Abstract
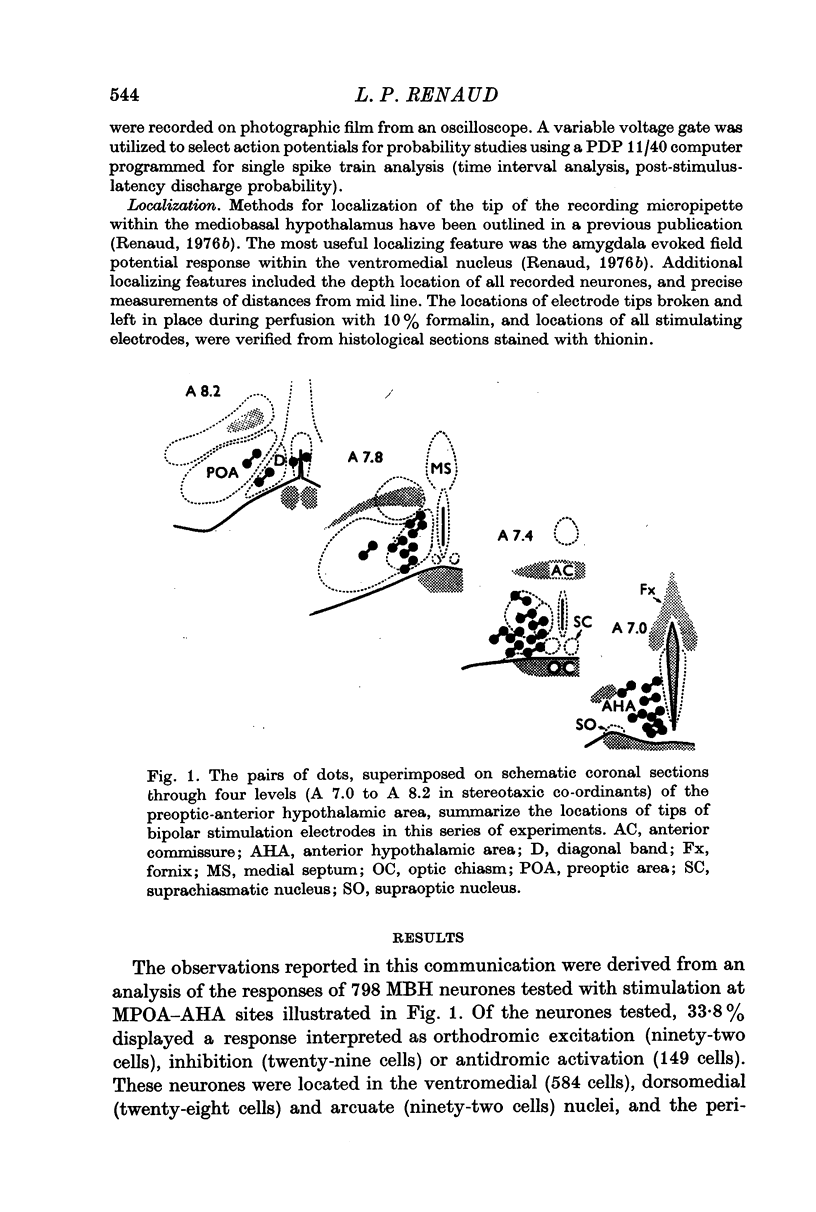
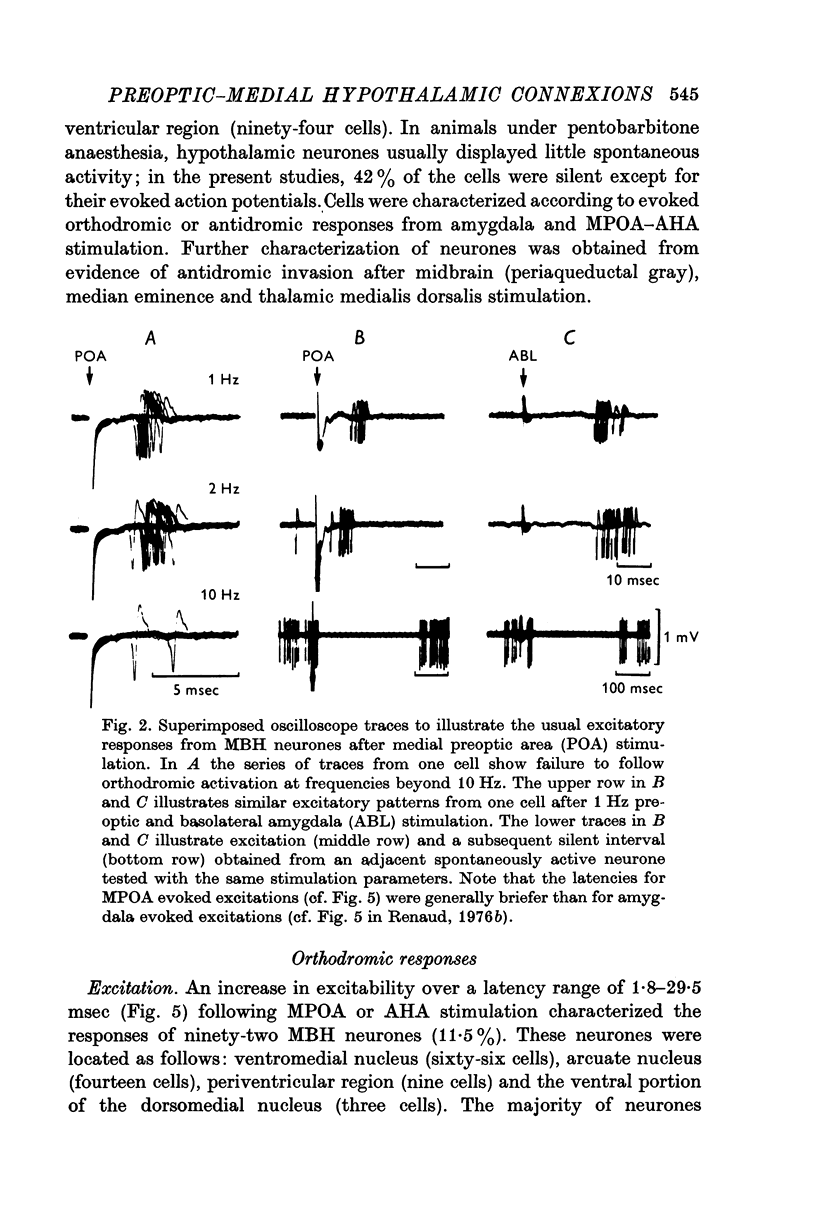
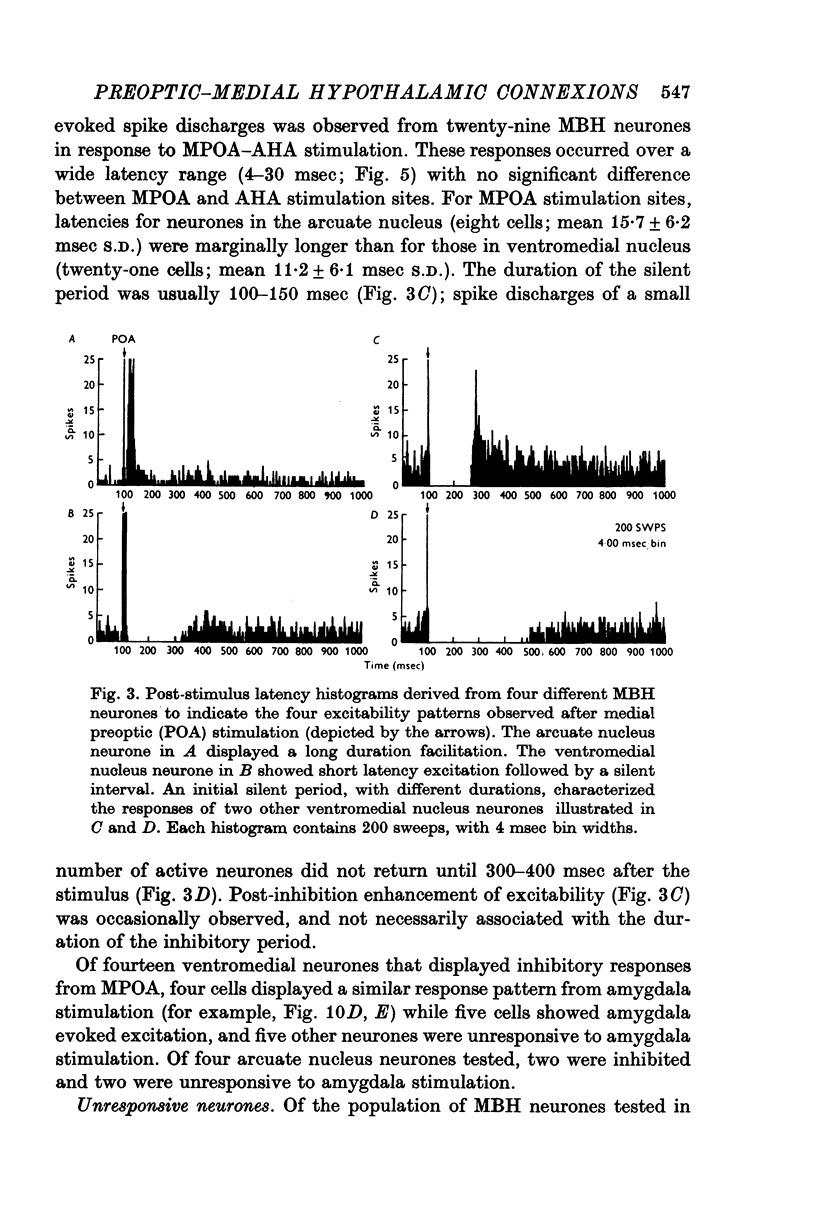
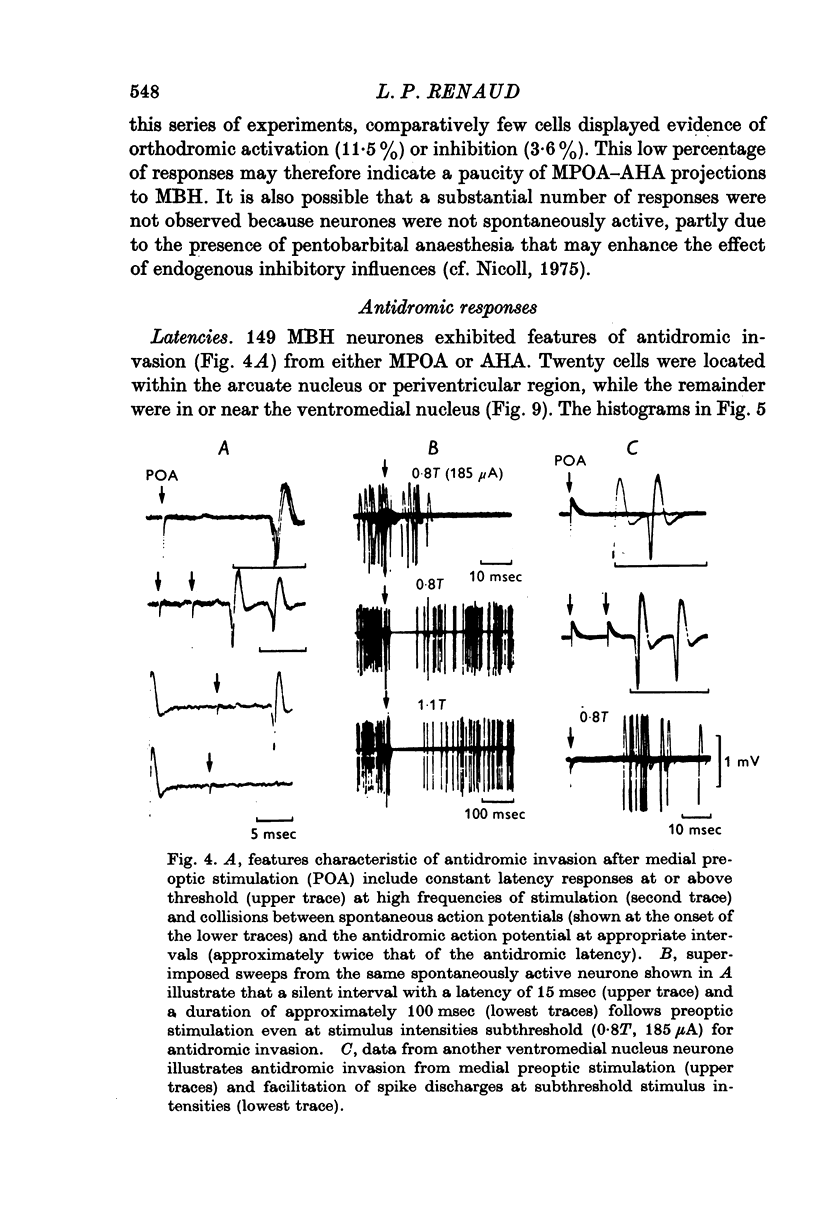
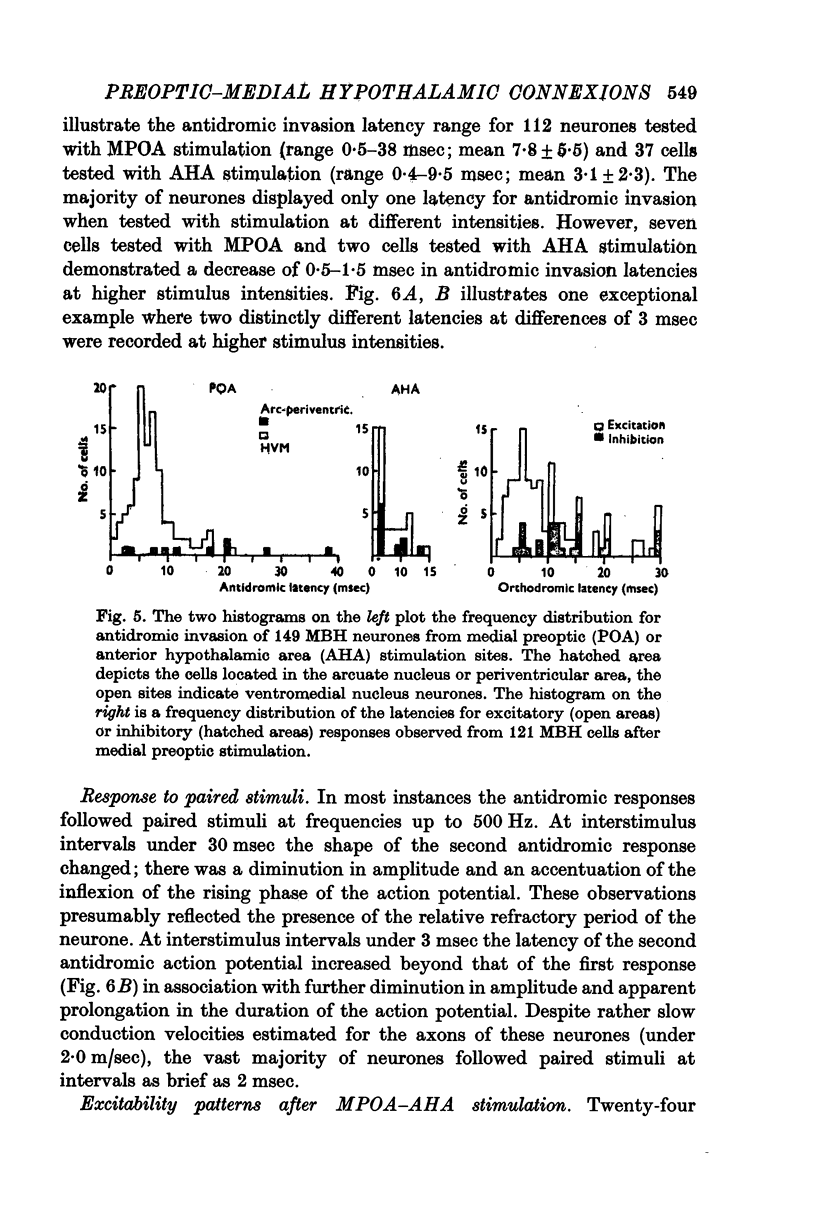
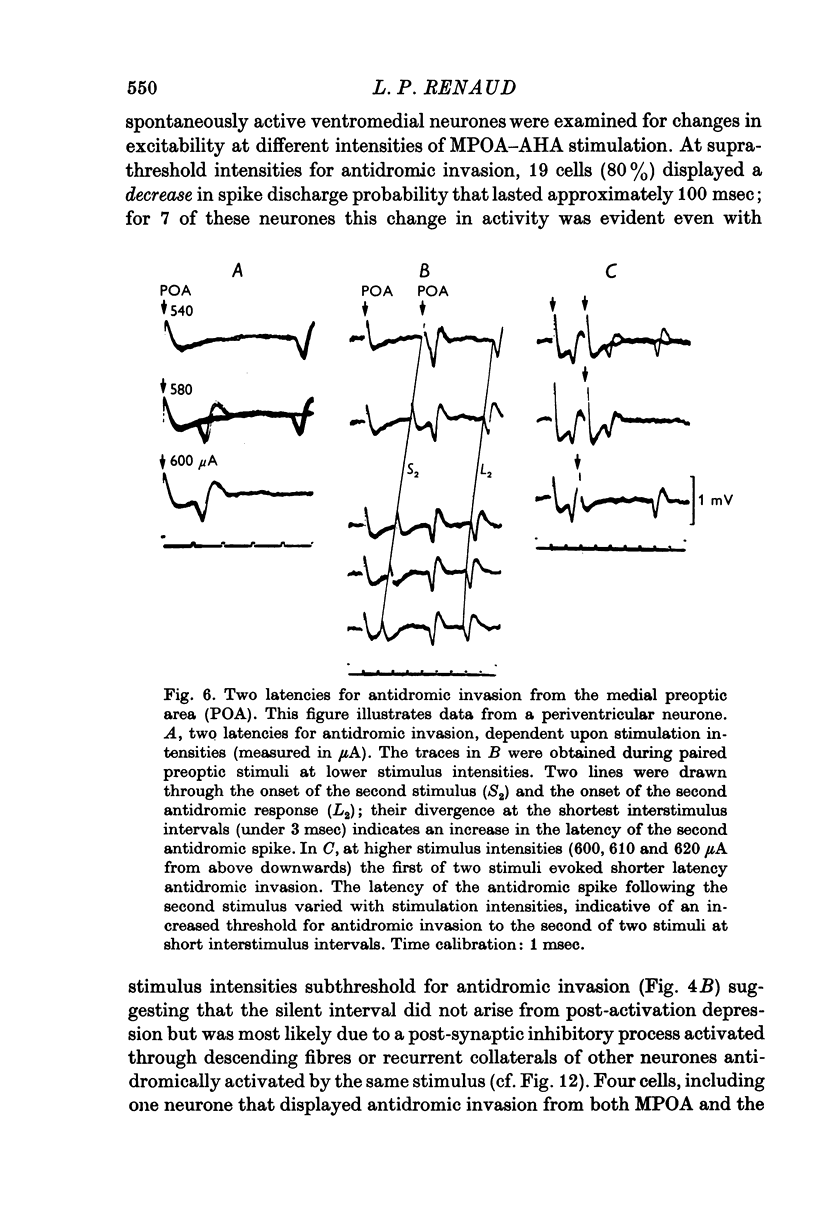
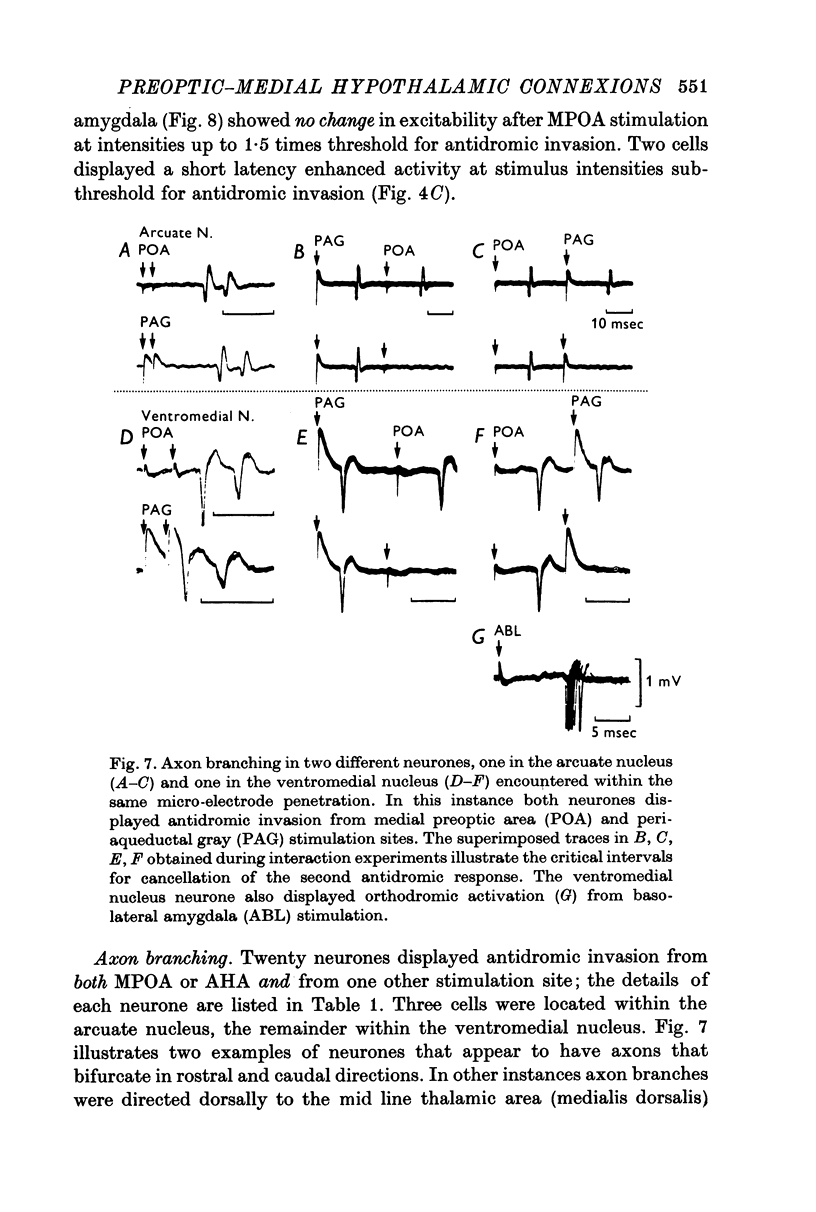
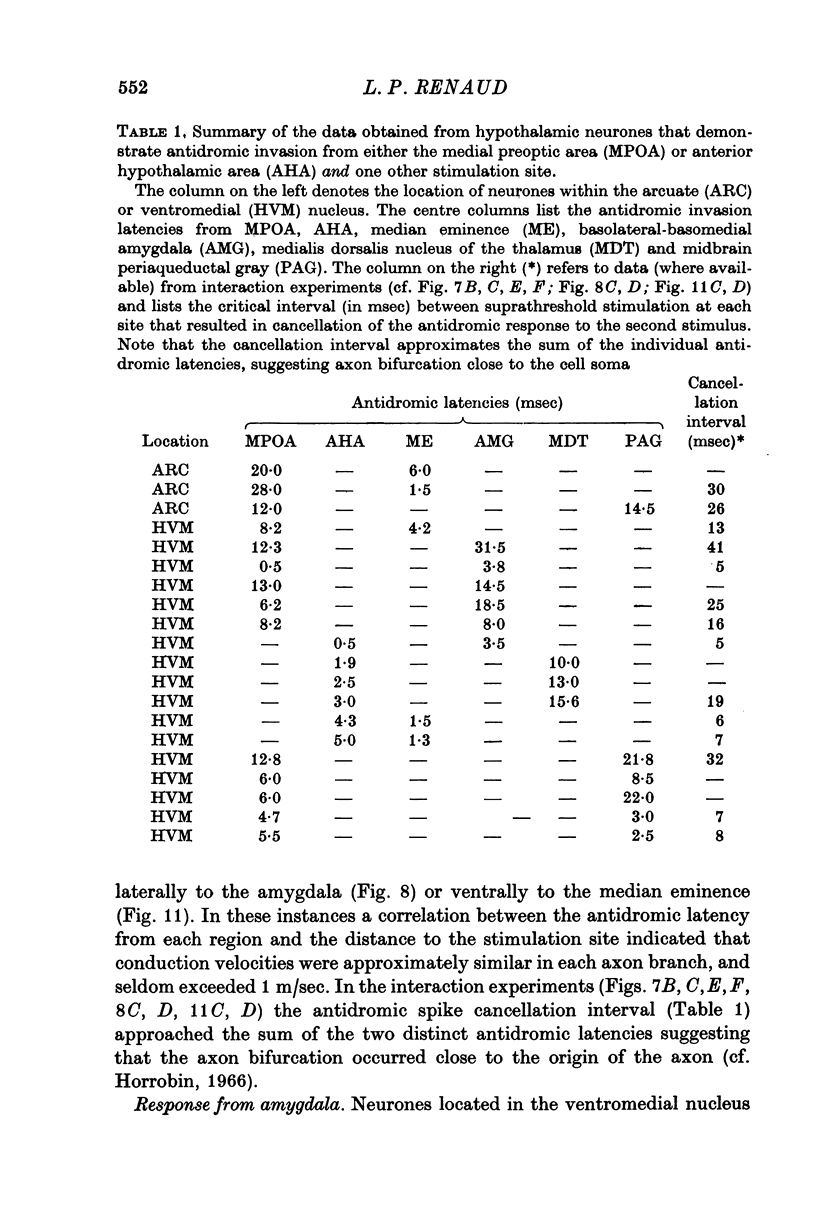
1. Extracellular action potentials recorded from 798 neurones in the mediobasal hypothalamus (MBH) of pentobarbitone anaesthetized male rats were analysed for a change in excitability following stimulation in the medial preoptic and anterior hypothalamic areas. 2. An increase in excitability characteristic of orthodromic excitation was observed from 11-5% (n=92) of MBH neurones. Latencies for excitation were shorter for cells tested with anterior