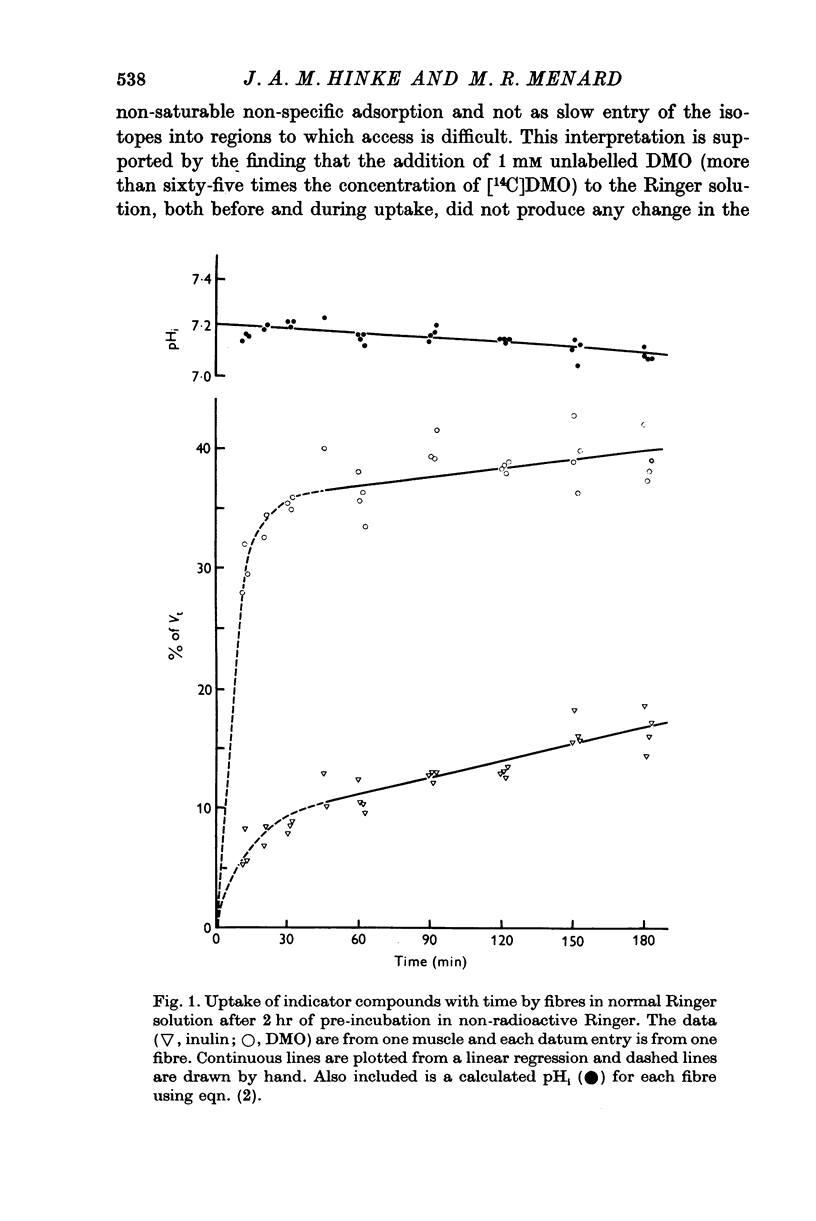
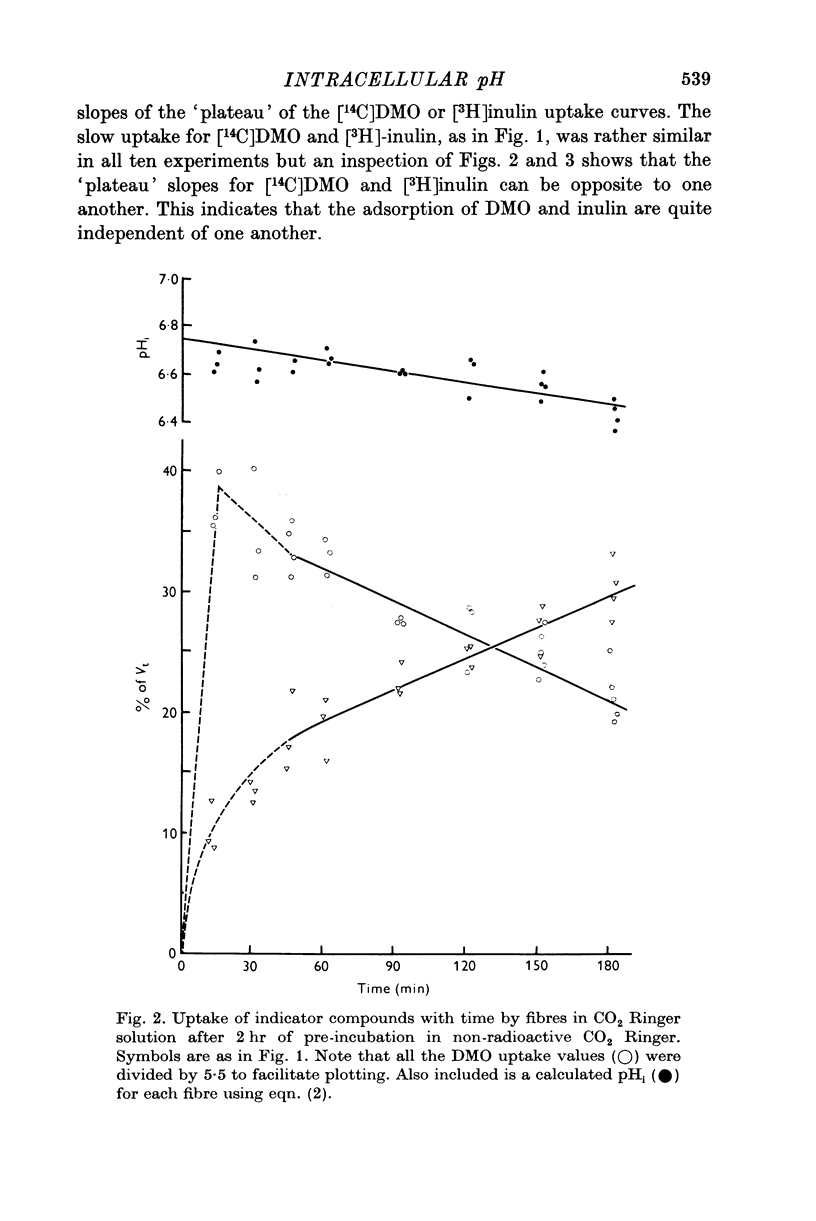
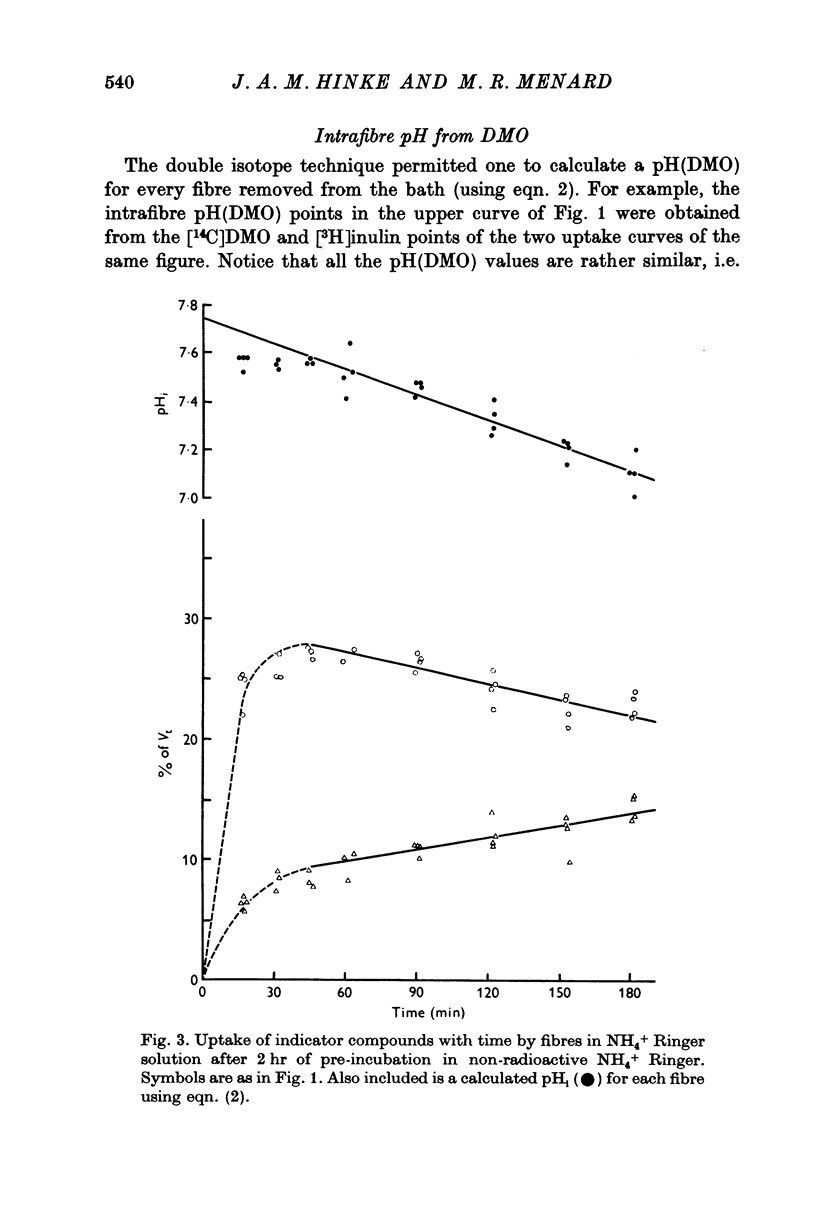
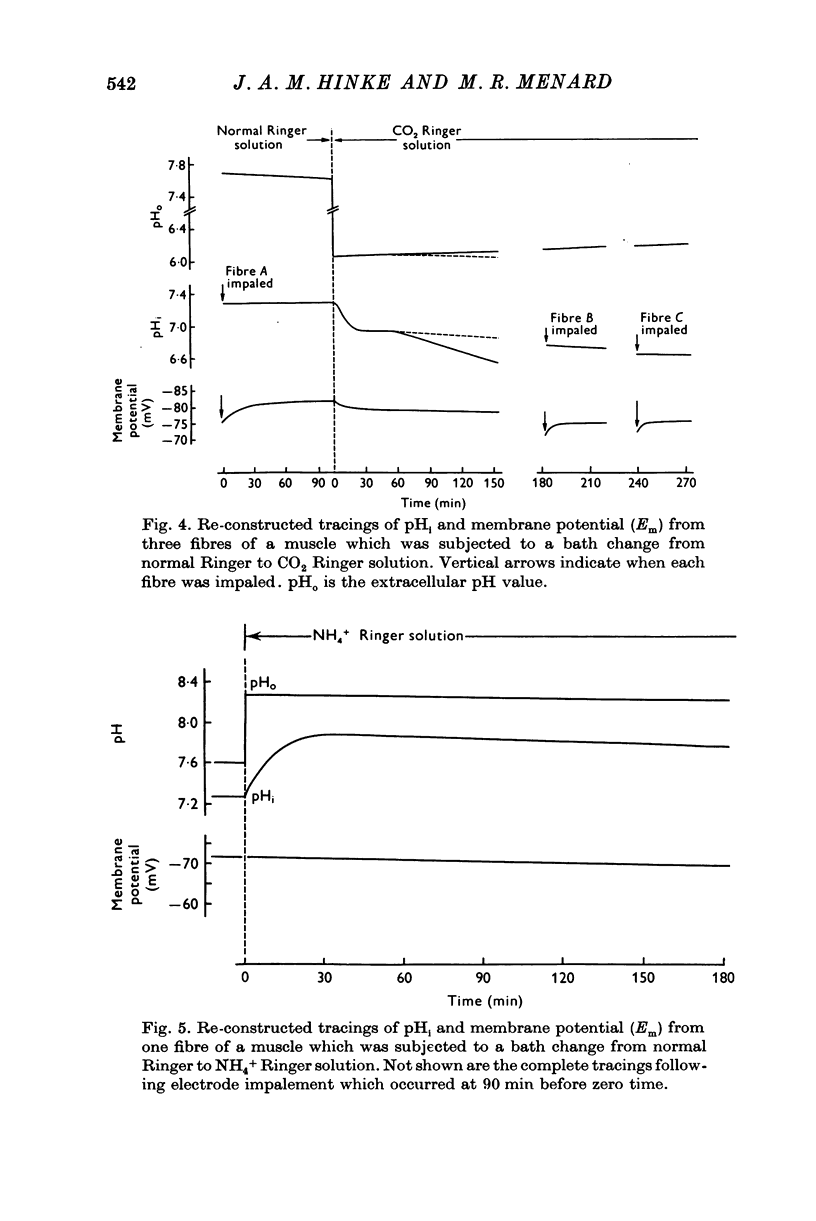
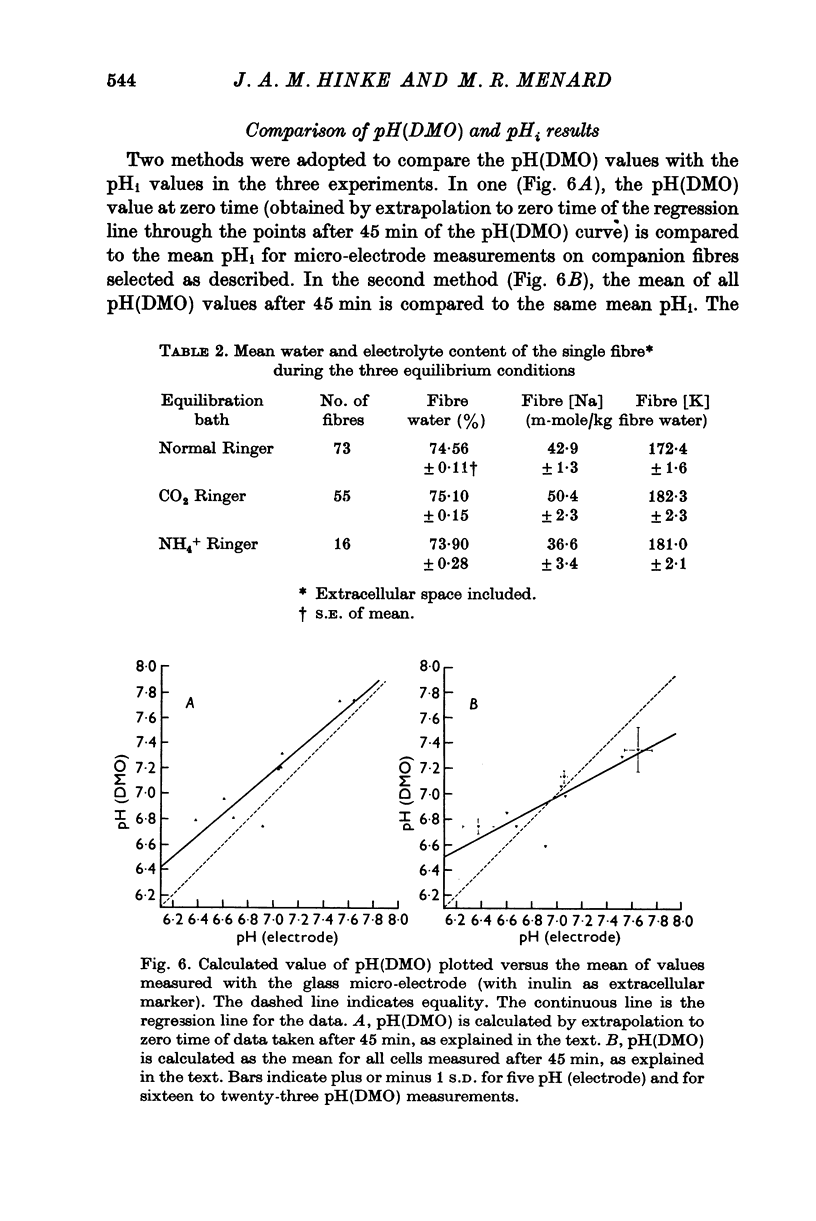
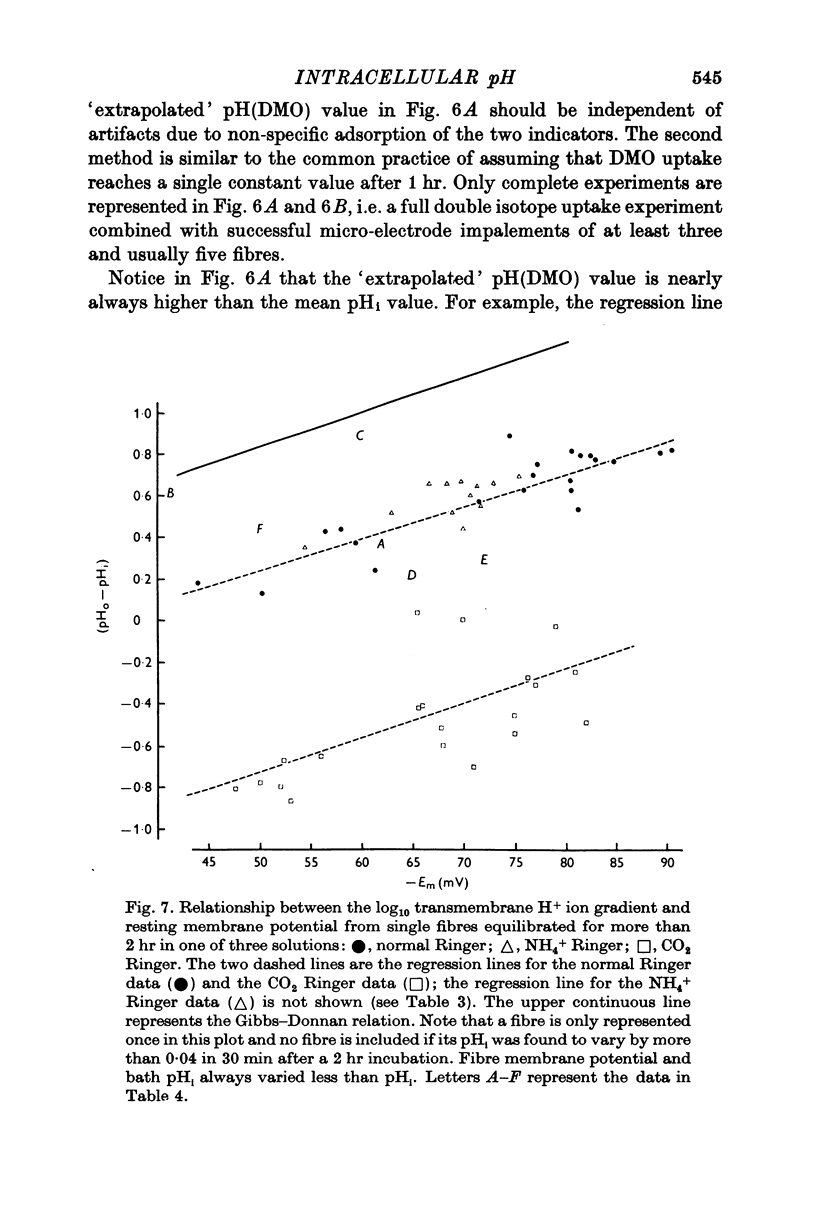
Abstract
1. The intracellular pH of intact single muscle fibres of the giant barnacle was measured directly with a glass micro-electrode following prolonged (2-5 hr) equilibration in one of three solutions: normal Ringer, CO2 Ringer and NH4+ Ringer. 2. The intracellular pH of identically-prepared fibres from the same specimen was measured indirectly from the distribution of DMO following prolonged equilibration in the same solutions. 