Abstract
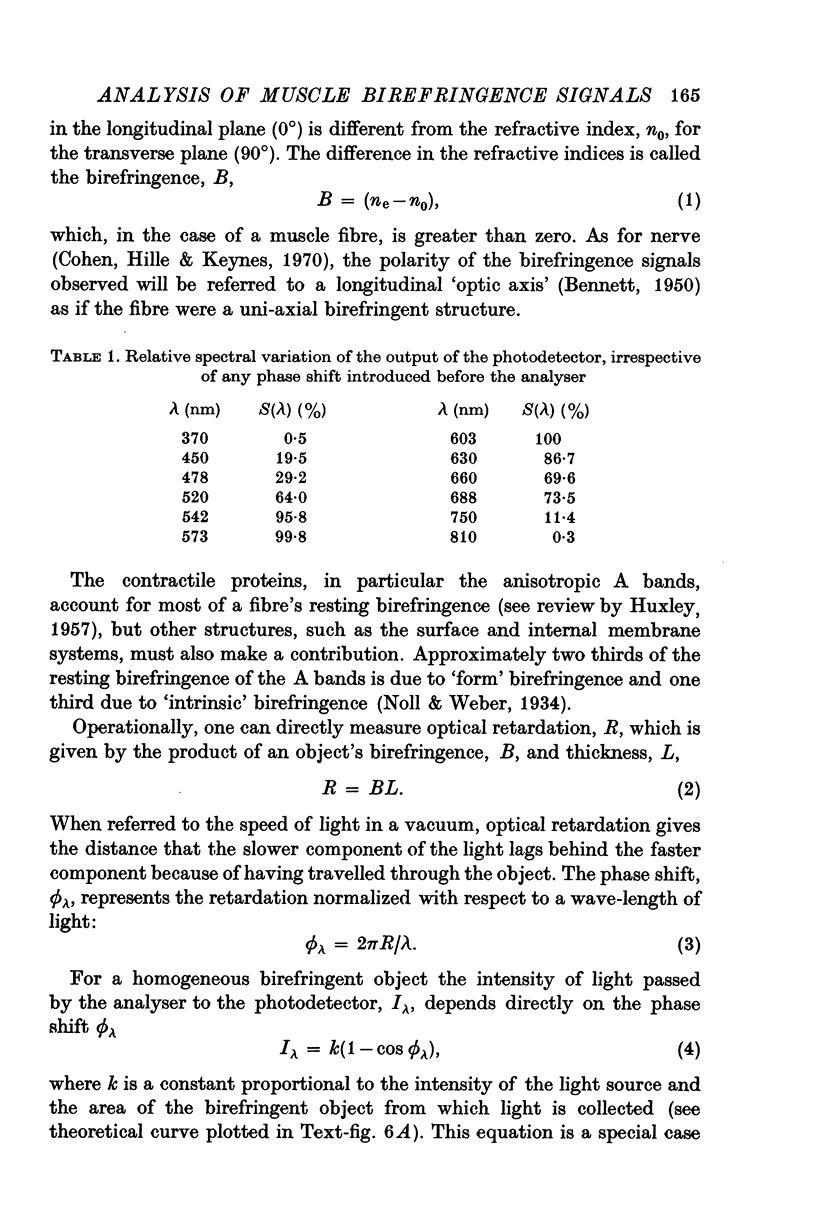
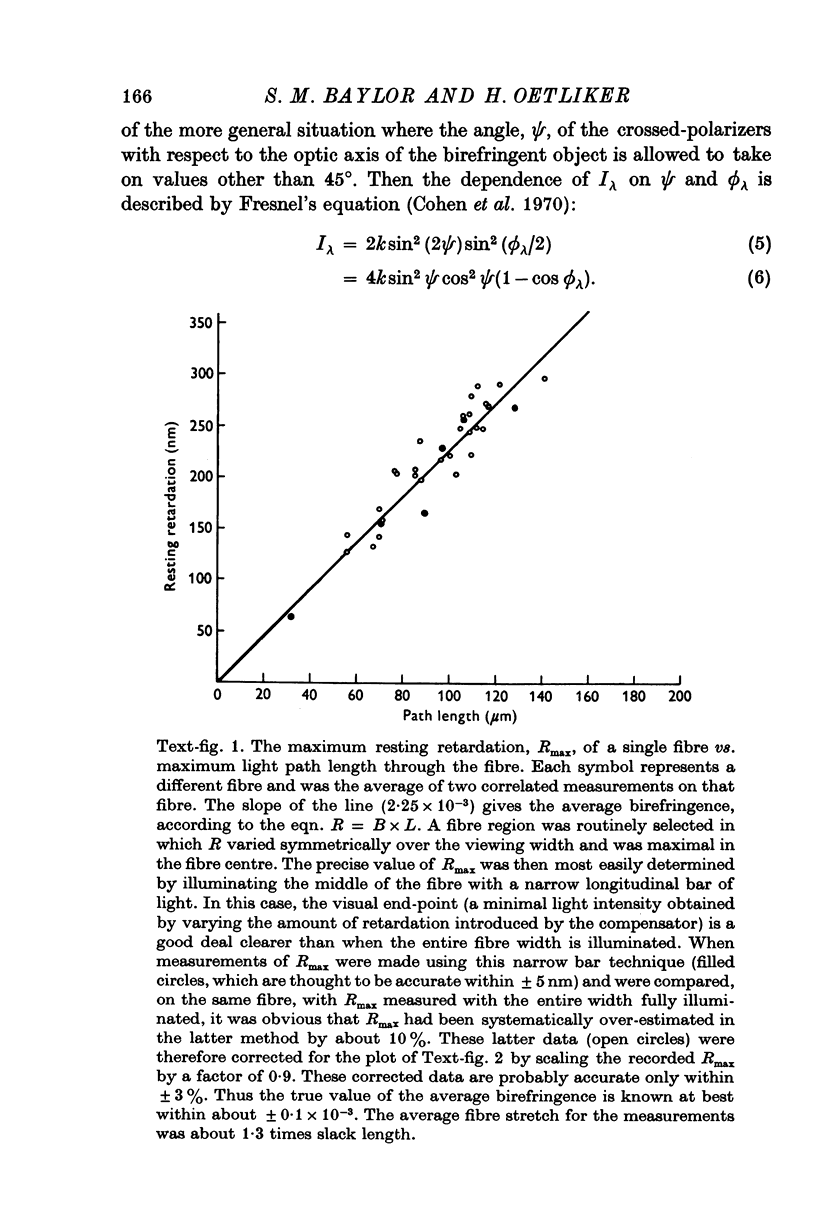
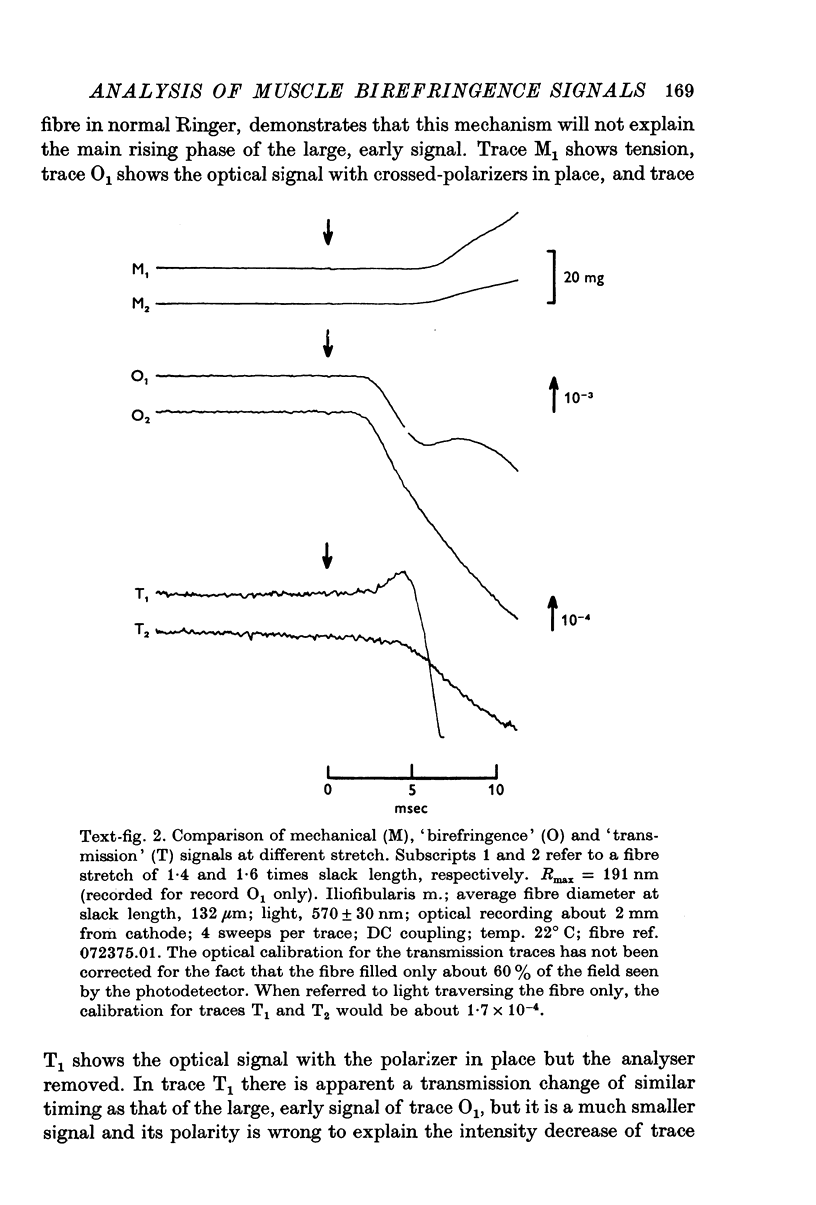
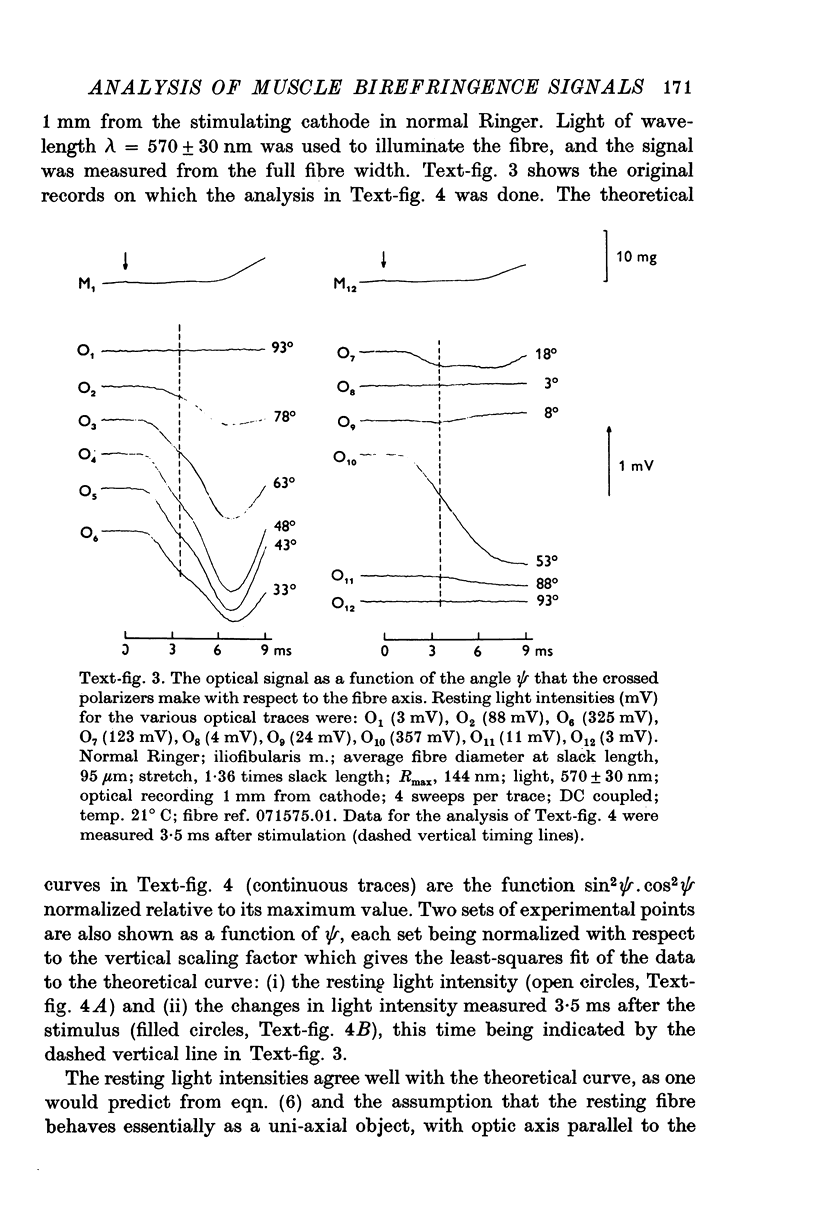
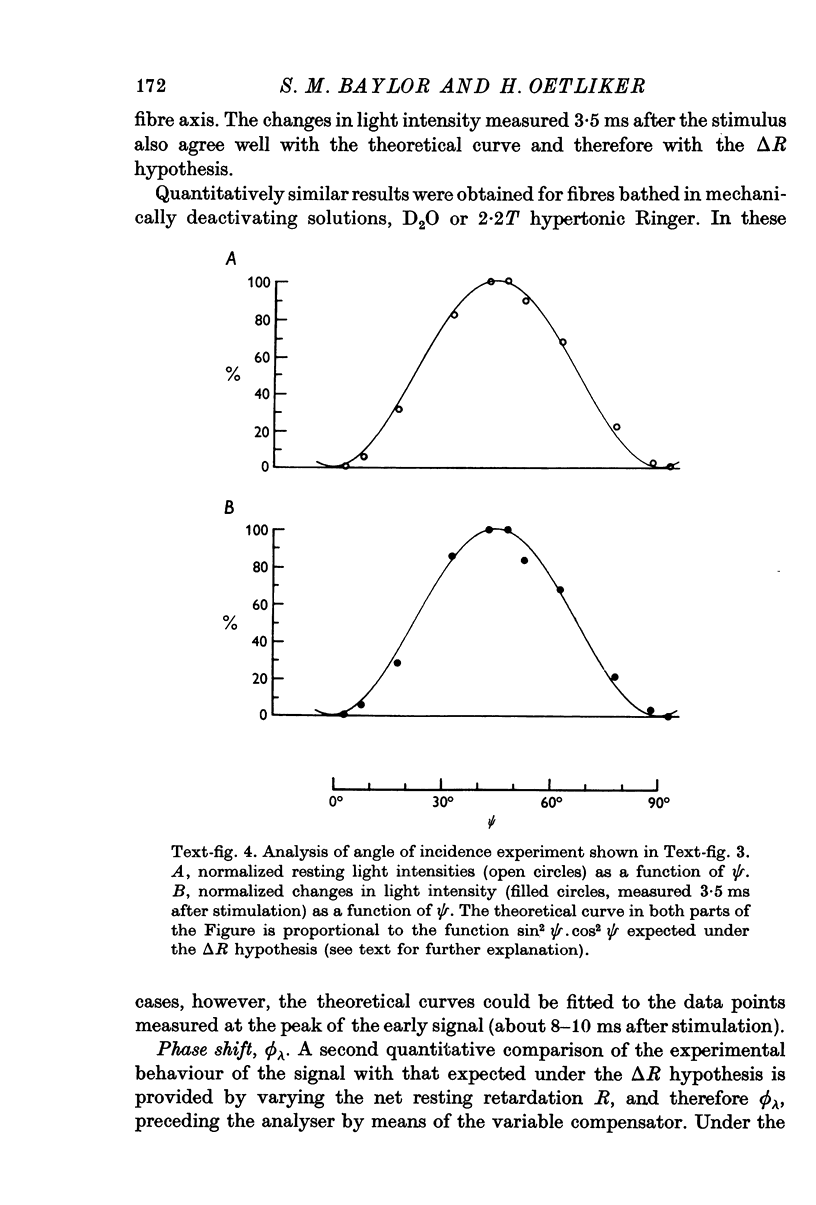
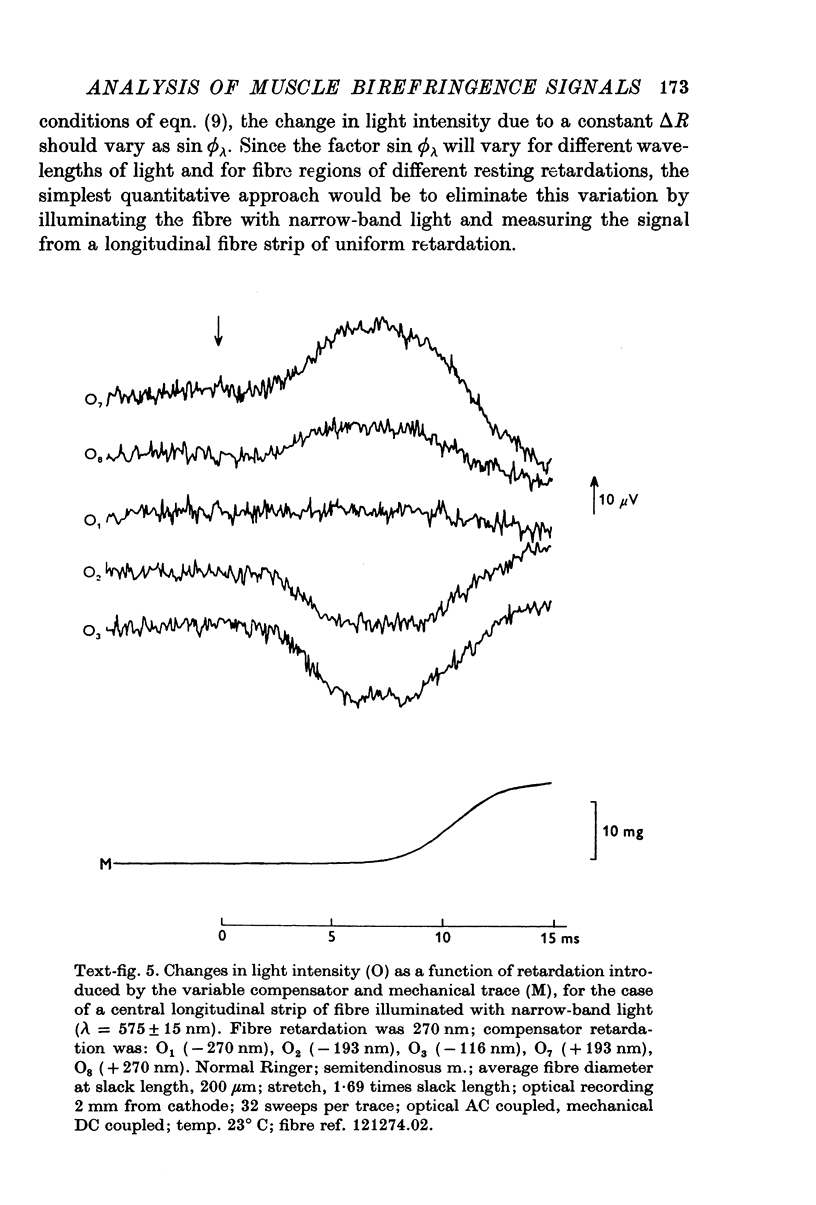
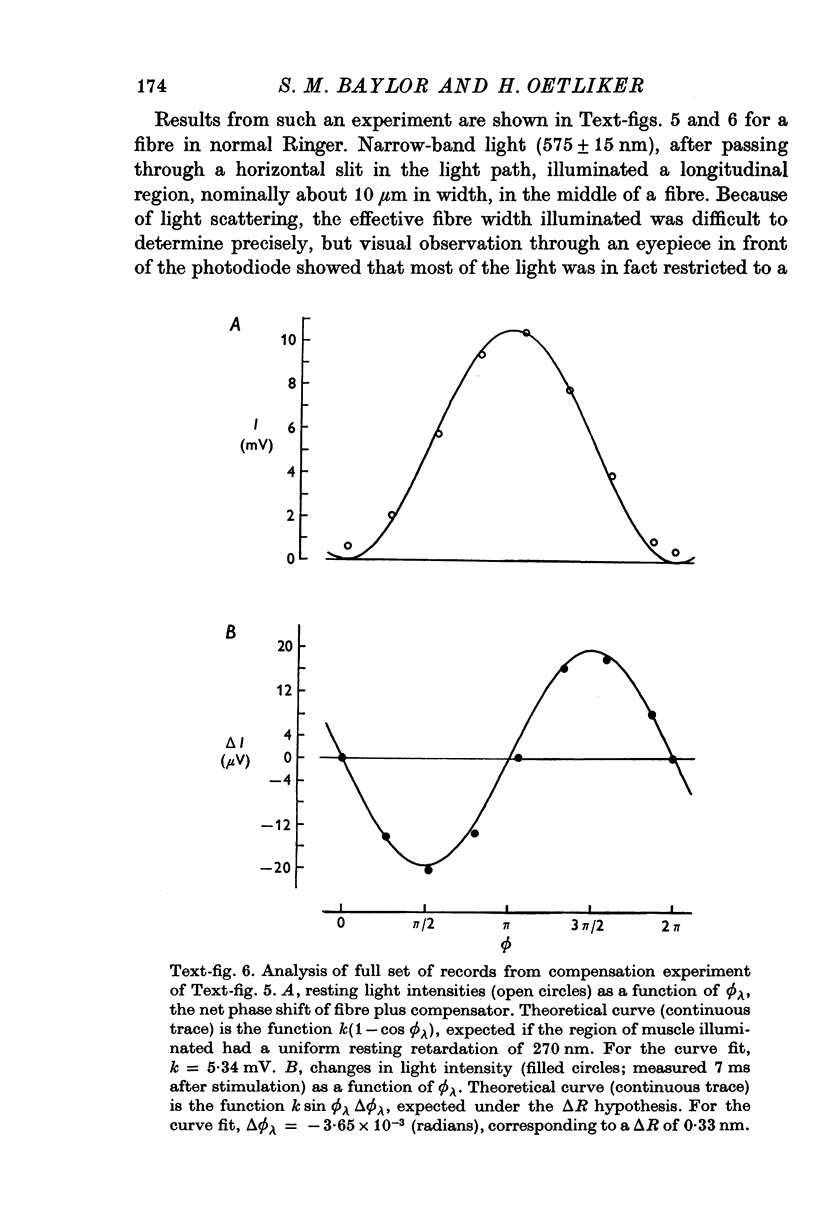
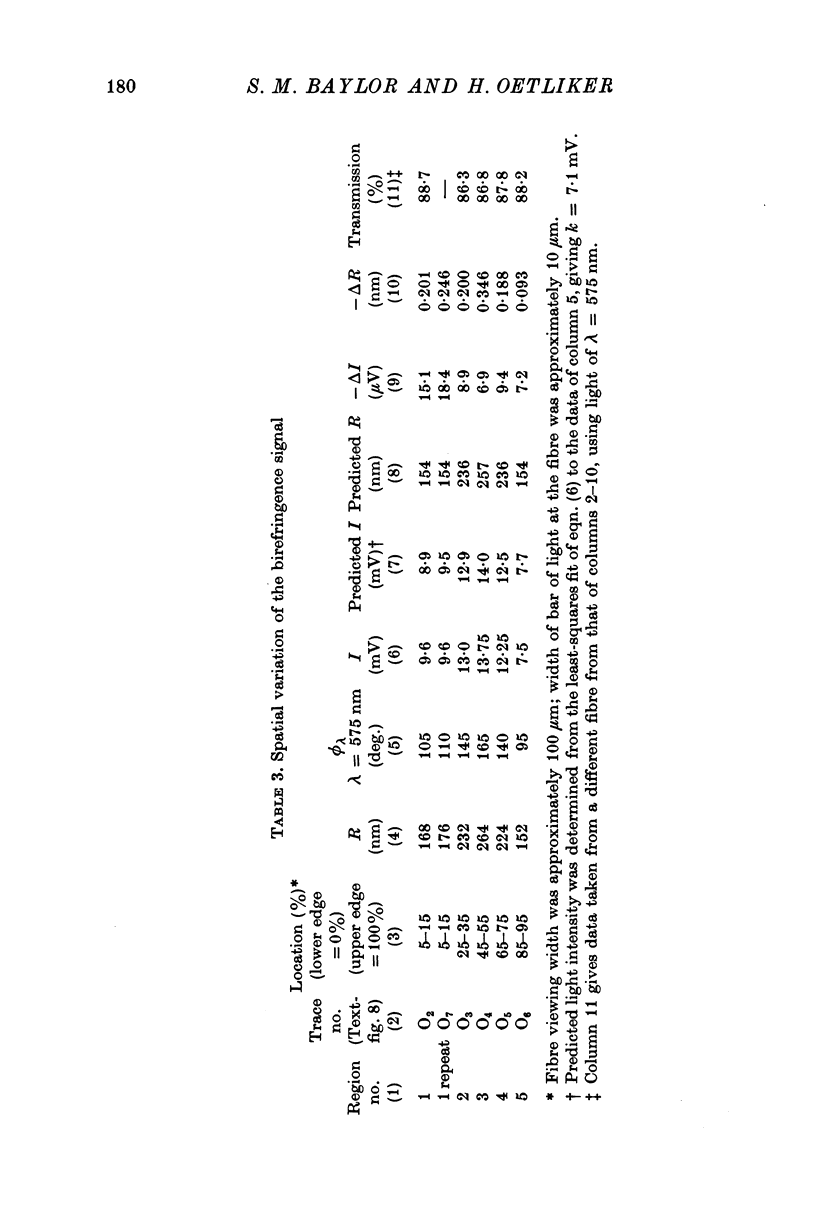
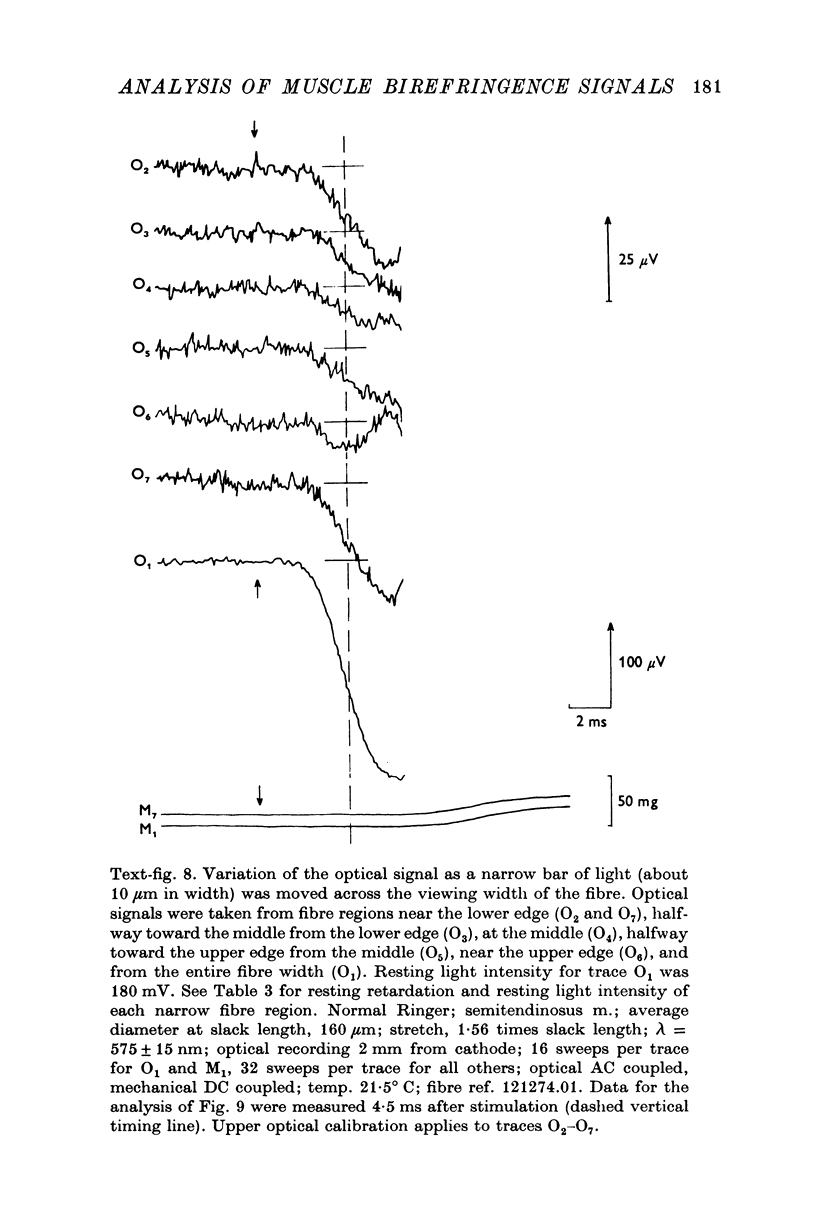
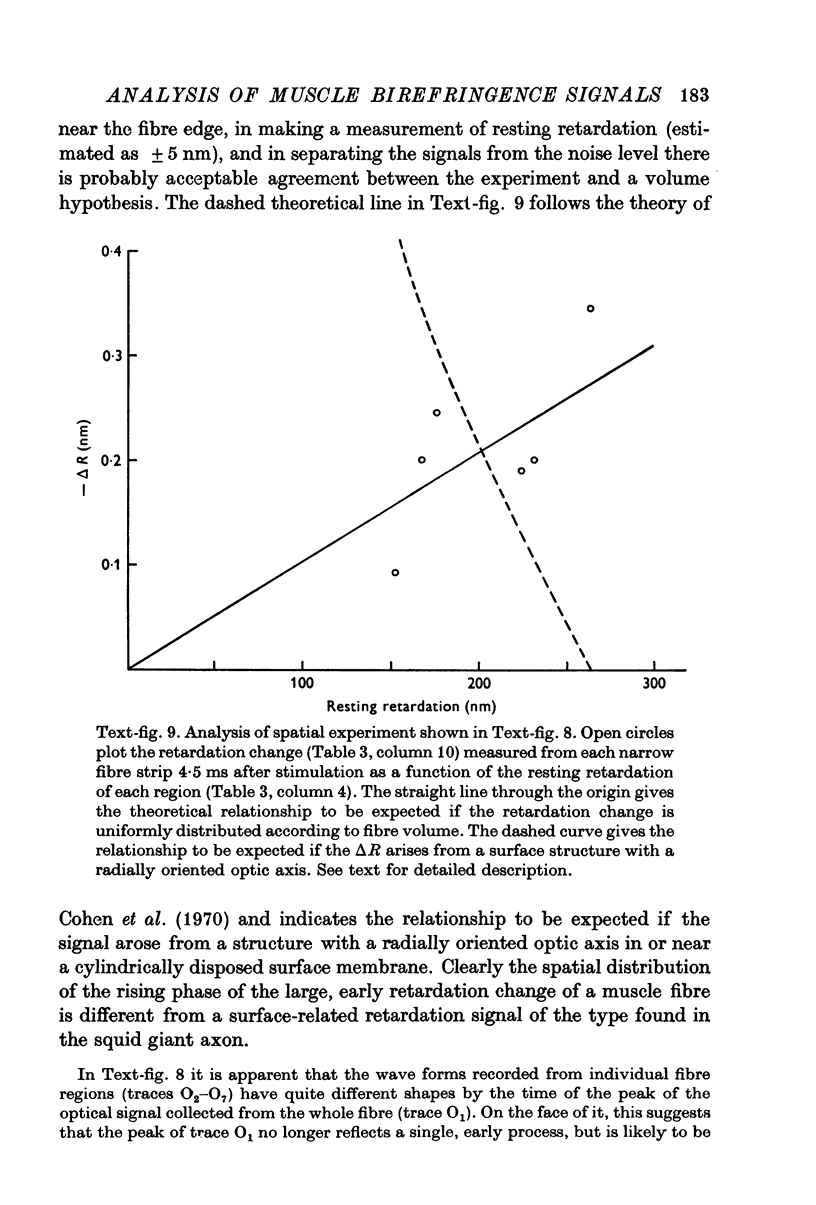