hypothalamic area stimulation (n=42; mean 5-4 +/- 2-6 msec S.D.) than for cells tested with medial preoptic stimulation (n=50; mean 15-2 +/- 7-2 msec S.D.). With spontaneously active neurones, excitation was followed by a decrease in excitability lasting 150-250 msec. An initial decrease in excitability, suggestive of post-synaptic inhibition, over a wide latency range (4-30 msec) and with duration of 100-400 msec was observed from 3-6% of MBH neurones. 3. Features of antidromic invasion were observed from 149 MBH neurones. From the medial preoptic area, the latency range was 0-5-38 msec (mean 7-8 +/- 5-5); from the anterior hypothalamic area the latency range was 0-4-9-5 msec (mean 3-1 +/- 2-3). Occasionally an abrupt decrease in latency followed an increase in stimulus intensity. Most cells followed paired stimuli at frequencies up to 500 Hz. Axon conduction velocities were estimated to be under 2-0 m/sec. Antidromic invasion was usually followed by a decrease in excitability lasting approximately 100-150 msec. 4. Twenty MBH neurons displayed antidromic invasion from both the medial preoptic or anterio hypothalamic areas and one other stimulation site: the median eminence (five cells); the amygdala (six cells); the region of thalamic nucleus medialis dorsalis (three cells) and the midbrain periaqueductal gray (six cells). Interaction studies indicated that the axons of these cells branched close to the origin of the axon itself. 5. Antidromic invasion from the surface of the median eminence identified thirty-nine tuberoinfundibular neurones. Stimulation in the medial preoptic and anterior hypothalamic area produced orthodromic excitatory (n = 5) and inhibitory (n = 4) actions on HVM neurones, but was without an action on most other neurones (n = 30). Tuberoinfundibular neurones in the ventromedial nucleus also responded to stimulation in the amygdala, but usually at latencies greater than that for medial preoptic area evoked responses. 6. These observations indicate a close relationship between MBH neurones and cells located in both the amygdala and the medial preoptic-anterior hypothalamic area. The data for tuberoinfundibular neurones indicates that several extrahypothalamic areas may send fibres to these cells. These pathways may be important for the adaptive neuroendocrine responses reported in the literature.
