Abstract
1. The hypothesis is advanced that the joint occurrence of unitary excitatory post-synaptic potentials e.p.s.p.s) evoked in motoneurones by branches of common stem pre-synaptic fibres causes short-term synchronization of their discharge during the rising phases of the unitary e.p.s.p.s. 2. This hypothesis was tested using the pre- and post-stimulus time (PPST) histogram to detect synchronized firing among groups of intercostal motoneurones discharging in response to their natural synaptic drives. 3. Motor nerve action potentials were recorded monophasically from nerve filaments of the external intercostal muscles of anaesthetized, paralysed cats maintained on artificial ventilation. 4. Computer methods were used to measure peak spike amplitude, spike amplitude, spike interval and filament identification for simultaneous recordings from four filaments. The spike amplitude histograms were derived for each filament and groups of spikes were selected for analysis. 5. With spikes of one group designated as 'stimuli' (occurring at zero time) and those of a second as 'response' the PPST histogram was computed with different time bin widths. 6. With bin widths of 100 and 10 msec the central respiratory periodicity was apparent in the PPST histogram. With 1.0 msec bins the PPST histogram showed a narrow central peak extending to +/- 3.0 msec at its base. This 'short-term synchronization' supports the hypothesis of joint firing due to common presynaptic connectivity. 7. It was shown that detection of short-term synchronization was critically dependent on a sufficient quantity of data but that provided a simple criterion of adequate