1. The optical retardation of single muscle fibres at rest and the optical properties of the large, early birefringence signal detectable during a twitch (Baylor & Oetliker, 1975, 1977a) were investigated. 2. The resting birefringence, B, which is the factor relating resting retardation, R, to the light path length through the fibre, L, was found to be 2.25 x 10-3 (i.e. R = 2.25 X 10-3 X L) and to be independent of wavelength ( lambda = 480-660 nm). 3. When the angle of incidence, psi, of the crossed polarizers with respect to the fibre axis was varied, the resting light intensity and large, early change in light intensity were related by the function sin2 psi-cos2psi. When the net phase shift, phi lambda, of a narrow longitudinal strip of fibre plus compensator was varied, the resting light intensity was described by the function (1-cos phi lambda), whereas the early change in light intensity followed sin phi lambda. These results make it likely that the optical mechanism underlying the early birefringence signal is a change in retardation. 4. When a narrow longitudinal strip of fibre was illuminated by monochromatic light in the range 480-690 nm, the magnitude of the signal varied approximately as expected if the retardation change is independent of wave-length. 5. The spatial characteristics of the signal were examined by moving a small slit of light across the fibre width as well as by measuring the signal collected from the entire fibre width as a function of wave-length. The results from both experiments support the idea that the large, early change in retardation is due to a volume-related rather than surface-related structure. 6. Under the assumption that the retardation change is distributed as fibre volume, its average magnitude was calculated. For fibres in normal Ringer the peak of the early retardation change compared with resting is about 1.7 x 10-3, and for fibres in D2O Ringer about 0.7 x 10-3.