Full text
PDF



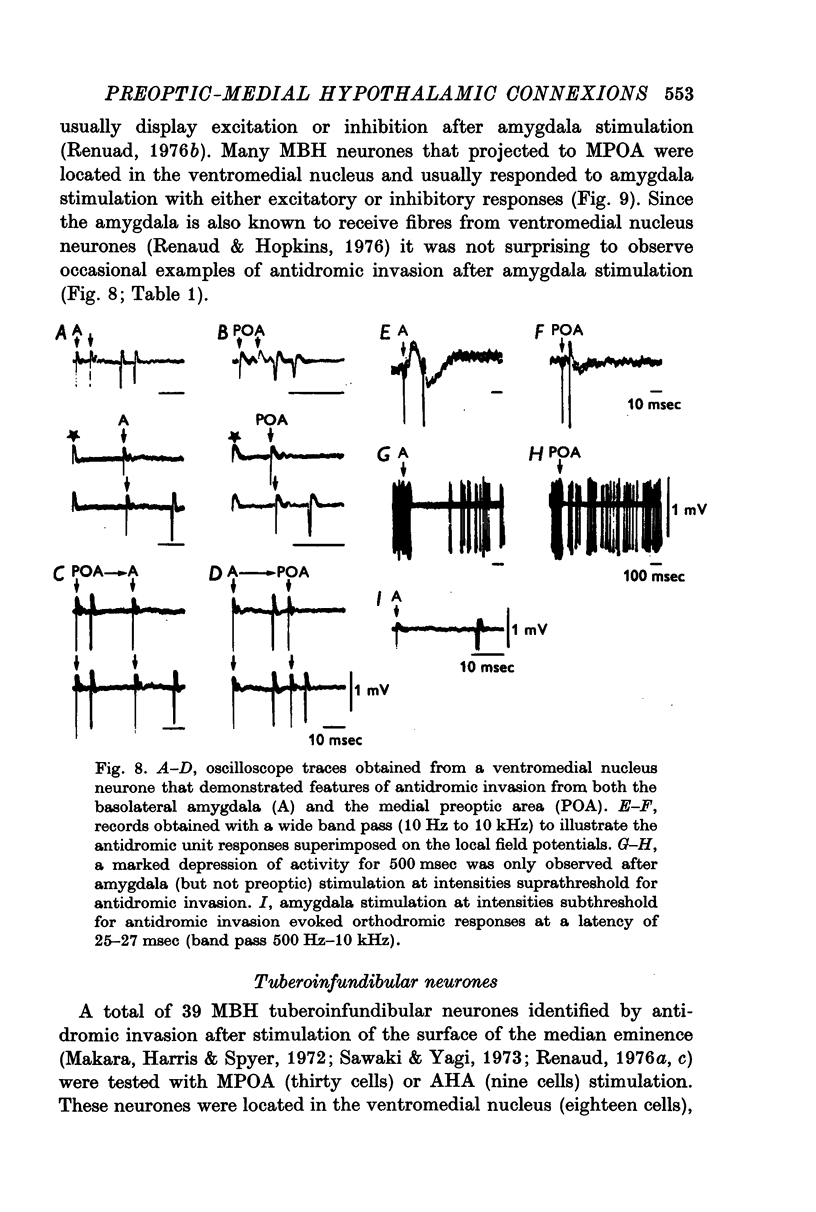
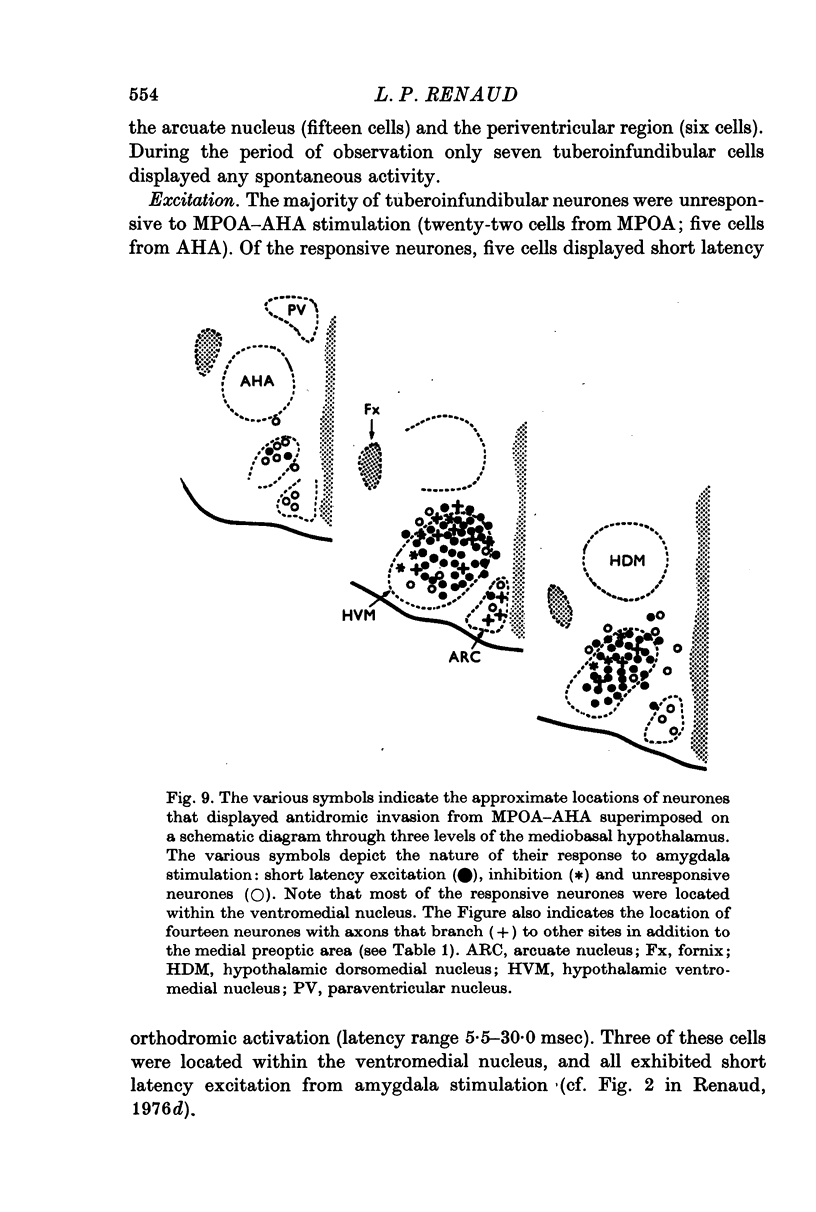
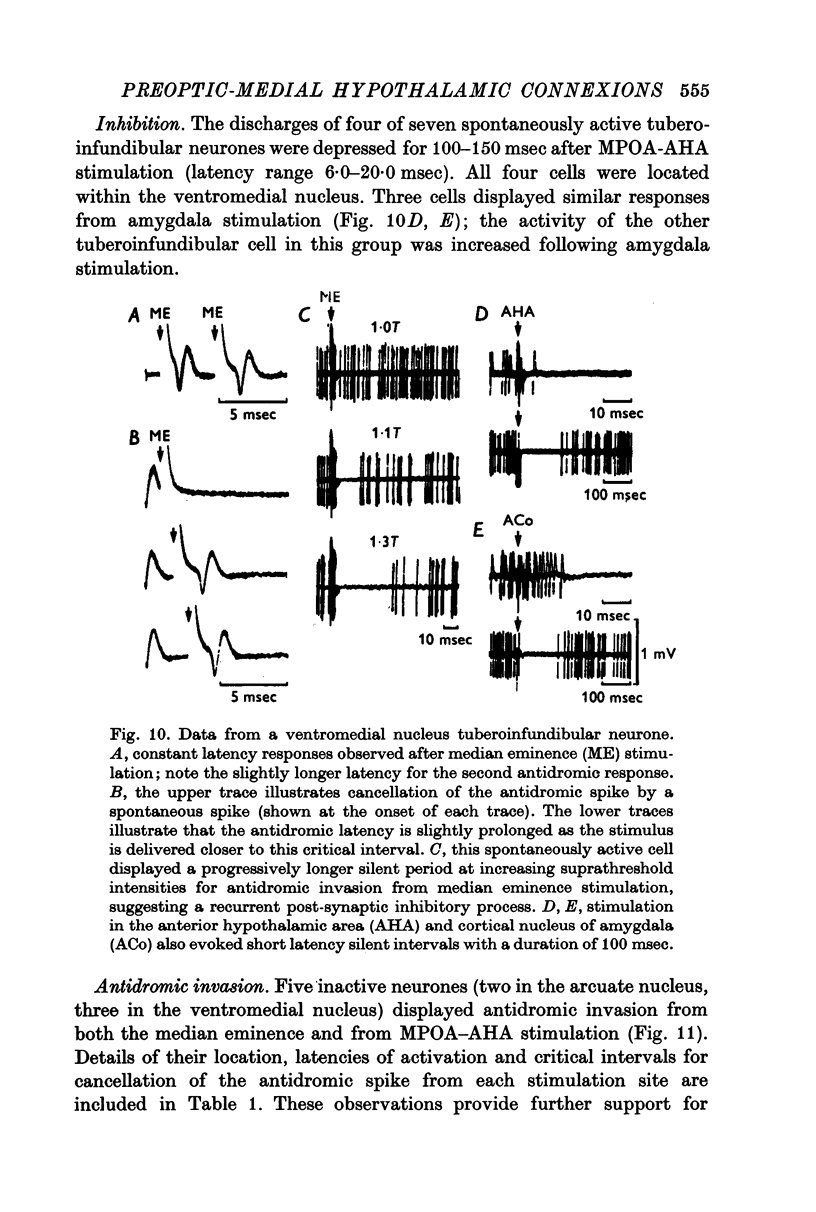
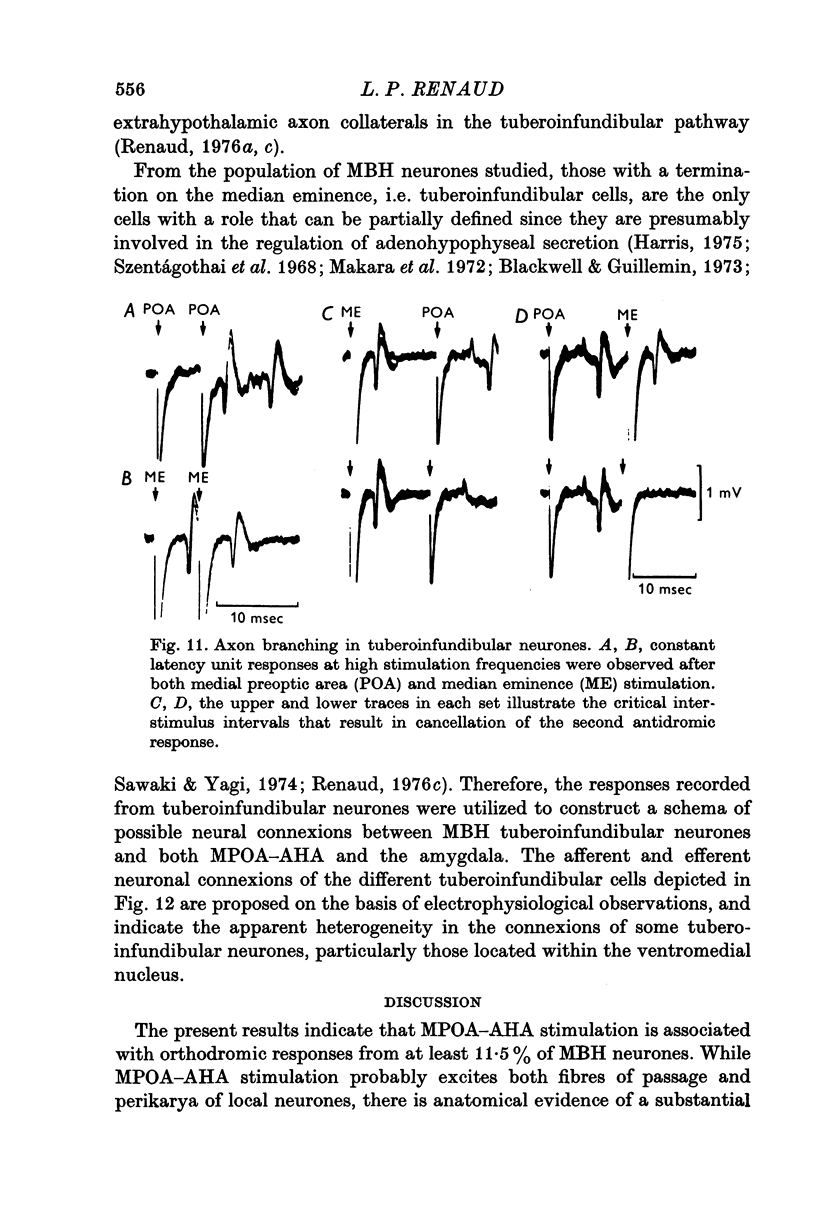







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert L. C., Brawer J. R., Patel Y. C., Reichlin S. Somatostatinergic neurons in anterior hypothalamus: immunohistochemical localization. Endocrinology. 1976 Jan;98(1):255–258. doi: 10.1210/endo-98-1-255. [DOI] [PubMed] [Google Scholar]
- Barnea A., Ben-Jonathan N., Colston C., Johnston J. M., Porter J. C. Differential sub-cellular compartmentalization of thyrotropin releasing hormone (TRH) and gonadotropin releasing hormone (LRH) in hypothalamic tissue. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3153–3157. doi: 10.1073/pnas.72.8.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry J., Dubois M. P., Carette B. Immunofluorescence study of the preoptico-infundibular LRF neurosecretory pathway in the normal, gastrated or testosterone-treated male guinea pig. Endocrinology. 1974 Nov;95(5):1416–1423. doi: 10.1210/endo-95-5-1416. [DOI] [PubMed] [Google Scholar]
- Blackwell R. E., Guillemin R. Hypothalamic control of adenohypophysial secretions. Annu Rev Physiol. 1973;35:357–390. doi: 10.1146/annurev.ph.35.030173.002041. [DOI] [PubMed] [Google Scholar]
- Blake C. A., Sawyer C. H. Effects of hypothalamic deafferentation on the pulsatile rhythm in plasma concentrations of luteinizing hormone in ovariectomized rats. Endocrinology. 1974 Mar;94(3):730–736. doi: 10.1210/endo-94-3-730. [DOI] [PubMed] [Google Scholar]
- Brawer J. R., Sonnenschein C. Cytopathological effects of estradiol on the arcuate nucleus of the female rat. A possible mechanism for pituitary tumorigenesis. Am J Anat. 1975 Sep;144(1):57–88. doi: 10.1002/aja.1001440105. [DOI] [PubMed] [Google Scholar]
- Brawer J. R. The fine structure of the ependymal tanycytes at the level of the arcuate nucleus. J Comp Neurol. 1972 May;145(1):25–41. doi: 10.1002/cne.901450103. [DOI] [PubMed] [Google Scholar]
- Brownstein M., Arimura A., Sato H., Schally A. V., Kizer J. S. The regional distribution of somatostatin in the rat brain. Endocrinology. 1975 Jun;96(6):1456–1461. doi: 10.1210/endo-96-6-1456. [DOI] [PubMed] [Google Scholar]
- Clemente C. D., Chase M. H. Neurological substrates of aggressive behavior. Annu Rev Physiol. 1973;35:329–356. doi: 10.1146/annurev.ph.35.030173.001553. [DOI] [PubMed] [Google Scholar]
- Conrad L. C., Pfaff D. W. Axonal projections of medial preoptic and anterior hypothalamic neurons. Science. 1975 Dec 12;190(4219):1112–1114. doi: 10.1126/science.1188390. [DOI] [PubMed] [Google Scholar]
