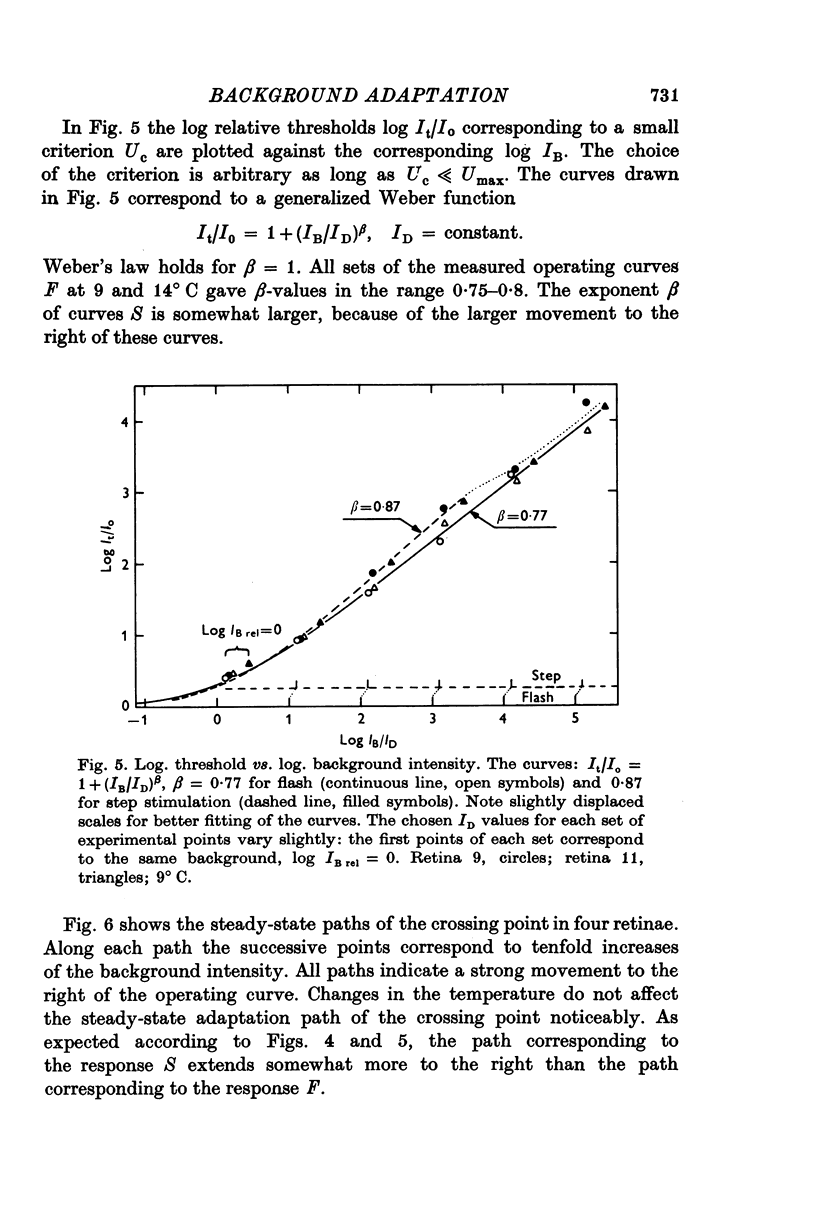
