Abstract
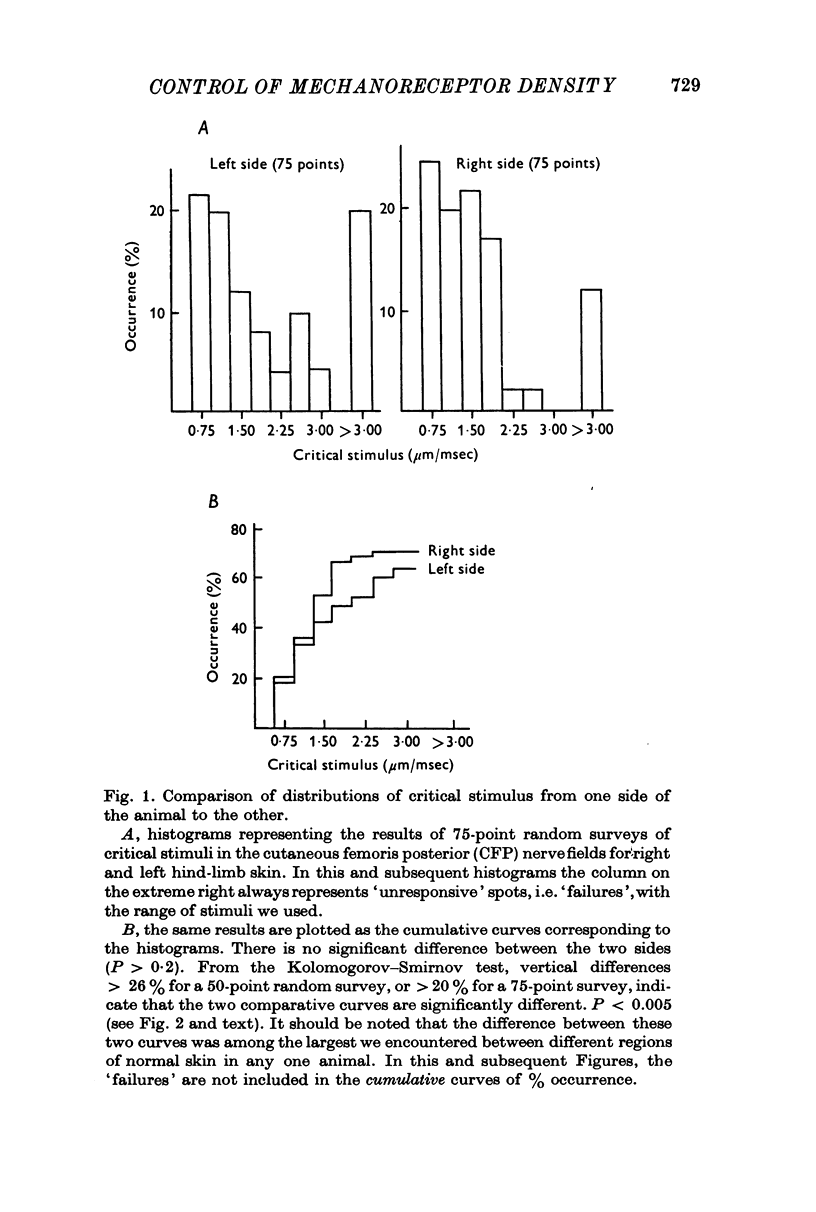
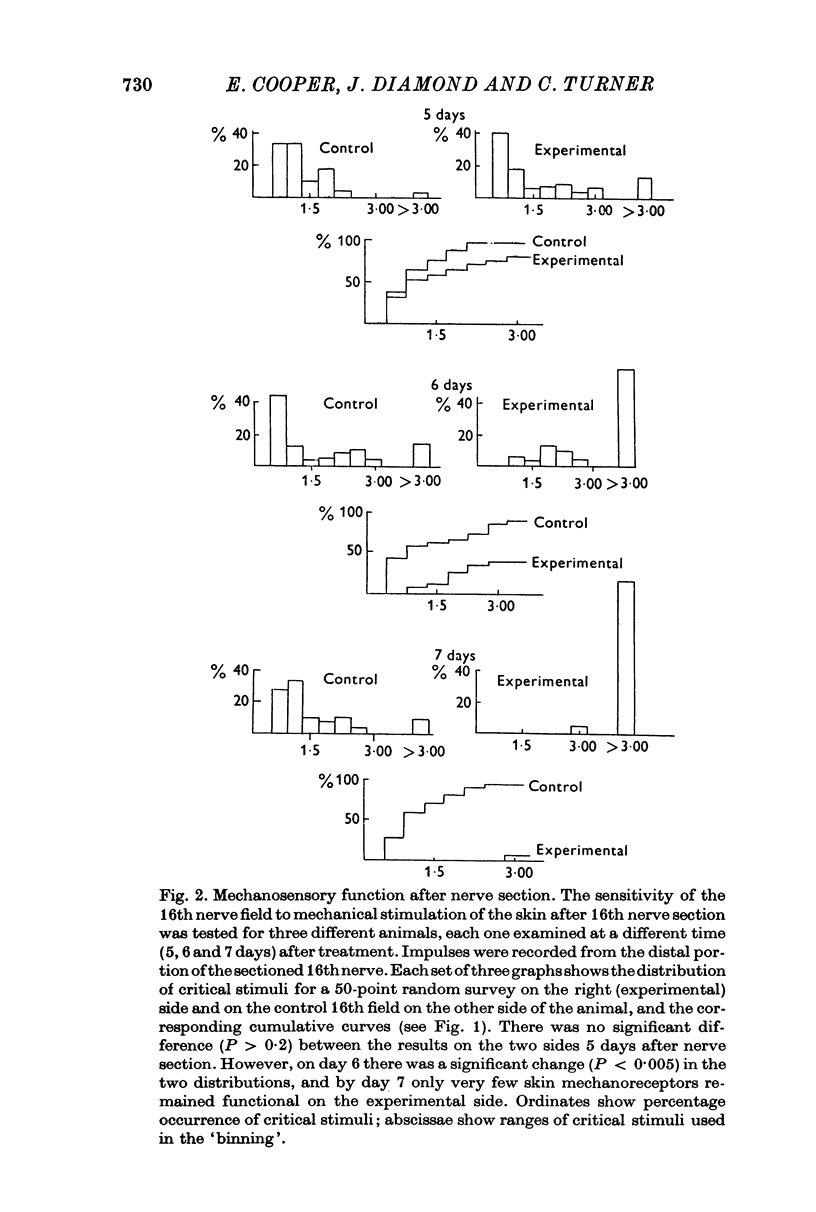
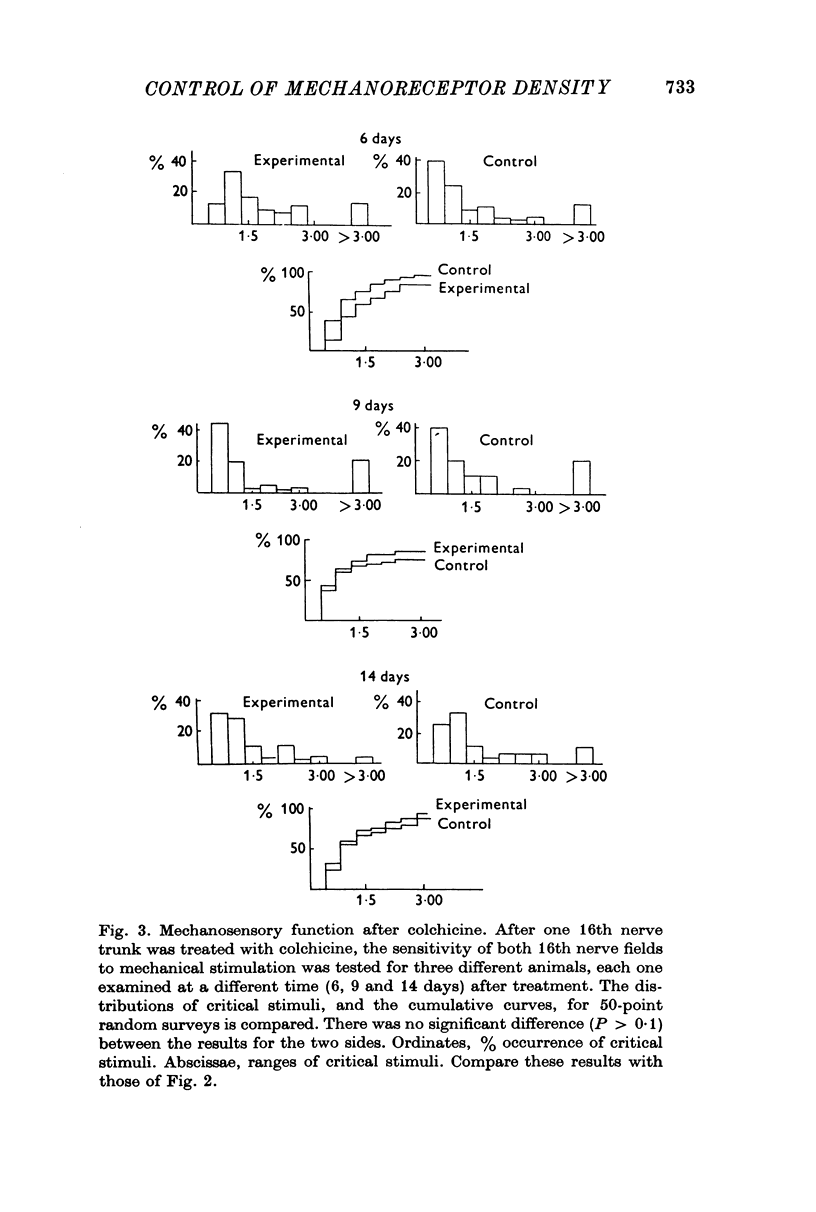
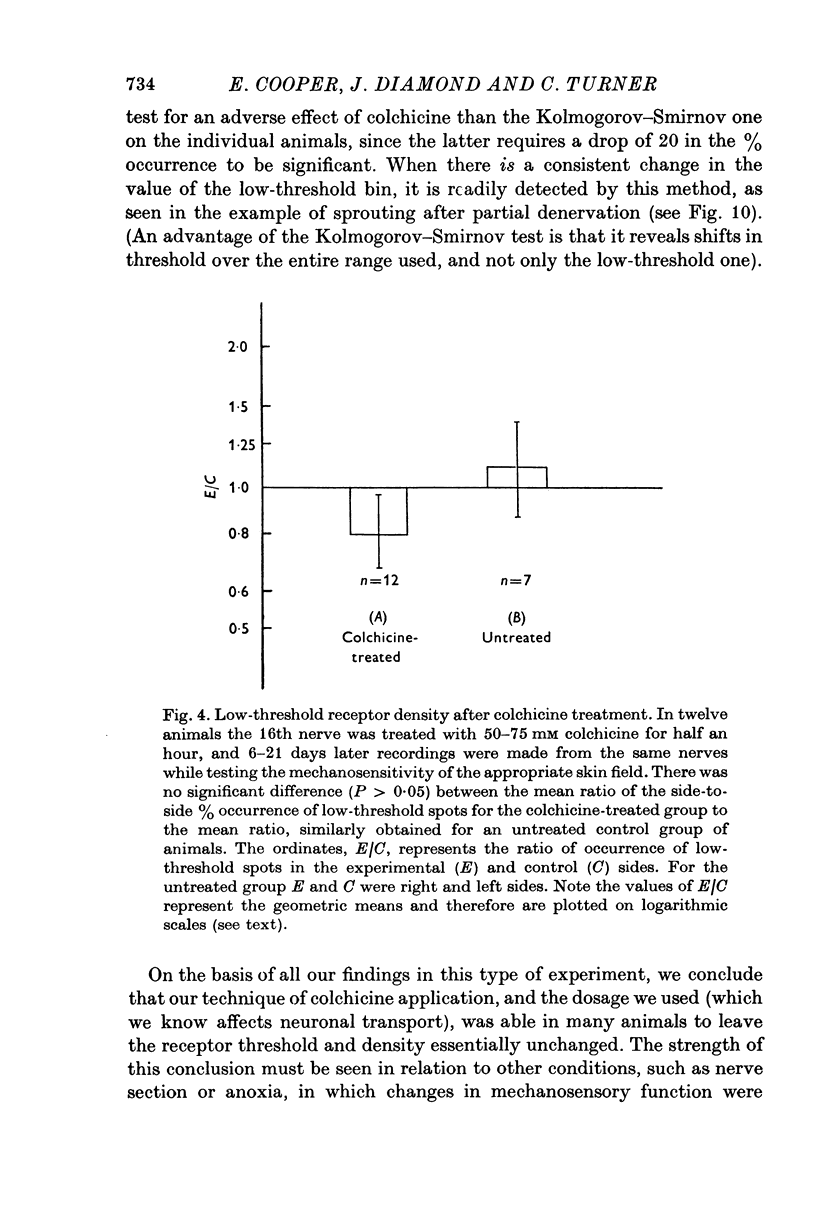
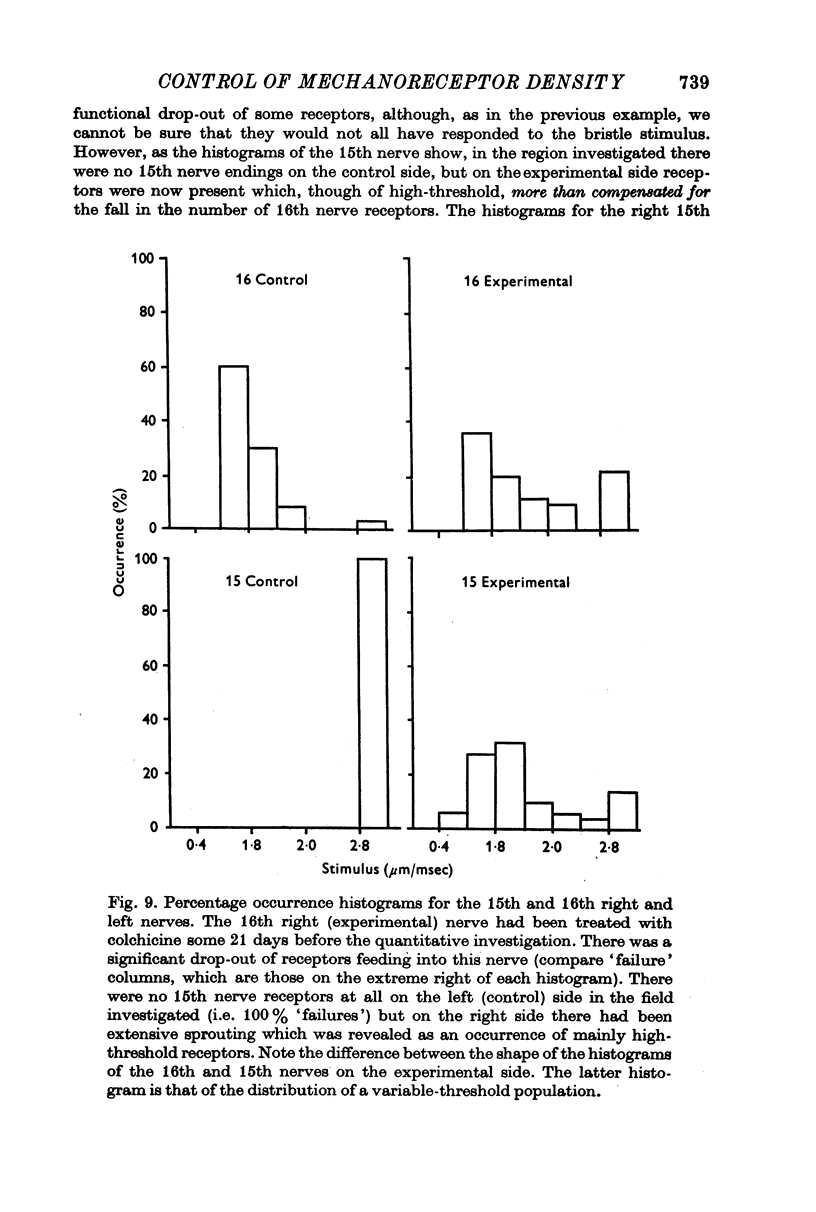
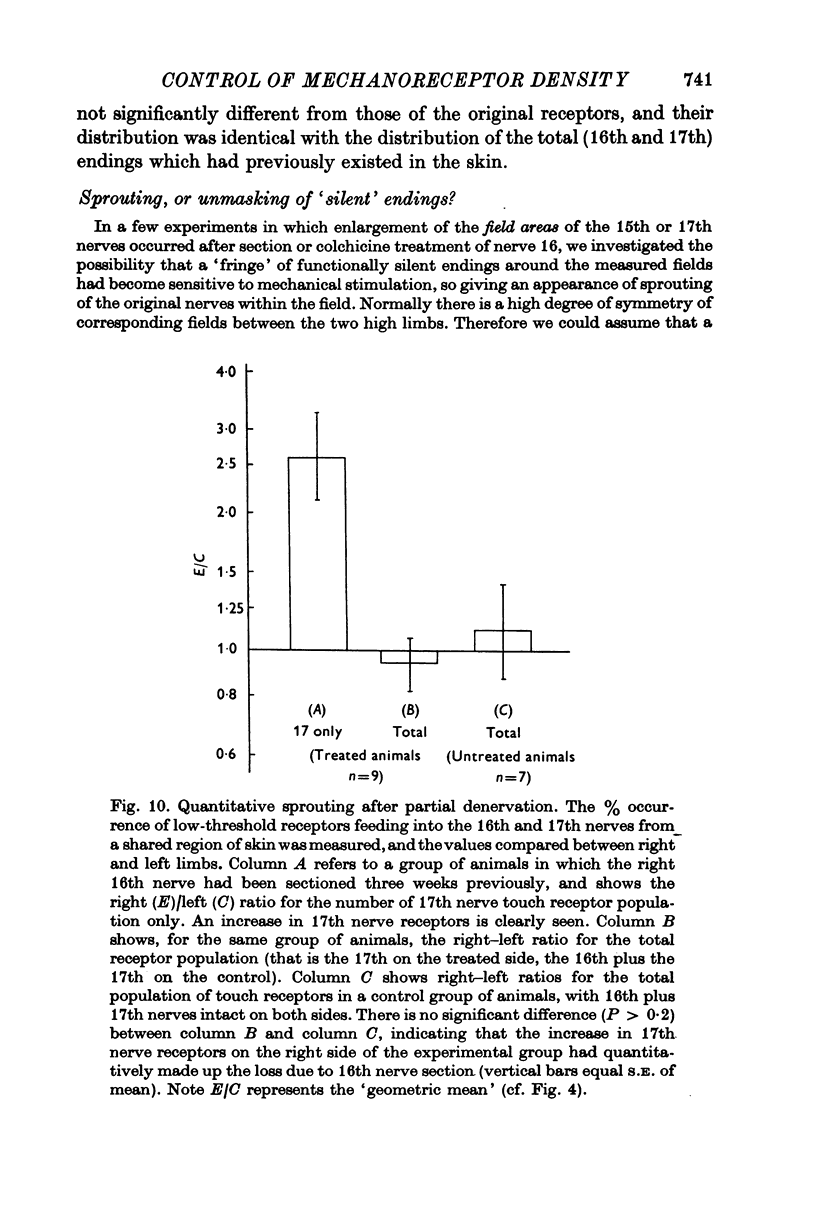
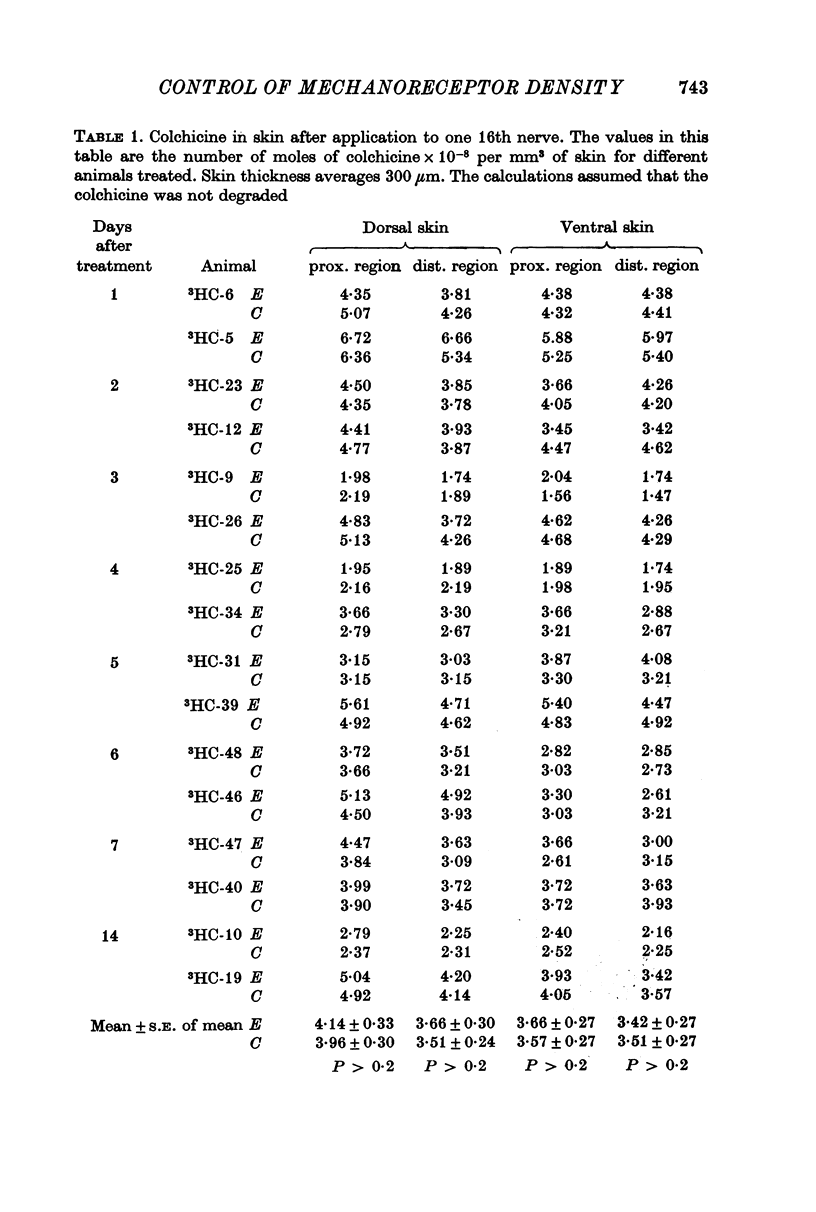
We have shown that when one of the spinal nerves supplying the salamander hind limb is cut or treated with colchicine, the fields of the remaining nerves enlarge in area; whereas nerve section produces Wallerian degeneration, the colchicine-treated nerves conducted action potentials normally and their peripheral fields remained unchanged in area (Aguilar, Bisby, Cooper & Diamond, 1973). Since colchicine-treatment reduced neuronal transport, and nerve-section eliminated it, we proposed that nerve sprouting is regulated by factors normally conveyed to the endings by axoplasmic transport. 