- Cramer O. M., Barraclough C. A. Effects of preoptic electrical stimulation on pituitary LH release following interruption of components of the preoptico-tuberal pathway in rats. Endocrinology. 1973 Aug;93(2):369–376. doi: 10.1210/endo-93-2-369. [DOI] [PubMed] [Google Scholar]
- Dyer R. G. An electrophysiological dissection of the hypothalamic regions which regulate the pre-ovulatory secretion of luteinizing hormone in the rat. J Physiol. 1973 Oct;234(2):421–442. doi: 10.1113/jphysiol.1973.sp010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer R. G., Dyball R. E. Evidence for a direct effect of LRF and TRF on single unit activity in the rostral hypothalamus. Nature. 1974 Dec 6;252(5483):486–488. doi: 10.1038/252486a0. [DOI] [PubMed] [Google Scholar]
- Field P. M. A quantitative ultrastructural analysis of the distribution of amygdaloid dibres in the preoptic area and the entromedial hypothalamic nucleus. Exp Brain Res. 1972 Apr 27;14(5):527–538. doi: 10.1007/BF00236594. [DOI] [PubMed] [Google Scholar]
- Fitzsimons J. T. Thirst. Physiol Rev. 1972 Apr;52(2):468–561. doi: 10.1152/physrev.1972.52.2.468. [DOI] [PubMed] [Google Scholar]
- Grossman Y., Spira M. E., Parnas I. Differential flow of information into branches of a single axon. Brain Res. 1973 Dec 21;64:379–386. doi: 10.1016/0006-8993(73)90191-1. [DOI] [PubMed] [Google Scholar]
- Harris M. C., Sanghera M. Projection of medial basal hypothalamic neurones to the preoptic anterior hypothalamic areas and the paraventricular nucleus in the rat. Brain Res. 1974 Dec 13;81(3):401–411. doi: 10.1016/0006-8993(74)90839-7. [DOI] [PubMed] [Google Scholar]
- Hayward J. N., Baker M. A. Diuretic and thermoregulatory responses to preoptic cooling in the monkey. Am J Physiol. 1968 Apr;214(4):843–850. doi: 10.1152/ajplegacy.1968.214.4.843. [DOI] [PubMed] [Google Scholar]
- Heimer L., Nauta W. J. The hypothalamic distribution of the stria terminalis in the rat. Brain Res. 1969 Apr;13(2):284–297. doi: 10.1016/0006-8993(69)90288-1. [DOI] [PubMed] [Google Scholar]
- Hoebel B. G. Feeding: neural control of intake. Annu Rev Physiol. 1971;33:533–568. doi: 10.1146/annurev.ph.33.030171.002533. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Sakuma Y. Electrophysiological evidences for possible participation of periventricular neurons in anterior pituitary regulation. Brain Res. 1976 Jan 9;101(1):79–94. doi: 10.1016/0006-8993(76)90989-6. [DOI] [PubMed] [Google Scholar]
- Krey L. C., Lu K. H., Bulter W. R., Hotchkiss J., Piva F., Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. II. GH and cortisol secretion. Endocrinology. 1975 May;96(5):1088–1093. doi: 10.1210/endo-96-5-1088. [DOI] [PubMed] [Google Scholar]
