Abstract
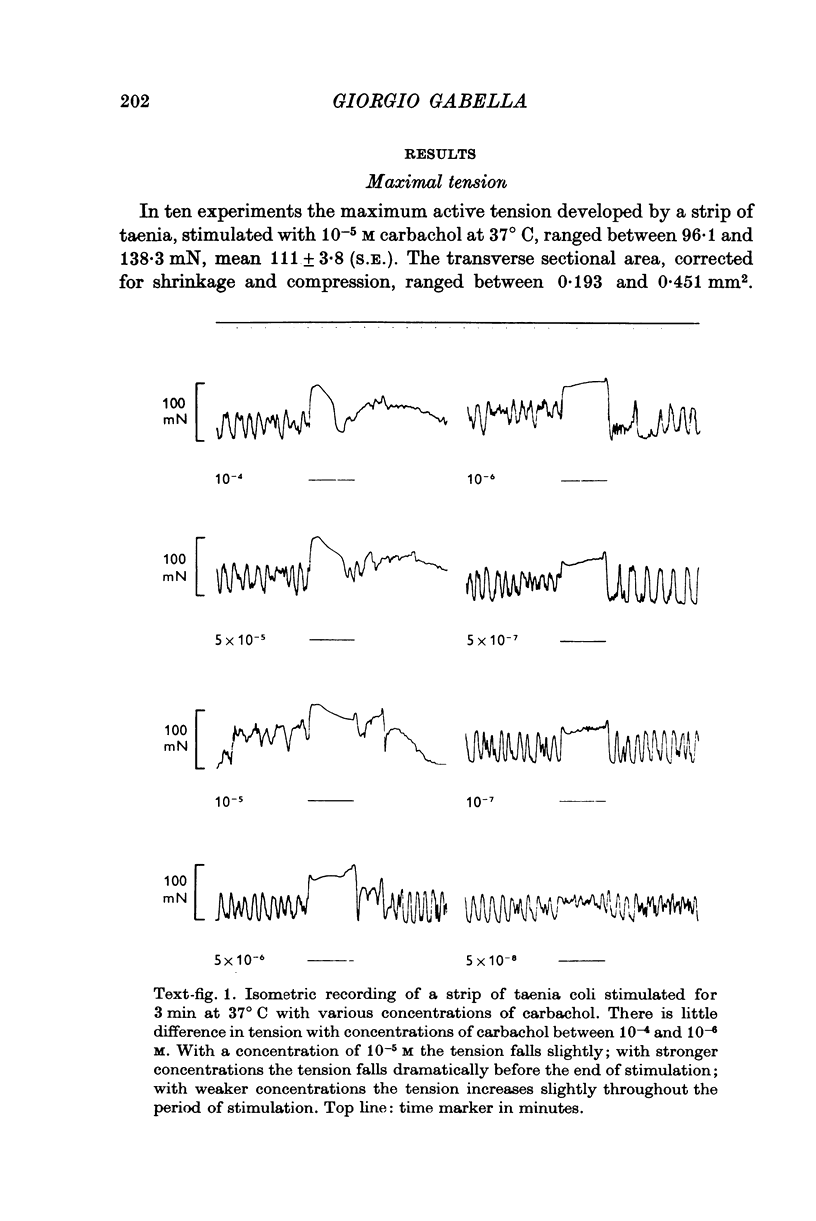
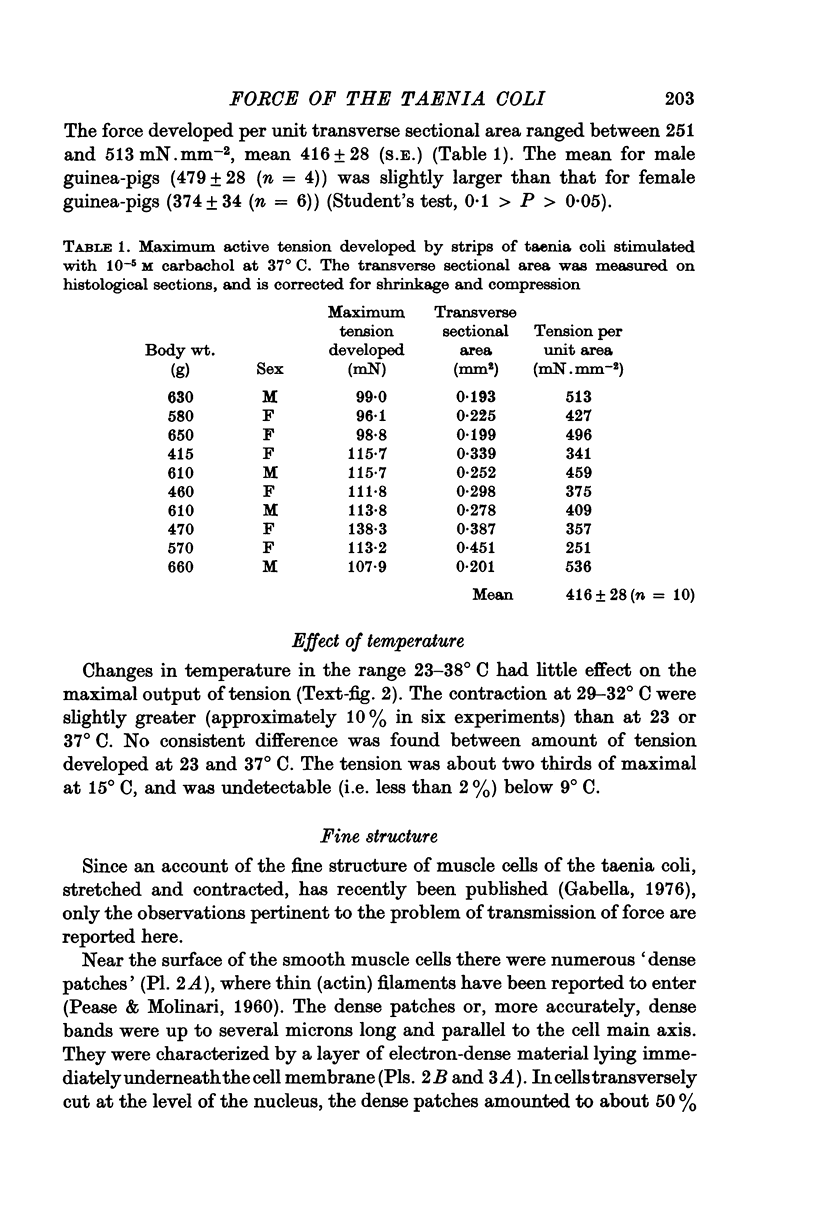
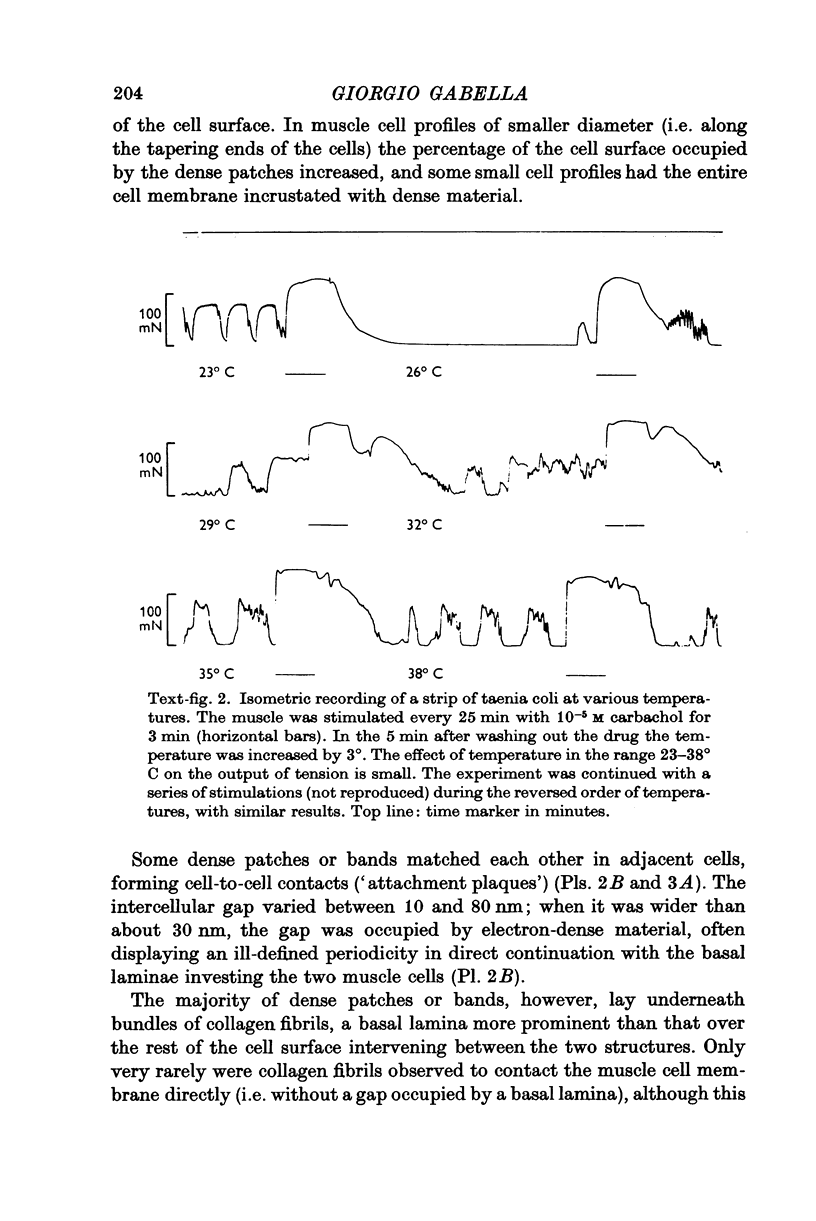
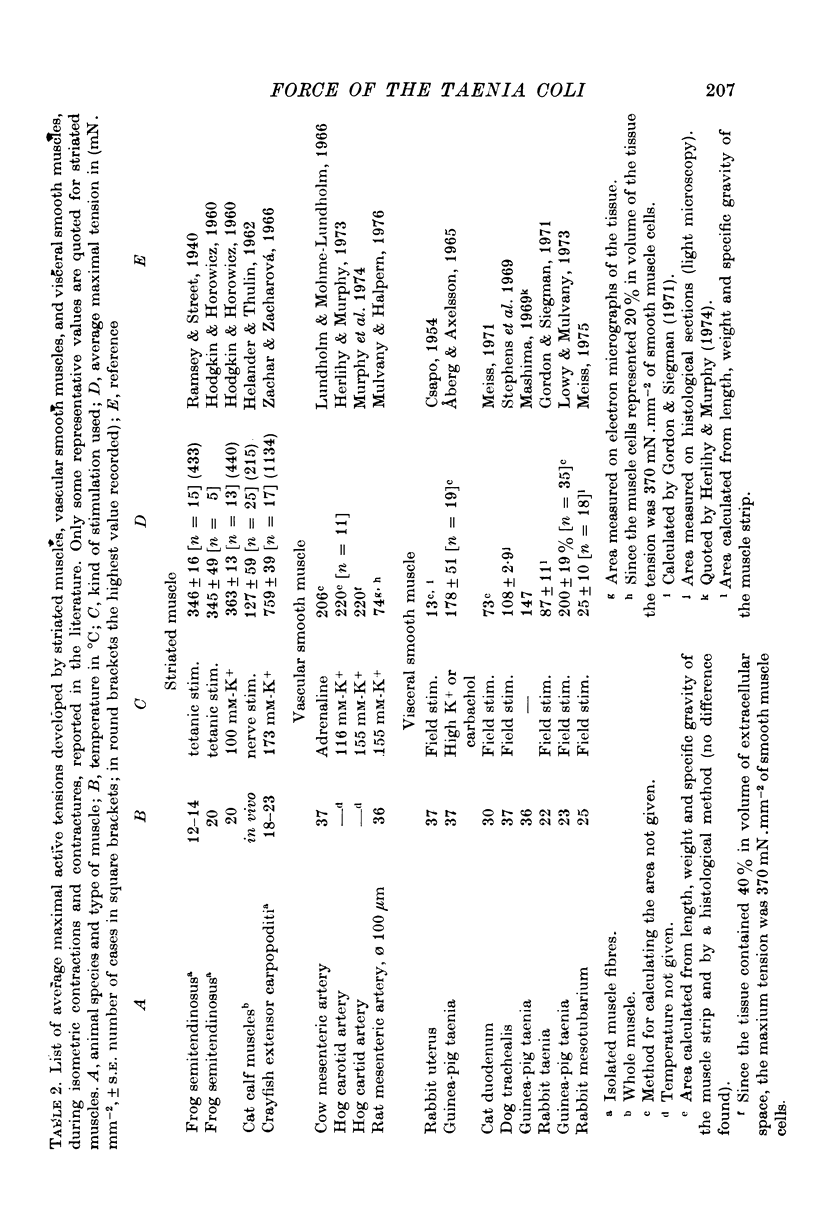
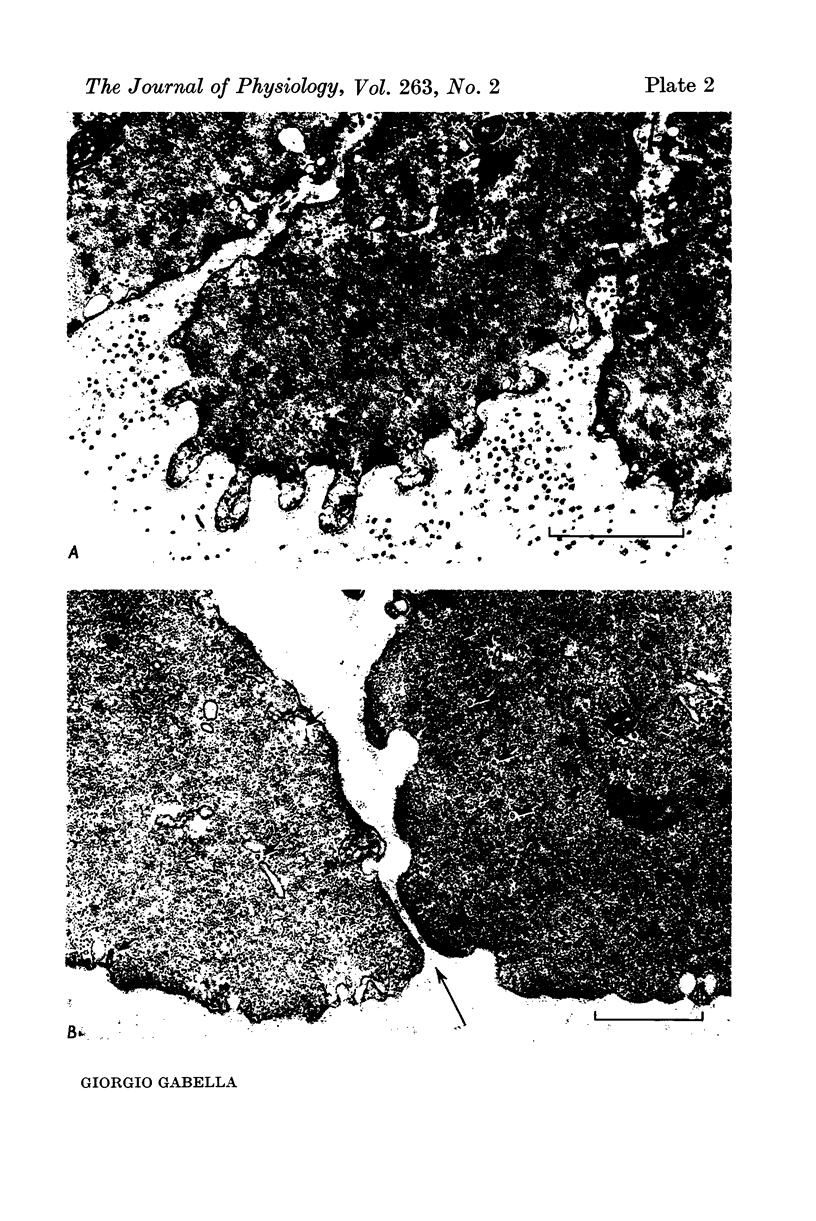
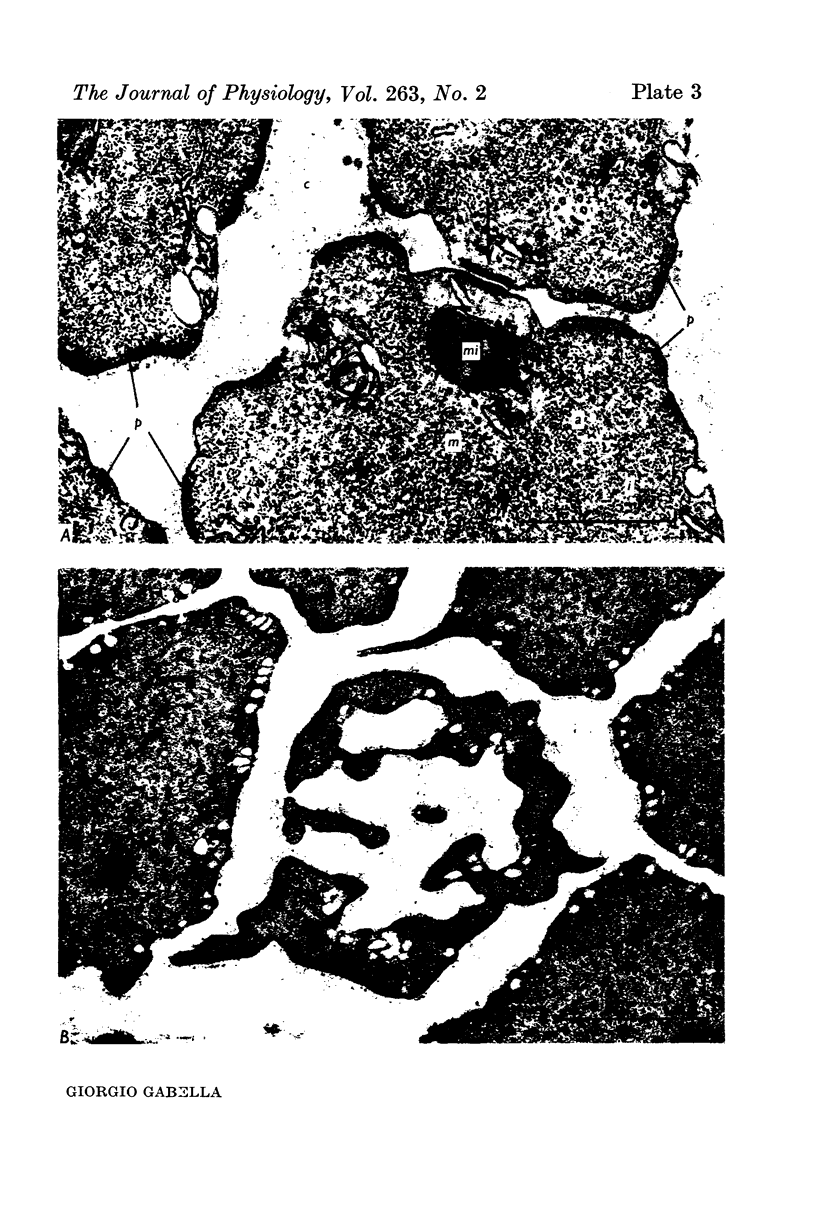
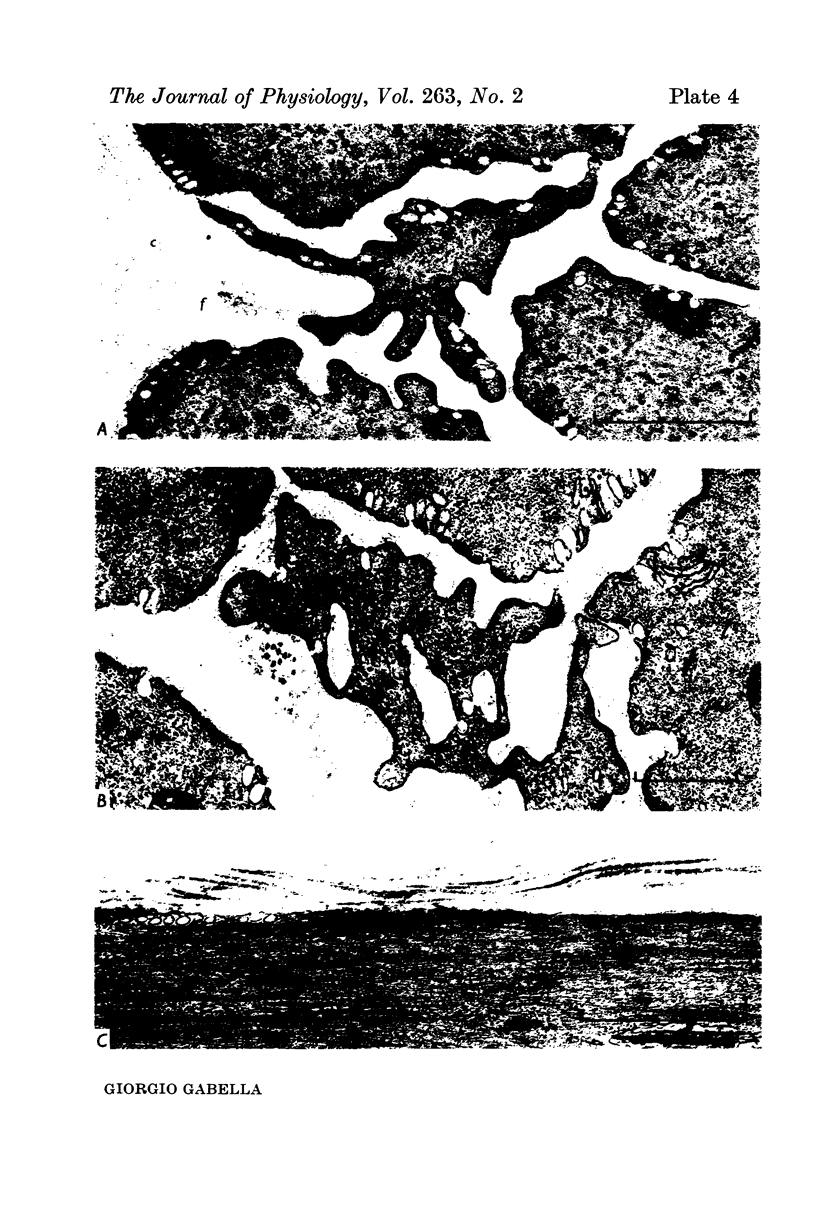
1. Strips of taenia coli from the caecum of the guinea-pig were mounted in an organ bath at 37 degrees C; isometric contractions were elicited with 10(5)M carbachol. Each taenia was stretched to the length at which it produced the maximum active tension; it was then fixed and embedded for measurement of the transverse sectional area. 2. The maximal force produced ranged between 96-1 and 138-3 mN. This corresponded to a force of between 251 and 513 mN.mm(2) (mean: 416 +/-28 [n = 10]). Temperature changes in the range 23-38 degrees C had little effect on the maximal force output.3. When allowance is made for the extracellular space (about 32% of the transverse sectional area), for the non-muscular cells present