1. We have now investigated the effects of colchicine on the thresholds and distribution of individual mechanosensory endings in the skin. If reduction of neuronal transport were enough to cause the threshold to be increased to the point of total unresponsiveness, then this could be a sign of an early stage of degernation in those terminals. It could then be hypothesized that products of degeneration were providing a stimulus for adjacent nerves to sprout. 2. Quantitative physiological studies of the effects of colchicine doses known to interfere with fast axoplasmic transport, indicate that in some experiments the terminal field of the treated nerve was invaded by sprouting fibres from neighbouring axons, when its own endings were unchanged in number, distribution and sensory thresholds. In other experiments the colchicine-treated nerve endings showed an increase in threshold but their function was otherwise unchanged; a similar adjacent nerve sprouting occurred. In a final group, colchicine caused total unresponsiveness of some endings of the treated nerve. 3. When a region of skin was partially denervated by nerve section, the physiological analysis indicated that the number of new mechanosensory endings which sprouted from the remaining axons exactly matched the number lost by nerve degeneration: furthermore the distribution of the endings was normal. It therefore appears that sprouting ceased when the original density of mechanosensory endings in the skin was restored. 4. The possibility that the drug induced sprouting as a consequense of a direct action on the skin is unlikely. With [3H]colchicine, we found that the accumulation of label in the skin of the untreated limb, in which sprouting