- Makara G. B., Harris M. C., Spyer K. M. Identification and distribution of tuberoinfundibular neurones. Brain Res. 1972 May 26;40(2):283–290. doi: 10.1016/0006-8993(72)90134-5. [DOI] [PubMed] [Google Scholar]
- Makara G. B., Hodåcs L. Rostral projections from the hypothalamic arcuate nucleus. Brain Res. 1975 Jan 24;84(1):23–29. doi: 10.1016/0006-8993(75)90797-0. [DOI] [PubMed] [Google Scholar]
- Martin J. B., Reichlin S. Plasma thyrotropin (TSH) response to hypothalamic electrical stimulation and to injection of synthetic thyrotropin releasing hormone (TRH). Endocrinology. 1972 Apr;90(4):1079–1085. doi: 10.1210/endo-90-4-1079. [DOI] [PubMed] [Google Scholar]
- Martin J. B., Renaud L. P., Brazeau P. Hypothalamic peptides: New evidence for "peptidergic" pathways in the C.N.S. Lancet. 1975 Aug 30;2(7931):393–395. doi: 10.1016/s0140-6736(75)92901-3. [DOI] [PubMed] [Google Scholar]
- Millhouse O. E. The organization of the ventromedial hypothalamic nucleus. Brain Res. 1973 May 30;55(1):71–87. [PubMed] [Google Scholar]
- Murphy J. T., Renaud L. P. Mechanisms of inhibition in the ventromedial nucleus of the hypothalamus. J Neurophysiol. 1969 Jan;32(1):85–102. doi: 10.1152/jn.1969.32.1.85. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A. Pentobarbital: action on frog motoneurons. Brain Res. 1975 Oct 10;96(1):119–123. doi: 10.1016/0006-8993(75)90582-x. [DOI] [PubMed] [Google Scholar]
- Ramirez V. D., Gautron J. P., Epelbaum J., Pattou E., Zamora A., Kordon C. Distribution of LH-RH in subcellular fractions of the basomedial hypothalamus. Mol Cell Endocrinol. 1975 Nov;3(5):339–350. doi: 10.1016/0303-7207(75)90035-0. [DOI] [PubMed] [Google Scholar]
- Renaud L. P. An electrophysiological study of amygdalohypothalamic projections to the ventromedial nucleus of the rat. Brain Res. 1976 Mar 19;105(1):45–58. doi: 10.1016/0006-8993(76)90921-5. [DOI] [PubMed] [Google Scholar]
- Renaud L. P. Influence of amygdala stimulation on the activity of identified tuberoinfundibular neurones in the rat hypothalamus. J Physiol. 1976 Aug;260(1):237–252. doi: 10.1113/jphysiol.1976.sp011513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud L. P., Martin J. B., Brazeau P. Depressant action of TRH, LH-RH and somatostatin on activity of central neurones. Nature. 1975 May 15;255(5505):233–235. doi: 10.1038/255233a0. [DOI] [PubMed] [Google Scholar]
- Renaud L. P., Martin J. B. Electrophysiological studies of connections of hypothalamic ventromedial nucleus neurons in the rat: evidence for a role in neuroendocrine regulation. Brain Res. 1975 Jul 25;93(1):145–151. doi: 10.1016/0006-8993(75)90293-0. [DOI] [PubMed] [Google Scholar]