3. The DMO-pH compared favourably with the electrode-pHi provided DMO-pHi was calculated from the values of the indicator compounds, [14C]DMO and [3H]inulin, obtained by extrapolating the slow uptake phase to time zero. 4. Following prolonged equilibration, the transmembrane H+ ion distribution was found to vary with the membrane potential but not in accordance with a simple Gibbs-Donnan equilibrium. 5. A model which recognizes the existence of two independent net fluxes for H+ across the membrane in developed to explain the results. One of the fluxes represents passive diffusion and the other represents the so called H+-pump. The model predicts the H+-pump rate increases by two orders of magnitude when pHi is reduced from 7-2 to 6-7.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C., Thomas R. C. Micro-electrode measurement of the internal pH of crab muscle fibres. J Physiol. 1975 Nov;252(3):803–815. doi: 10.1113/jphysiol.1975.sp011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. A., Sjodin R. A. Sodium extrusion by giant muscle fibres from the barnacle. Nature. 1967 Sep 16;215(5107):1307–1308. doi: 10.1038/2151307a0. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr Sodium and potassium fluxes in isolated barnacle muscle fibers. J Gen Physiol. 1968 Apr;51(4):445–477. doi: 10.1085/jgp.51.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter N. W., Rector F. C., Jr, Campion D. S., Seldin D. W. Measurement of intracellular pH of skeletal muscle with pH-sensitive glass microelectrodes. J Clin Invest. 