in the taenia (about 5%), and for the non-contractile material present in the muscle cells (about 10%), the maximal force generated was about 734 mN.mm(2) of contractile material (or almost twice as large as in skeletal muscle).4. Electron microscopy revealed terminal apparatuses at the ends of muscle cells, anchoring the cells to the connective tissue, and cell-to-cell junctions (attachment plaques). In addition, many dense patches of dense bands, sites near the cell surface where filaments are seen to end, were scattered along the entire length of the muscle cell and lay close to bundles of collagen fibrils. 5. It is suggested that the production of such a large force by this smooth muscle is partly explained by the lateral attachment of some contractile units to sites along the entire cell length, which in their turn are anchored to the collagen network; the latter may be considered a sort of intramuscular tendon.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERG A. K., AXELSSON J. SOME MECHANICAL ASPECTS OF AN INTESTINAL SMOOTH MUSCLE. Acta Physiol Scand. 1965 May-Jun;64:15–27. doi: 10.1111/j.1748-1716.1965.tb04150.x. [DOI] [PubMed] [Google Scholar]
- Ashton F. T., Somlyo A. V., Somlyo A. P. The contractile apparatus of vascular smooth muscle: intermediate high voltage stereo electron microscopy. J Mol Biol. 1975 Oct 15;98(1):17–29. doi: 10.1016/s0022-2836(75)80098-2. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E., PROSSER C. L. Electrophysiology of smooth muscle. Physiol Rev. 1963 Jul;43:482–527. doi: 10.1152/physrev.1963.43.3.482. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Jones A. W. Distribution and kinetics of CoEDTA in smooth muscle, and its use as an extracellular marker. J Physiol. 1969 Feb;200(2):387–401. doi: 10.1113/jphysiol.1969.sp008700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Rand M. J. The inhibitory innervation of the taenia of the guinea-pig caecum. J Physiol. 