- Renaud L. P. Tuberoinfundibular neurons in the basomedial hypothalamus of the rat: electrophysiological evidence for axon collaterals to hypothalamic and extrahypothalamic areas. Brain Res. 1976 Mar 19;105(1):59–72. doi: 10.1016/0006-8993(76)90922-7. [DOI] [PubMed] [Google Scholar]
- Schubert P., Lee K., West M., Deadwyler S., Lynch G. Stimulation-dependent release of 3H-adenosine derivatives from central axon terminals to target neurones. Nature. 1976 Apr 8;260(5551):541–542. doi: 10.1038/260541a0. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the association between transmitter secretion and the release of adenine nucleotides from mammalian motor nerve terminals. J Physiol. 1975 May;247(1):145–162. doi: 10.1113/jphysiol.1975.sp010925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W. An autoradiographic study of the efferent connections of the preoptic region in the rat. J Comp Neurol. 1976 May 15;167(2):227–256. doi: 10.1002/cne.901670207. [DOI] [PubMed] [Google Scholar]
- Sétáló G., Vigh S., Schally A. V., Arimura A., Flerkó B. GH-RIH-containing neural elements in the rat hypothalamus. Brain Res. 1975 Jun 13;90(2):352–356. doi: 10.1016/0006-8993(75)90319-4. [DOI] [PubMed] [Google Scholar]
- Waxman S. G. Integrative properties and design principles of axons. Int Rev Neurobiol. 1975;18:1–40. doi: 10.1016/s0074-7742(08)60032-x. [DOI] [PubMed] [Google Scholar]
- Wilber J. F., Montoya E., Plotnikoff N. P., White W. F., Gendrick R., Renaud L., Martin J. B. Gonadotropin-releasing hormone and thyrotropin-releasing hormone: distribution and effects in the central nervous system. Recent Prog Horm Res. 1976;32:117–159. doi: 10.1016/b978-0-12-571132-6.50013-0. [DOI] [PubMed] [Google Scholar]
- Wilson M. M., Critchlow V. Absence of a circadian rhythm in persisting corticosterone fluctuations following surgical isolation of the medial basal hypothalamus. Neuroendocrinology. 1975;19(2):185–192. doi: 10.1159/000122439. [DOI] [PubMed] [Google Scholar]
- Zimmerman E. A., Hsu K. C., Ferin M., Kozlowski G. P. Localization of gonadotropin-releasing hormone (Gn-RH) in the hypothalamus of the mouse by immunoperoxidase technique. Endocrinology. 1974 Jul;95(1):1–8. doi: 10.1210/endo-95-1-1. [DOI] [PubMed] [Google Scholar]
- van Gemert M., Miller M., Carey R. J., Moses A. M. Polyuria and imparied ADH release following medial preoptic lesioning in the rat. Am J Physiol. 1975 May;228(5):1293–1297. doi: 10.1152/ajplegacy.1975.228.5.1293. [DOI] [PubMed] [Google Scholar]