did not occur, equalled that of the opposite limb. 5. The present results lend support to the original hypothesis of Aguilar et al. (1973), which proposed that collateral sprouting of intact nerves occurs when the supply of neuronally transported factors becomes inadequate to balance out the effects of a postulated target-tissue stimulus. In the Discussion other examples of collateral nerve sprouting, such as that following adjacent denervation, are shown to be explainable by this hypothesis.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar C. E., Bisby M. A., Cooper E., Diamond J. Evidence that axoplasmic transport of trophic factors is involved in the regulation of peripheral nerve fields in salamanders. J Physiol. 1973 Oct;234(2):449–464. doi: 10.1113/jphysiol.1973.sp010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano A., Fried J. A. The production of denervation-like changes in rat muscle by colchicine, without interference with axonal transport or muscle activity. J Physiol. 1977 Feb;265(1):63–84. doi: 10.1113/jphysiol.1977.sp011705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Diamond J. A quantitative study of the mechanosensory innervation of the salmander skin. J Physiol. 1977 Jan;264(3):695–723. doi: 10.1113/jphysiol.1977.sp011690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Diamond J., Macintyre L., Turner C. Proceedings: Control of collateral sprouting in mechanosensory nerves of salamander skin. J Physiol. 1975 Nov;252(2):20P–21P. [PubMed] [Google Scholar]
- Diamond J., Cooper E., Turner C., Macintyre L. Trophic regulation of nerve sprouting. Science. 1976 Jul 30;193(4251):371–377. doi: 10.1126/science.935873. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Strich S. J. The effects of botulinum toxin on the pattern of innervation of skeletal muscle in the mouse. Q J Exp Physiol Cogn Med Sci. 1968 Jan;53(1):84–89. doi: 10.1113/expphysiol.1968.sp001948. [DOI] [PubMed] [Google Scholar]
- FITZGERALD M. J. Developmental changes in epidermal innervation. J Anat. 1961 Oct;95:495–514. [PMC free article] [PubMed] [Google Scholar]
- Grafstein B., Murray M., Ingoglia N. A. Protein synthesis and axonal transport in retinal ganglion cells of mice lacking visual receptors. Brain Res. 1972 Sep 15;44(1):37–48. doi: 10.1016/0006-8993(72)90364-2. [DOI] [PubMed] [Google Scholar]
- HOFFMAN H. Acceleration and retardation of the process of axon-sprouting in partially devervated muscles. Aust J Exp Biol Med Sci. 1952 Dec;30(6):541–566. doi: 10.1038/icb.1952.52. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. On the degeneration of rat neuromuscular junctions after nerve section. J Physiol. 1970 Apr;207(2):507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson L., Malmfors T. Growth characteristics of adrenergic nerves in the adult rat. Fluorescence histochemical and 3H-noradrenaline uptake studies using tissue transplantations to the anterior chamber of the eye. Acta Physiol Scand Suppl. 1970;348:1–112. [PubMed] [Google Scholar]
- Singer M., Steinberg M. C. Wallerian degeneration: a reevaluation based on transected and colchicine-poisoned nerves in the Amphibian, Triturus. Am J Anat. 1972 Jan;133(1):51–83. doi: 10.1002/aja.1001330105. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., Thoenen H. Retrograde axonal transport of nerve growth factor: specificity and biological importance. Brain Res. 1975 Feb 28;85(2):337–341. doi: 10.1016/0006-8993(75)90092-x. [DOI] [PubMed] [Google Scholar]