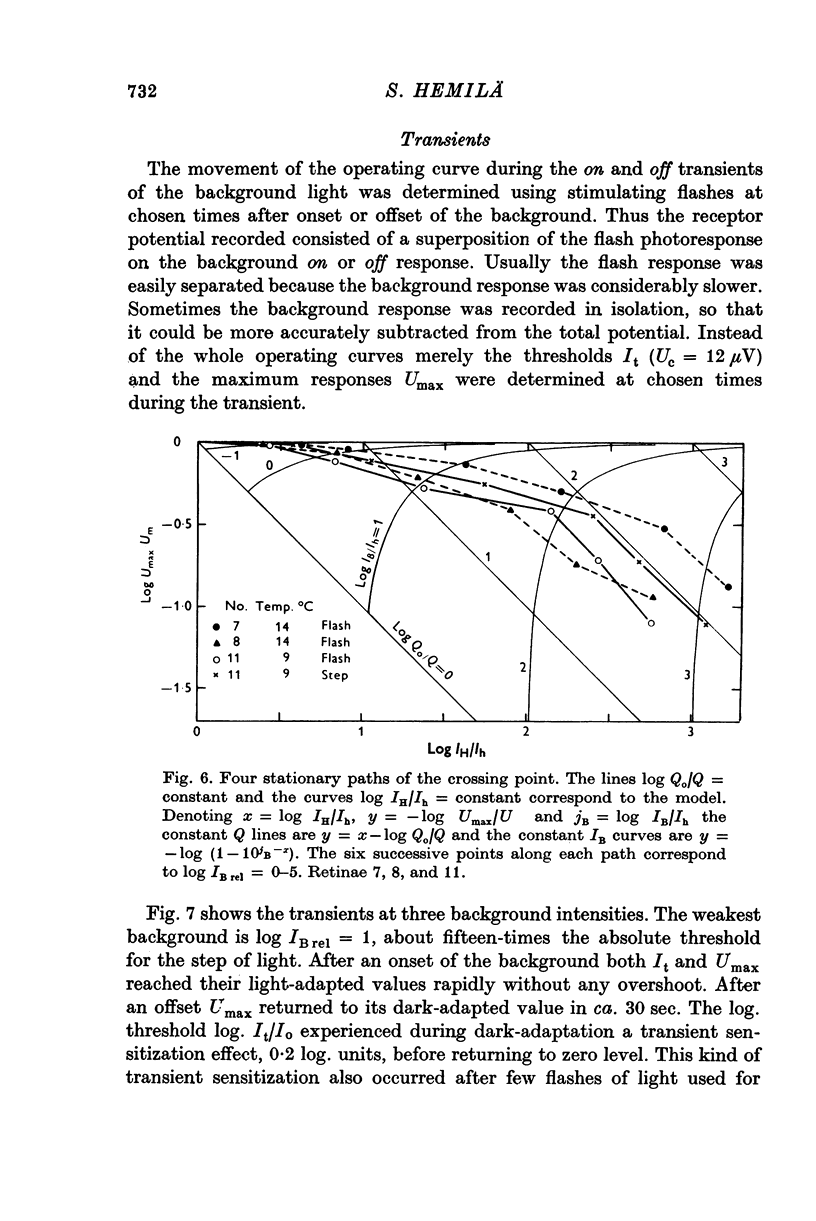
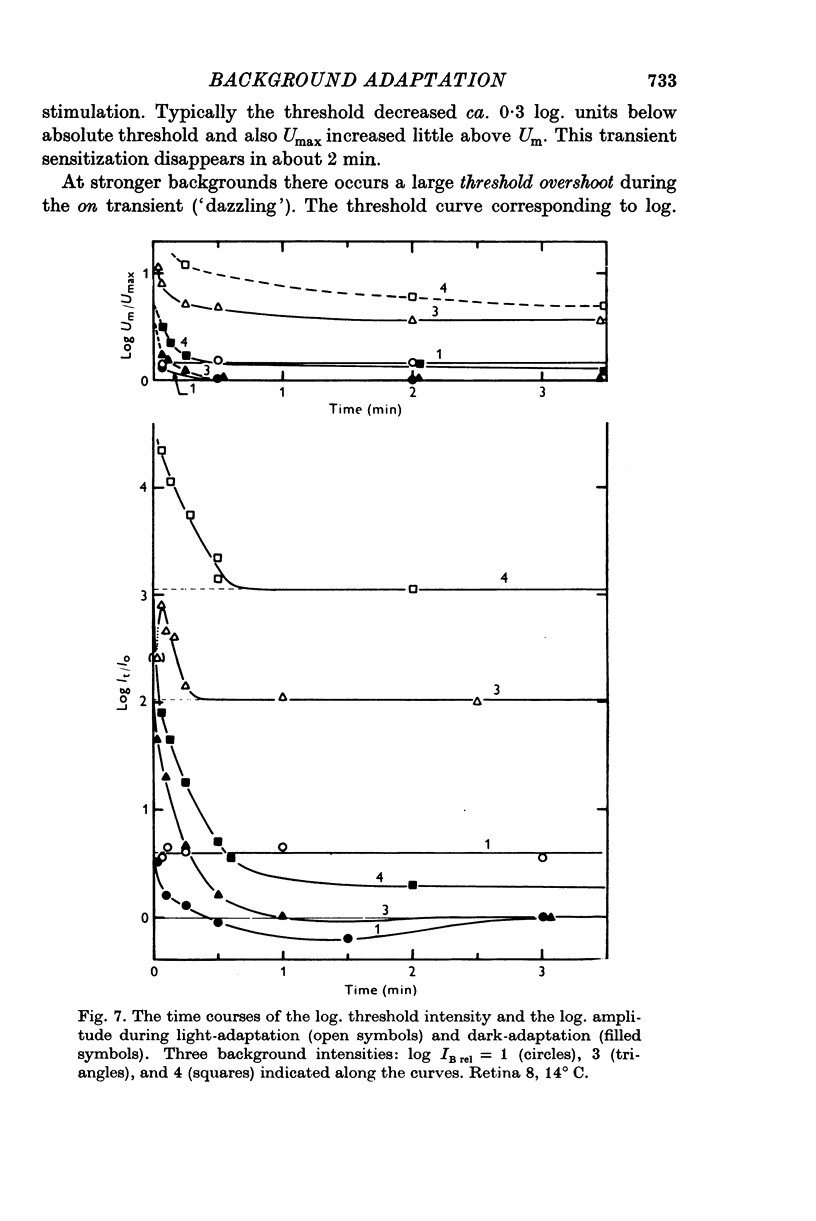