1966 Feb;182(3):504–526. doi: 10.1113/jphysiol.1966.sp007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSAPO A. Dependence of isometric tension and isotonic shortening of uterine muscle on temperature and on strength of stimulation. Am J Physiol. 1954 Jun;177(3):348–354. doi: 10.1152/ajplegacy.1954.177.3.348. [DOI] [PubMed] [Google Scholar]
- Canaday P. G., Fay F. S. An ultrasensitive isometric force transducer for single smooth muscle cell mechanics. J Appl Physiol. 1976 Feb;40(2):243–246. doi: 10.1152/jappl.1976.40.2.243. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Cooke P. A filamentous cytoskeleton in vertebrate smooth muscle fibers. J Cell Biol. 1976 Mar;68(3):539–556. doi: 10.1083/jcb.68.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay F. S., Delise C. M. Contraction of isolated smooth-muscle cells--structural changes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):641–645. doi: 10.1073/pnas.70.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Quantitative morphological study of smooth muscle cells of the guinea-pig taenia coli. Cell Tissue Res. 1976 Jul 26;170(2):161–186. doi: 10.1007/BF00224297. [DOI] [PubMed] [Google Scholar]
- Gordon A. R., Siegman M. J. Mechanical properties of smooth muscle. I. Length-tension and force-velocity relations. Am J Physiol. 1971 Nov;221(5):1243–1249. doi: 10.1152/ajplegacy.1971.221.5.1243. [DOI] [PubMed] [Google Scholar]
- HELANDER E., THULIN C. A. Isometric tension and myofilamental cross-sectional area in striated muscle. Am J Physiol. 1962 May;202:824–826. doi: 10.1152/ajplegacy.1962.202.5.824. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanak H., Böck P. Die Feinstruktur der Muskel-Sehnenverbindung von Skelett- und Herzmuskel. J Ultrastruct Res. 1971 Jul;36(1):68–85. doi: 10.1016/s0022-5320(71)80089-8. [DOI] [PubMed] [Google Scholar]
- Herlihy J. T., Murphy R. A. Force-velocity and series elastic characteristics of smooth muscle from the hog carotid artery. Circ Res. 1974 Apr;34(4):461–466. doi: 10.1161/01.res.34.4.461. [DOI] [PubMed] [Google Scholar]