Full text
PDF






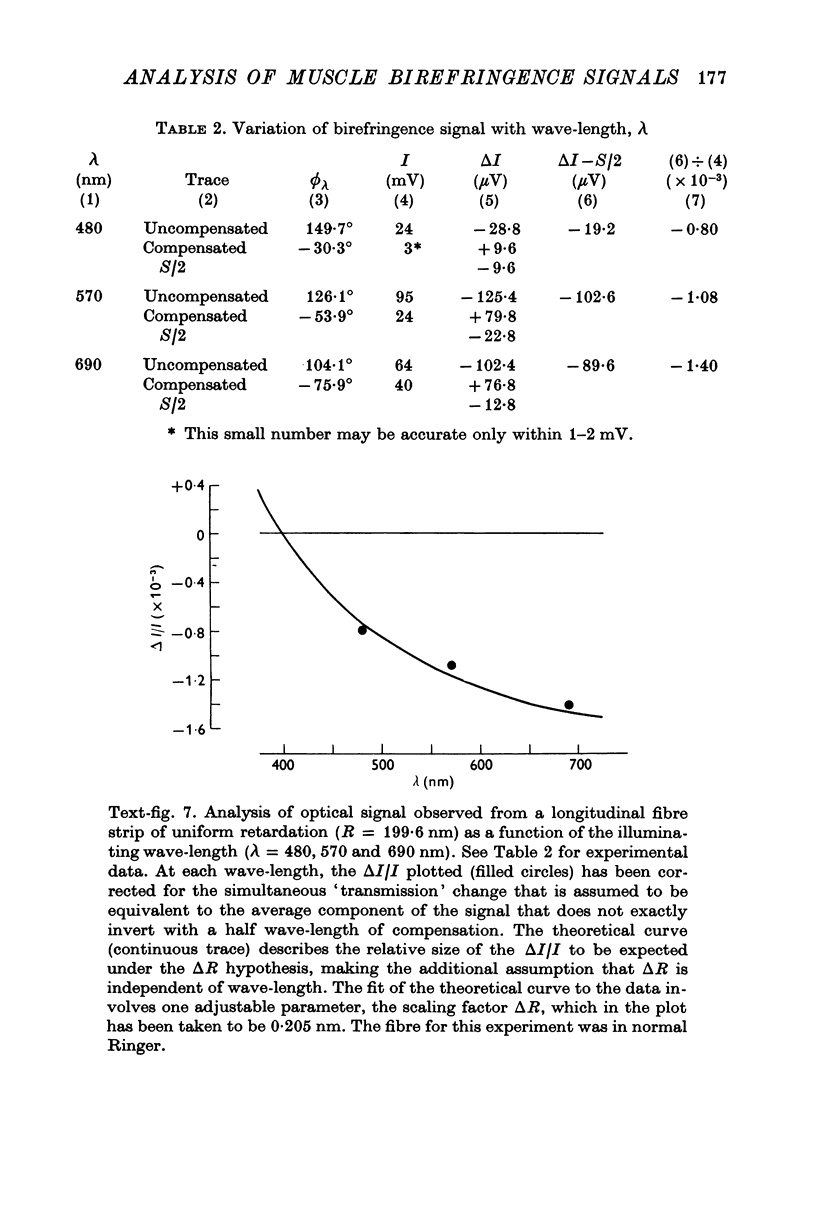





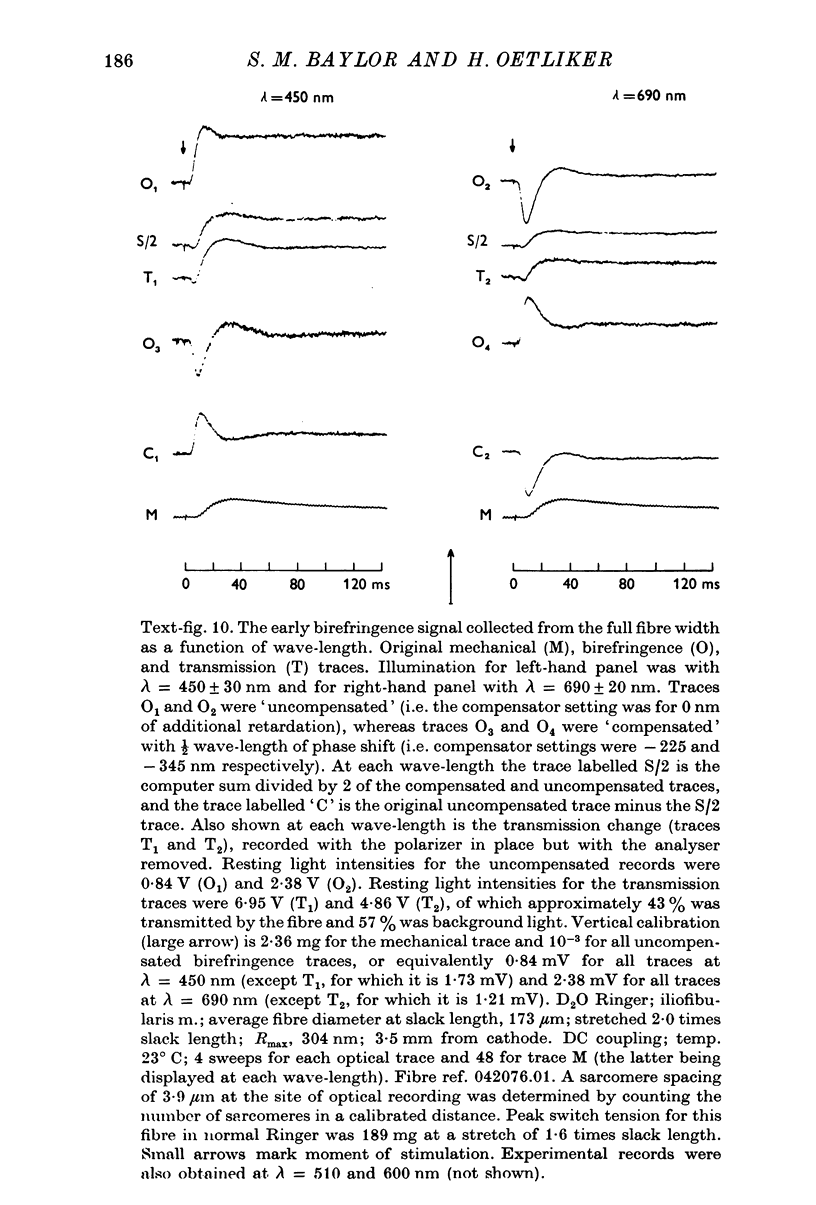
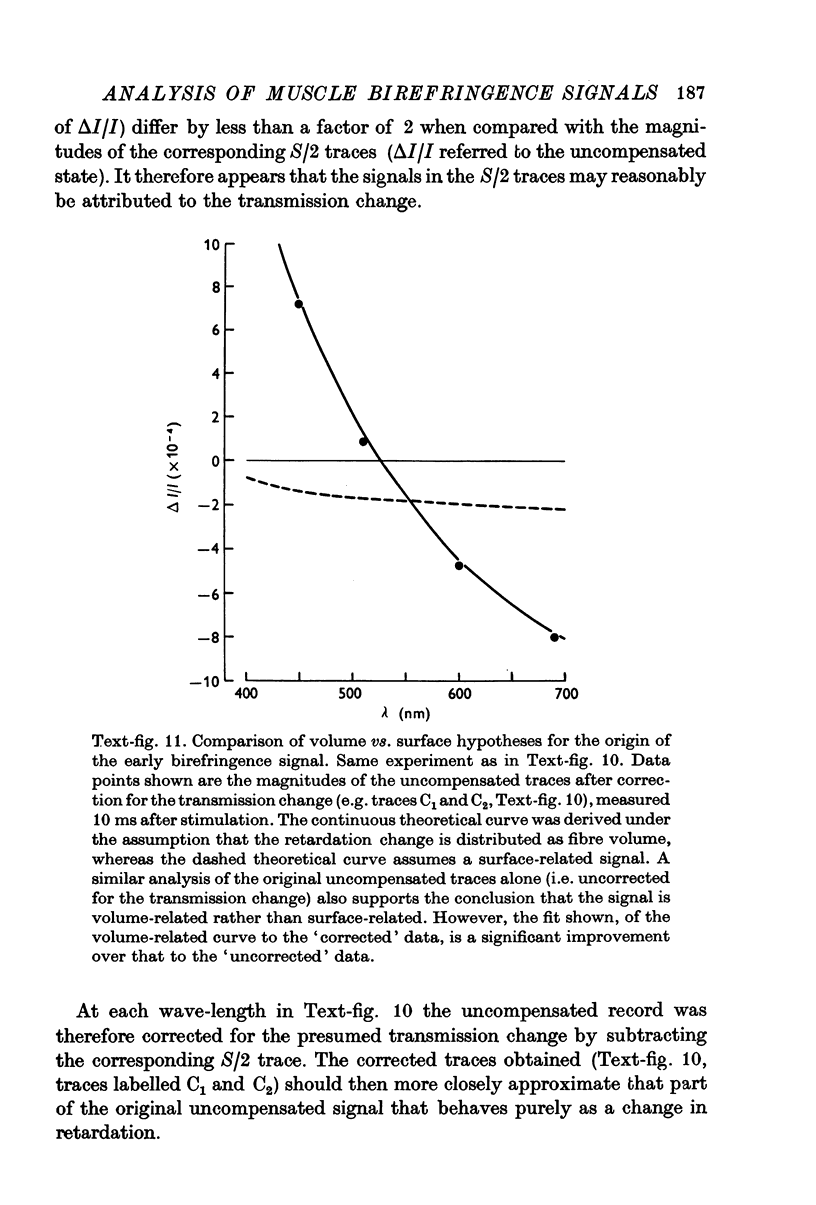



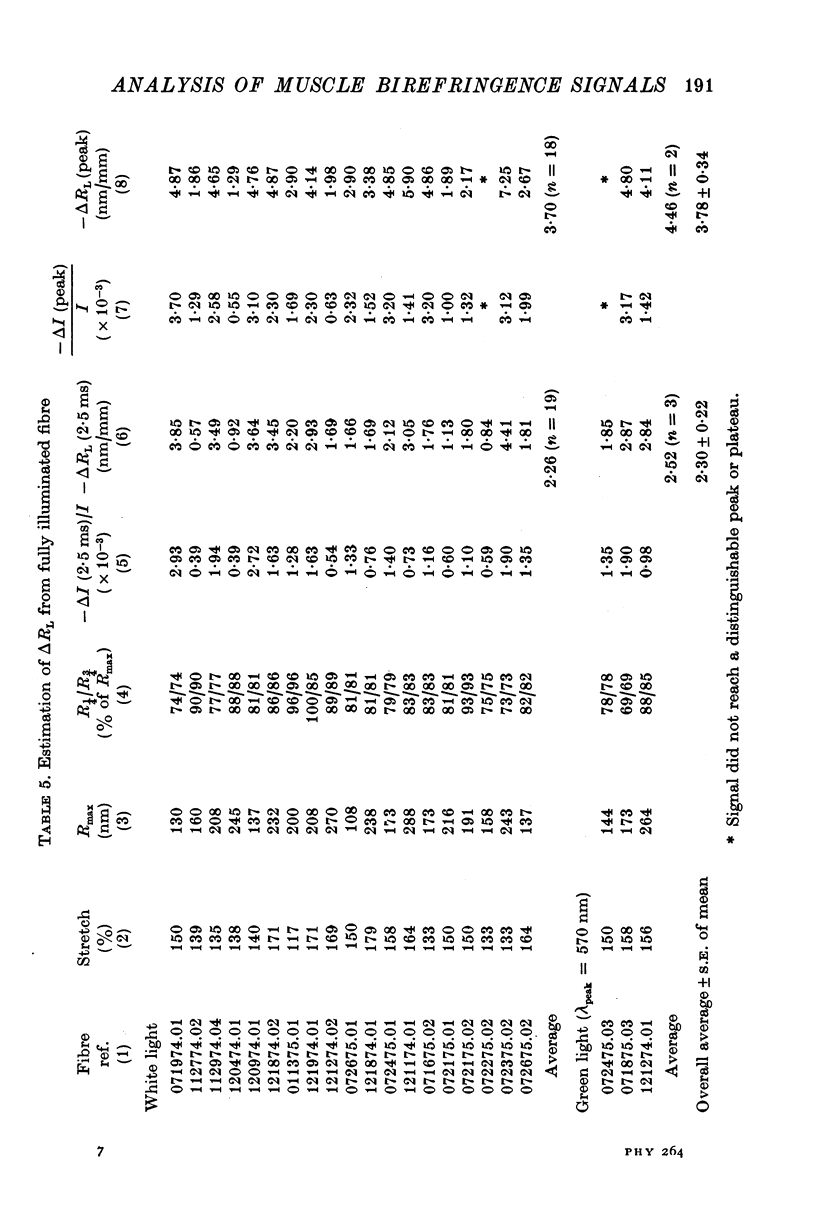
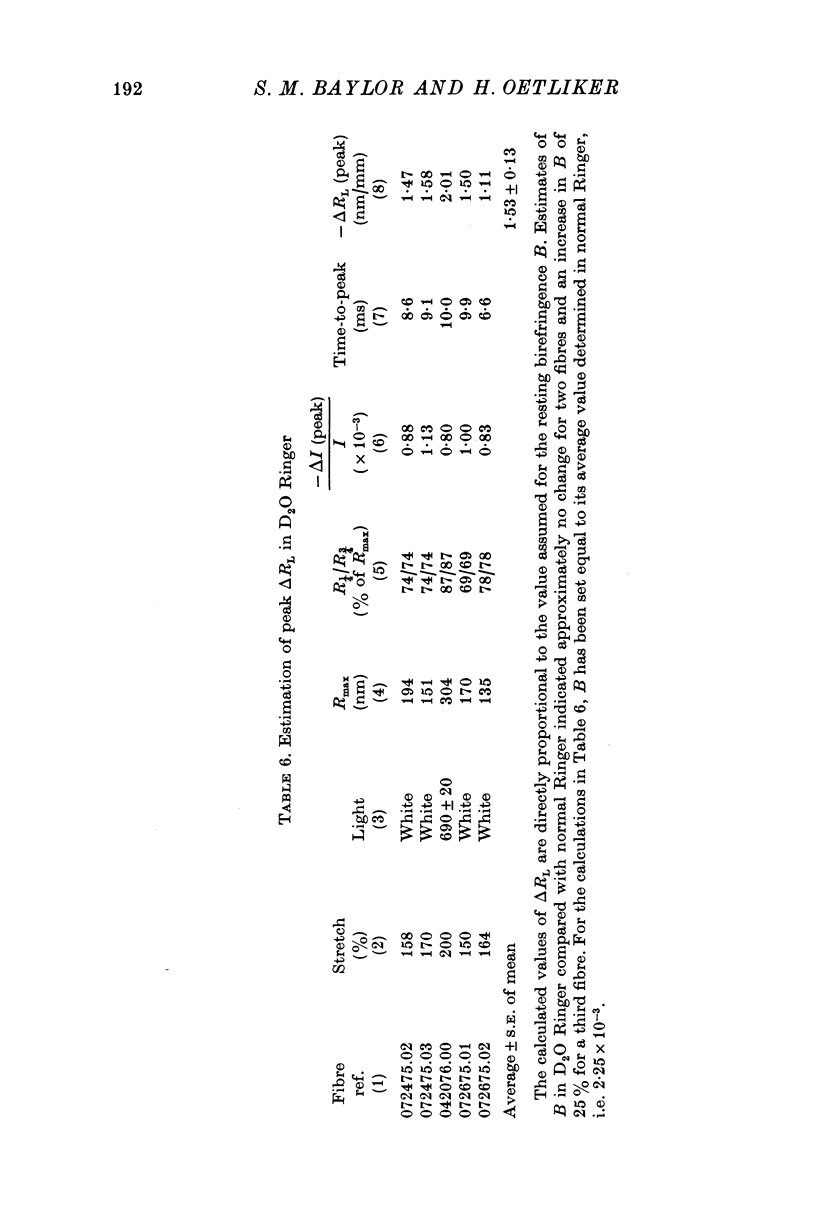







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Peachey L. D. Reconstruction of the action potential of frog sartorius muscle. J Physiol. 1973 Nov;235(1):103–131. doi: 10.1113/jphysiol.1973.sp010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry W. H., Carnay L. D. Changes in light scattered by striated muscle during excitation-contraction coupling. Am J Physiol. 1969 Nov;217(5):1425–1430. doi: 10.1152/ajplegacy.1969.217.5.1425. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. A large birefringence signal preceding contraction in single twitch fibres of the frog. J Physiol. 