counts per bin in the PPST histogram was met, short-term synchronization could be detected between intercostal motoneurones of the same and adjacent segments.
Full text
PDF


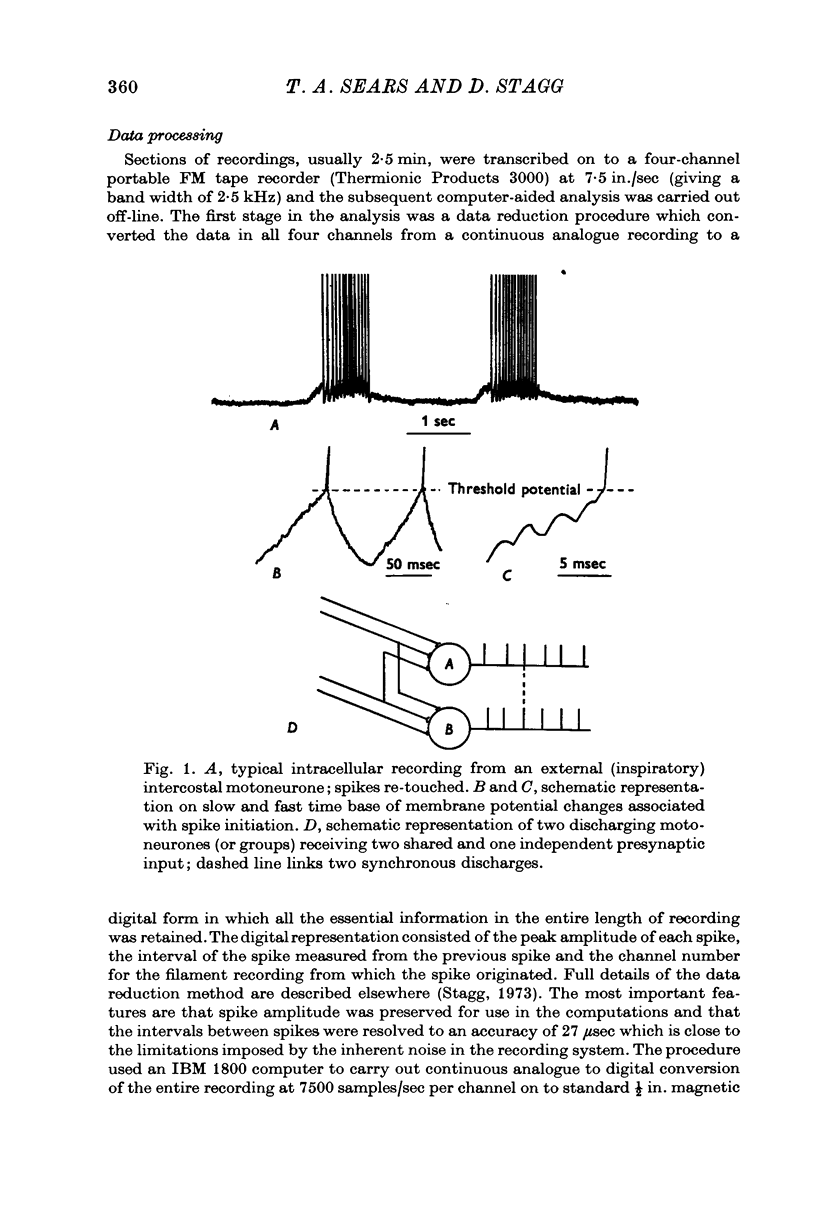
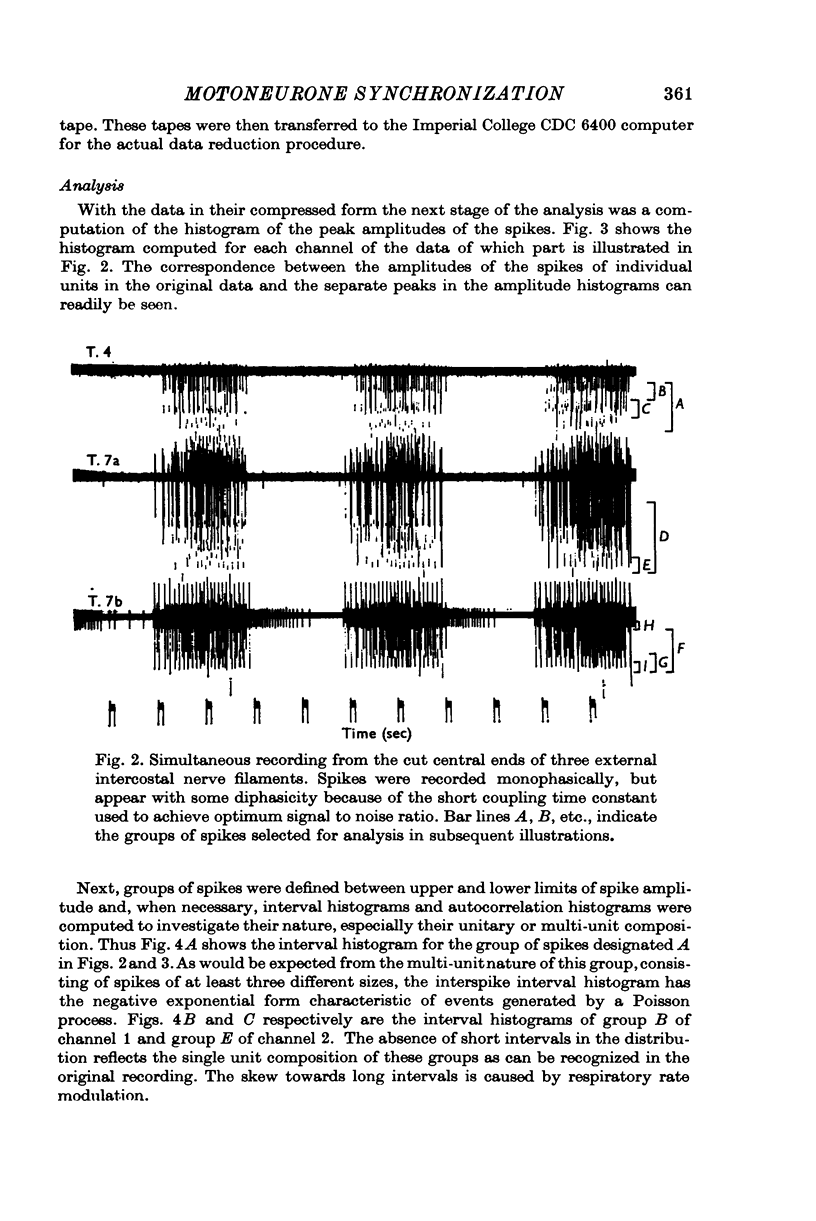
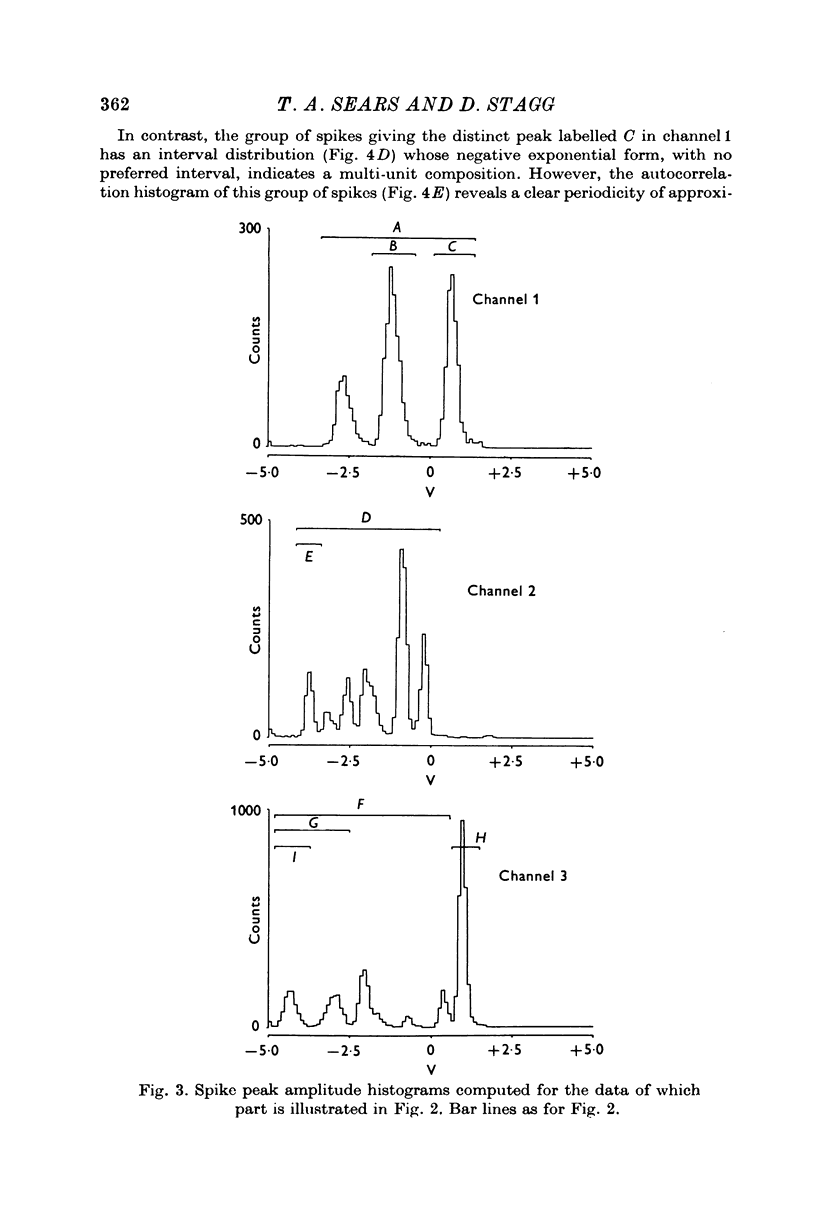

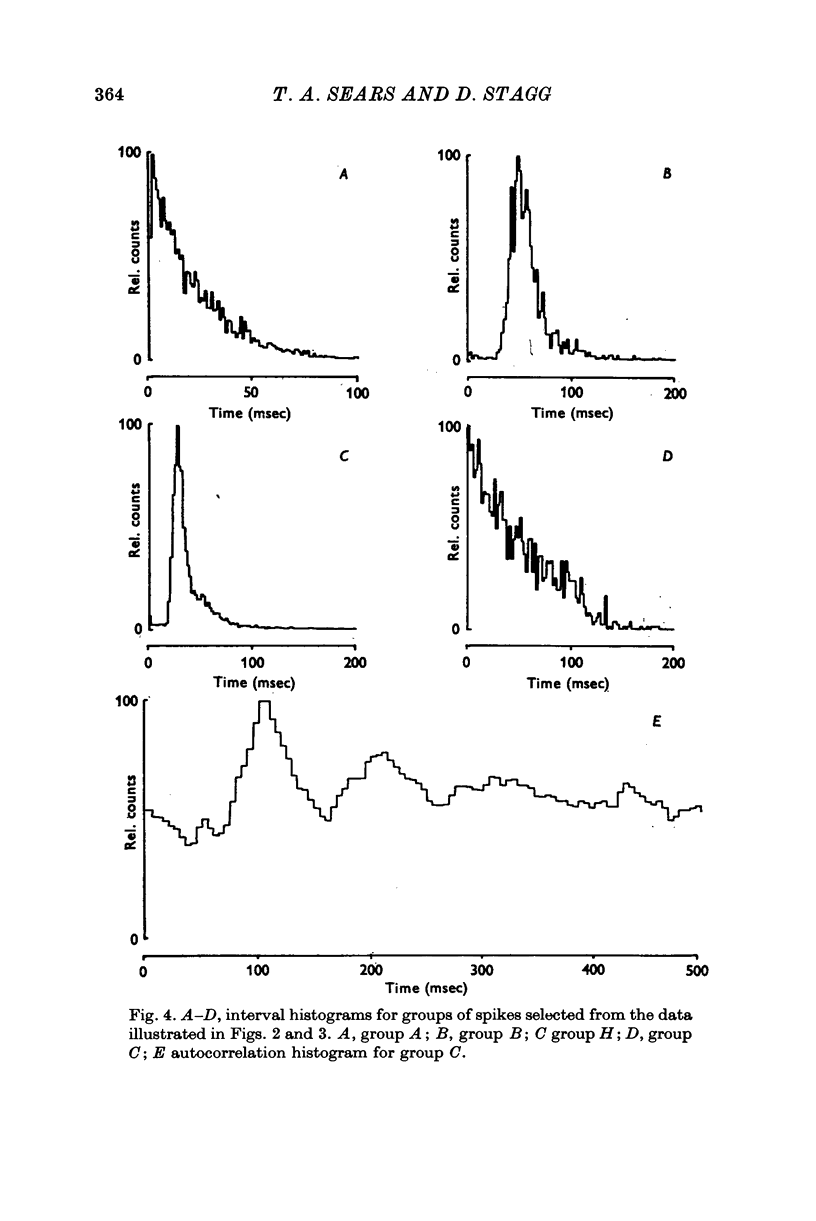
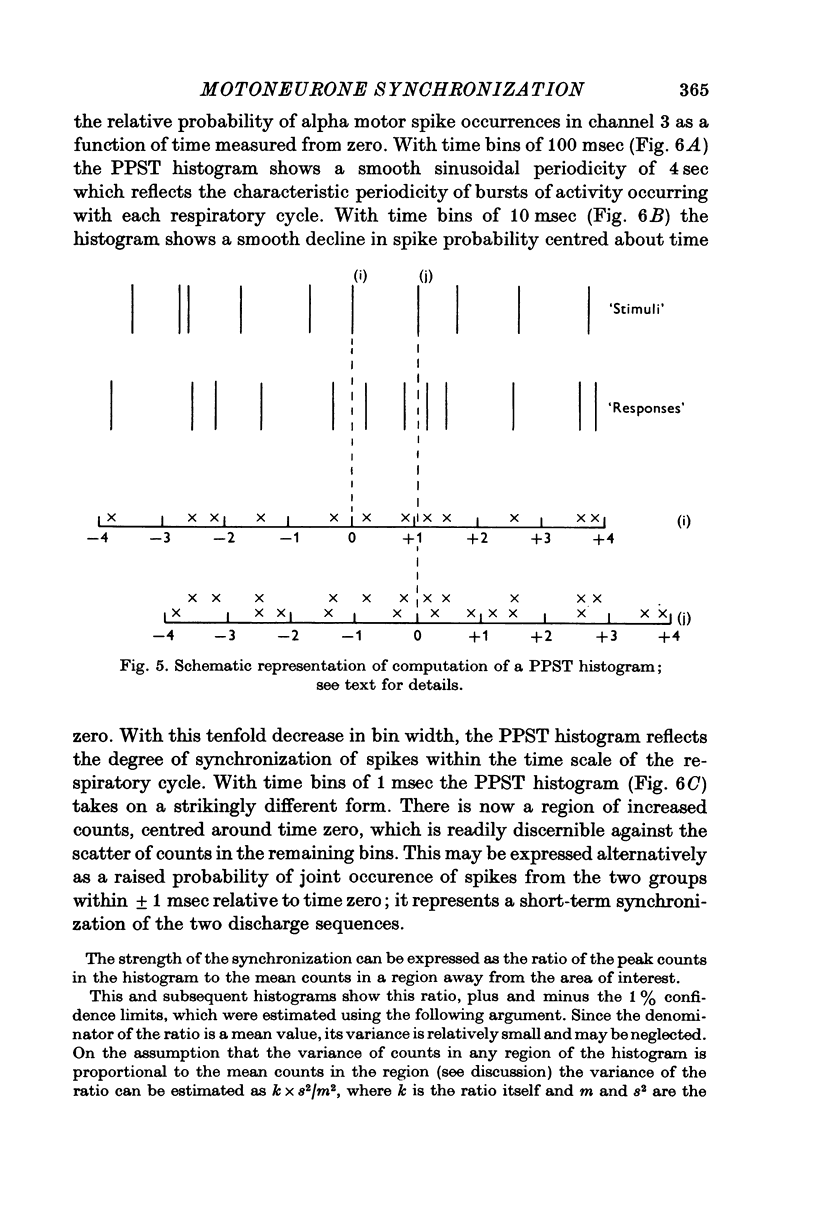
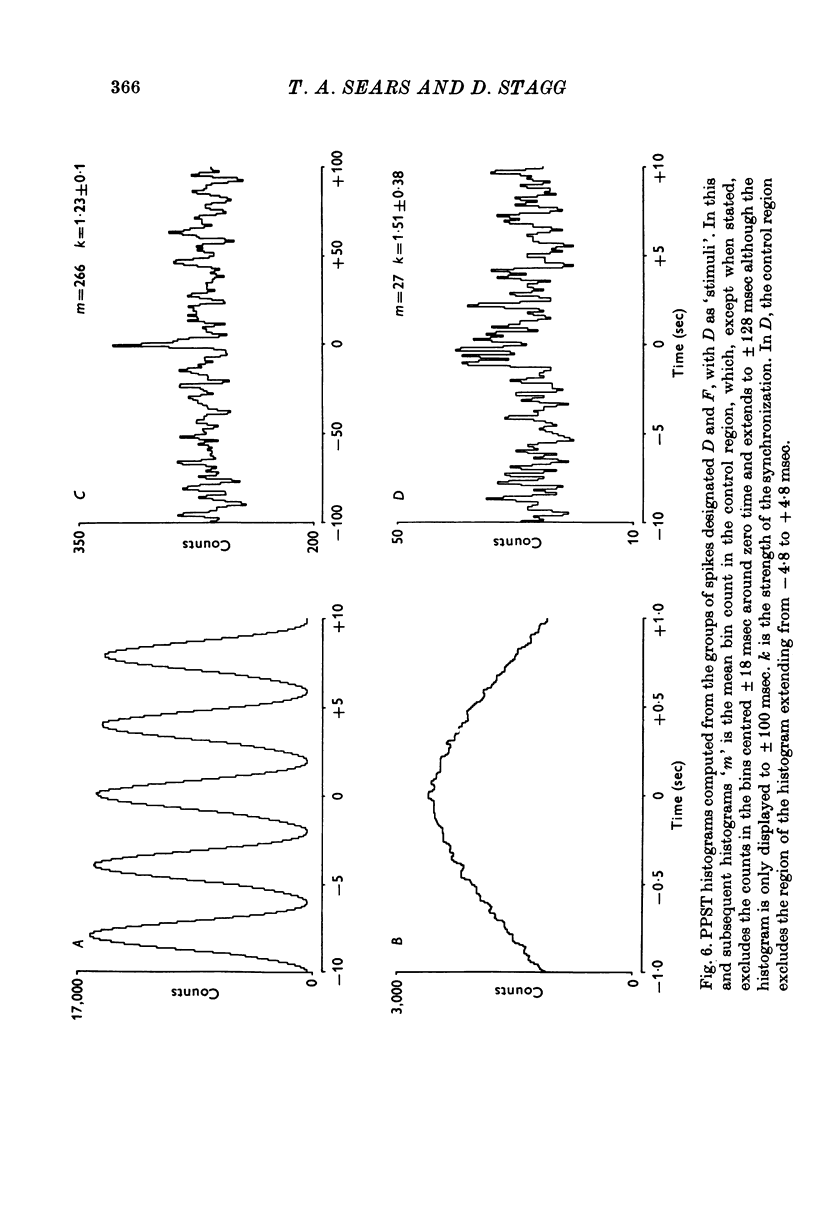

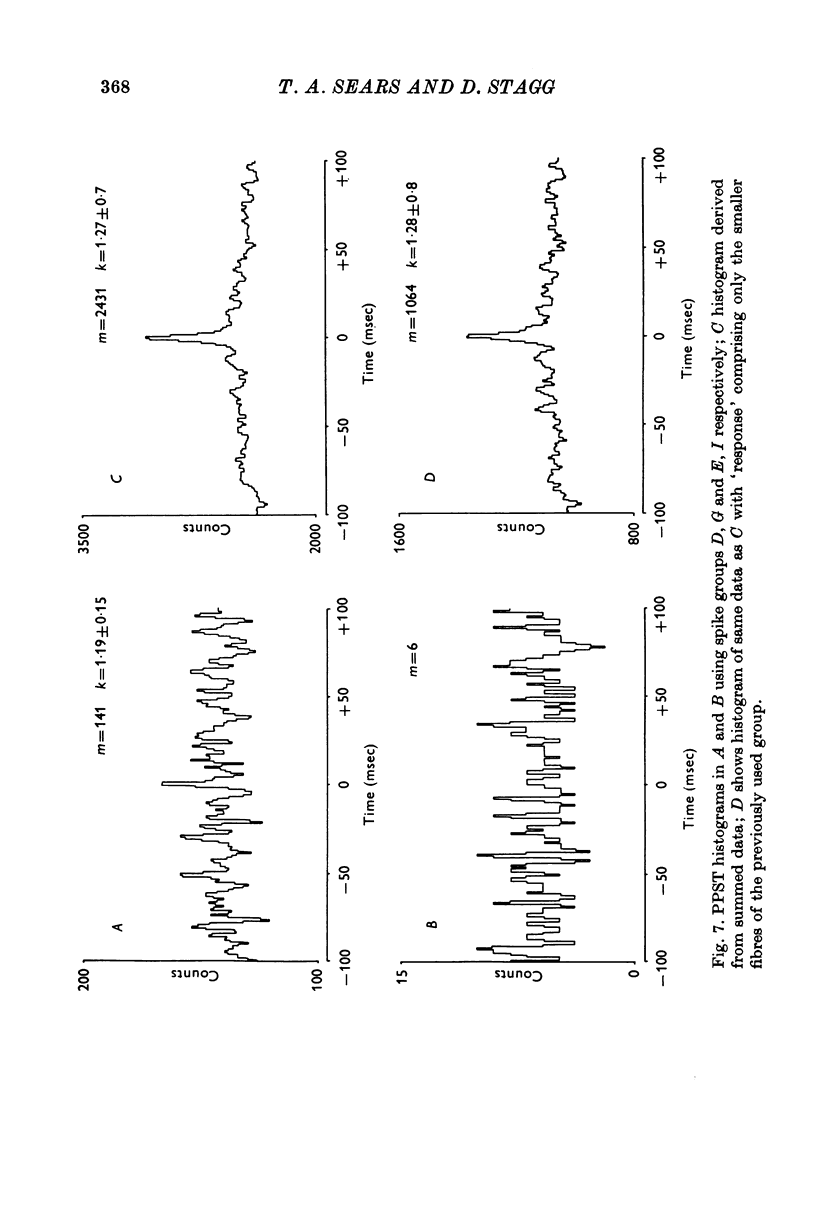

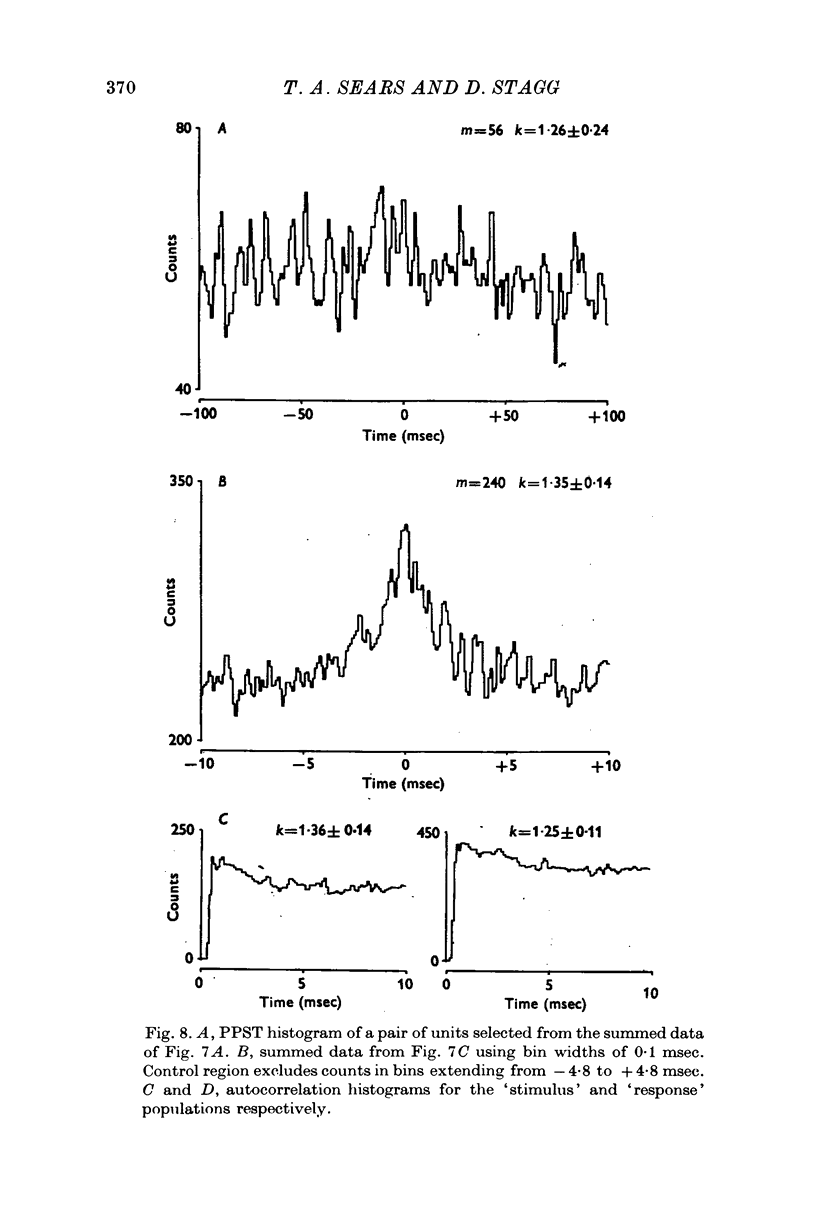









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. N., Crill W. E. Specific membrane properties of cat motoneurones. J Physiol. 1974 Jun;239(2):301–324. doi: 10.1113/jphysiol.1974.sp010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. I. Synchronization of discharge, spontaneous and evoked, between inspiratory neurons. Acta Neurobiol Exp (Wars) 1973;33(1):189–218. [PubMed] [Google Scholar]