1967 Jun;46(6):920–933. doi: 10.1172/JCI105598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayton D. C., Allen R. D., Hinke J. A. The intracellular concentration and activity of sodium in giant barnacle muscle fibers. J Gen Physiol. 1969 Sep;54(3):433–435. doi: 10.1085/jgp.54.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., CHICHIBU S., NAKA K. I. THE EFFECTS OF VARIOUS IONS ON RESTING AND SPIKE POTENTIALS OF BARNACLE MUSCLE FIBERS. J Gen Physiol. 1964 Sep;48:163–179. doi: 10.1085/jgp.48.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle G., McNeill P. A., Selverston A. I. Ultrastructure of barnacle giant muscle fibers. J Cell Biol. 1973 Jan;56(1):74–91. doi: 10.1083/jcb.56.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izutsu K. T. Intracellular pH, H ion flux and H ion permeability coefficient in bullfrog toe muscle. J Physiol. 1972 Feb;221(1):15–27. doi: 10.1113/jphysiol.1972.sp009735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. G., Hinke J. A. Optical density changes of single muscle fibres in sodium-free solutions. Can J Physiol Pharmacol. 1968 Mar;46(2):247–260. doi: 10.1139/y68-041. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Hinke J. A. Sodium and water binding in single striated muscle fibers of the giant barnacle. Can J Physiol Pharmacol. 1966 Sep;44(5):837–848. doi: 10.1139/y66-102. [DOI] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Paillard M. Direct intracellular pH measurement in rat and crab muscle. J Physiol. 1972 Jun;223(2):297–319. doi: 10.1113/jphysiol.1972.sp009848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and distribution of weak acids across cell membranes. A study of D- and L-lactate and of DMO in rat diaphragm. J Physiol. 1975 Jul;249(1):1–25. doi: 10.1113/jphysiol.1975.sp011000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose I. A. The state of magnesium in cells as estimated from the adenylate kinase equilibrium. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1079–1086. doi: 10.1073/pnas.61.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]