- Herlihy J. T., Murphy R. A. Length-tension relationship of smooth muscle of the hog carotid artery. Circ Res. 1973 Sep;33(3):275–283. doi: 10.1161/01.res.33.3.275. [DOI] [PubMed] [Google Scholar]
- Jones A. W., Somlyo A. P., Somlyo A. V. Potassium accumulation in smooth muscle and associated ultrastructural changes. J Physiol. 1973 Jul;232(2):247–273. doi: 10.1113/jphysiol.1973.sp010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy J., Mulvany M. J. Mechanical properties of guinea pig taenia coli muscles. Acta Physiol Scand. 1973 May;88(1):123–136. doi: 10.1111/j.1748-1716.1973.tb05439.x. [DOI] [PubMed] [Google Scholar]
- MERRILLEES N. C., BURNSTOCK G., HOLMAN M. E. CORRELATION OF FINE STRUCTURE AND PHYSIOLOGY OF THE INNERVATION OF SMOOTH MUSCLE IN THE GUINEA PIG VAS DEFERENS. J Cell Biol. 1963 Dec;19:529–550. doi: 10.1083/jcb.19.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiss R. A. Graded activation in rabbit mesotubarium smooth muscle. Am J Physiol. 1975 Aug;229(2):455–465. doi: 10.1152/ajplegacy.1975.229.2.455. [DOI] [PubMed] [Google Scholar]