1977 Jan;264(1):141–162. doi: 10.1113/jphysiol.1977.sp011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. Birefringence experiments on isolated skeletal muscle fibres suggest a possible signal from the sarcoplasmic reticulum. Nature. 1975 Jan 10;253(5487):97–101. doi: 10.1038/253097a0. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Oetliker H. Birefringence signals from surface and t-system membranes of frog single muscle fibres. J Physiol. 1977 Jan;264(1):199–213. doi: 10.1113/jphysiol.1977.sp011663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnay L. D., Barry W. H. Turbidity, birefringence, and fluorescence changes in skeletal muscle coincident with the action potential. Science. 1969 Aug 8;165(3893):608–609. doi: 10.1126/science.165.3893.608. [DOI] [PubMed] [Google Scholar]
- Cohen L. B., Hille B., Keynes R. D. Changes in axon birefringence during the action potential. J Physiol. 1970 Dec;211(2):495–515. doi: 10.1113/jphysiol.1970.sp009289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Hille B., Keynes R. D., Landowne D., Rojas E. Analysis of the potential-dependent changes in optical retardation in the squid giant axon. J Physiol. 1971 Oct;218(1):205–237. doi: 10.1113/jphysiol.1971.sp009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Serratos H. Inward spread of activation in vertebrate muscle fibres. J Physiol. 1971 Feb;212(3):777–799. doi: 10.1113/jphysiol.1971.sp009356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. The effect of stimulation on the diffraction of light by striated muscle. J Physiol. 1953 Mar;119(4):501–512. doi: 10.1113/jphysiol.1953.sp004862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hill D. K. Changes in transparency of muscle during a twitch. J Physiol. 1949 May 15;108(3):292–302. [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Eisenberg B. R. Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol. 1975 Jul;66(1):31–45. doi: 10.1085/jgp.66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]