- Conradi S. Ultrastructure and distribution of neuronal and glial elements on the motoneuron surface in the lumbosacral spinal cord of the adult cat. Acta Physiol Scand Suppl. 1969;332:5–48. [PubMed] [Google Scholar]
- Davis J. N., Sears T. A. The proprioceptive reflex control of the intercostal muscles during their voluntary activation. J Physiol. 1970 Aug;209(3):711–738. doi: 10.1113/jphysiol.1970.sp009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. Monosynaptic reflex response of spinal motoneurons to graded afferent stimulation. J Gen Physiol. 1955 Jul 20;38(6):813–852. doi: 10.1085/jgp.38.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Miller S., Porter R., Redman S. J. The time course of minimal excitory post-synaptic potentials evoked in spinal motoneurones by group Ia afferent fibres. J Physiol. 1971 Jun;215(2):353–380. doi: 10.1113/jphysiol.1971.sp009474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Monosynaptic excitation of motoneurones from secondary endings of muscle spindles. Nature. 1974 Nov 15;252(5480):243–244. doi: 10.1038/252243a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Proceedings: The average common excitation (ACE) potential and its significance. J Physiol. 1976 Jul;259(1):36P–37P. [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Stagg D. Proceedings: Synchronized firing of respiratory motoneurones during spontaneous breathing in the anaesthetized cat. J Physiol. 1974 May;239(1):11P–13P. [PubMed] [Google Scholar]