- Meiss R. A. Some mechanical properties of cat intestinal muscle. Am J Physiol. 1971 Jun;220(6):2000–2007. doi: 10.1152/ajplegacy.1971.220.6.2000. [DOI] [PubMed] [Google Scholar]
- Mobley B. A., Eisenberg B. R. Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol. 1975 Jul;66(1):31–45. doi: 10.1085/jgp.66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Halpern W. Mechanical properties of vascular smooth muscle cells in situ. Nature. 1976 Apr 15;260(5552):617–619. doi: 10.1038/260617a0. [DOI] [PubMed] [Google Scholar]
- Murphy R. A., Herlihy J. T., Megerman J. Force-generating capacity and contractile protein content of arterial smooth muscle. J Gen Physiol. 1974 Dec;64(6):691–705. doi: 10.1085/jgp.64.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGE S. G., HUXLEY H. E. FILAMENT LENGTHS IN STRIATED MUSCLE. J Cell Biol. 1963 Nov;19:369–390. doi: 10.1083/jcb.19.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEASE D. C., MOLINARI S. Electron microscopy of muscular arteries; pial vessels of43 the cat and monkey. J Ultrastruct Res. 1960 Jun;3:447–468. doi: 10.1016/s0022-5320(60)90022-8. [DOI] [PubMed] [Google Scholar]
- Peiper U., Laven R., Ehl M. Force velocity relationships in vascular smooth muscle. The influence of temperature. Pflugers Arch. 1975 Apr 9;356(1):33–45. doi: 10.1007/BF00583519. [DOI] [PubMed] [Google Scholar]
- ROSENBLUTH J. SMOOTH MUSCLE: AN ULTRASTRUCTURAL BASIS FOR THE DYNAMIC OF ITS CONTRACTION. Science. 1965 Jun 4;148(3675):1337–1339. doi: 10.1126/science.148.3675.1337. [DOI] [PubMed] [Google Scholar]
- Stephens N. L., Kroeger E., Mehta J. A. Force-velocity characteristics of respiratory airway smooth muscle. J Appl Physiol. 1969 Jun;26(6):685–692. doi: 10.1152/jappl.1969.26.6.685. [DOI] [PubMed] [Google Scholar]
- Zachar J., Zacharová D. Potassium contractures in single muscle fibres of the crayfish. J Physiol. 1966 Oct;186(3):596–618. doi: 10.1113/jphysiol.1966.sp008058. [DOI] [PMC free article] [PubMed] [Google Scholar]