- Knox C. K. Cross-correlation functions for a neuronal model. Biophys J. 1974 Aug;14(8):567–582. doi: 10.1016/S0006-3495(74)85936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD D. P., McINTYRE A. K. Mono-synaptic reflex responses of individual motoneurons. J Gen Physiol. 1955 Jul 20;38(6):771–787. doi: 10.1085/jgp.38.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B. The relative unimportance of the temporal pattern of the primary afferent input in determining the mean level of motor firing in the tonic vibration reflex. J Physiol. 1975 Oct;251(2):333–361. doi: 10.1113/jphysiol.1975.sp011096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. The contractile properties of human motor units during voluntary isometric contractions. J Physiol. 1973 Jan;228(2):285–306. doi: 10.1113/jphysiol.1973.sp010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A. Synchronized high frequency synaptic potentials in medullary respiratory neurons. Brain Res. 1974 Jul 26;75(2):350–355. doi: 10.1016/0006-8993(74)90760-4. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Segundo J. P., Perkel D. H., Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J. 1970 Sep;10(9):876–900. doi: 10.1016/S0006-3495(70)86341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967 Jul;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person R. S., Kudina L. P. Cross-correlation of electromyograms showing interference patterns. Electroencephalogr Clin Neurophysiol. 1968 Jul;25(1):58–68. doi: 10.1016/0013-4694(68)90087-4. [DOI] [PubMed] [Google Scholar]
- Rasminsky M., Sears T. A. Internodal conduction in undissected demyelinated nerve fibres. J Physiol. 1972 Dec;227(2):323–350. doi: 10.1113/jphysiol.1972.sp010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. EFFERENT DISCHARGES IN ALPHA AND FUSIMOTOR FIBRES OF INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Nov;174:295–315. doi: 10.1113/jphysiol.1964.sp007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. THE SLOW POTENTIALS OF THORACIC RESPIRATORY MOTONEURONES AND THEIR RELATION TO BREATHING. J Physiol. 1964 Dec;175:404–424. doi: 10.1113/jphysiol.1964.sp007524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg D. Computer acquisition of multiunit nerve-spike signals. Med Biol Eng. 1973 May;11(3):340–347. doi: 10.1007/BF02475544. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. The significance of grouping of motor unit activity. J Physiol. 1962 Jul;162:259–269. doi: 10.1113/jphysiol.1962.sp006930. [DOI] [PMC free article] [PubMed] [Google Scholar]