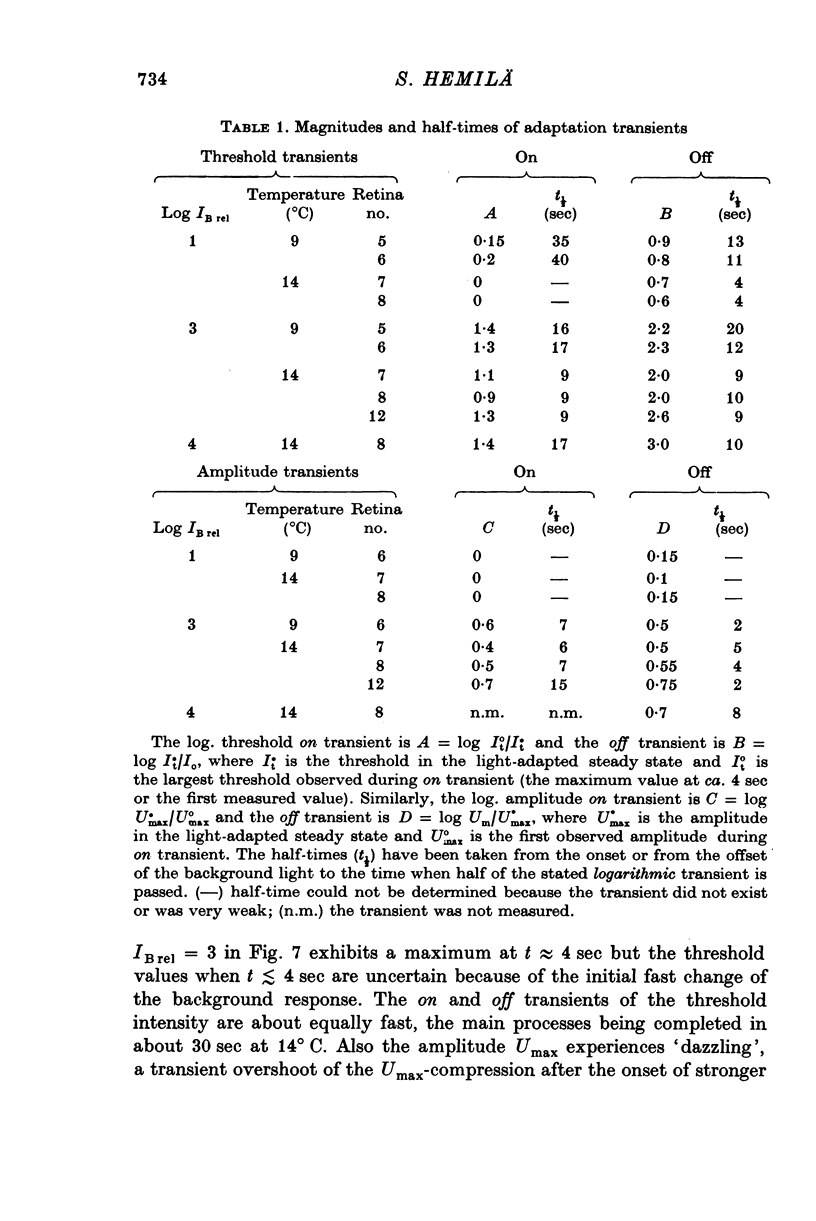
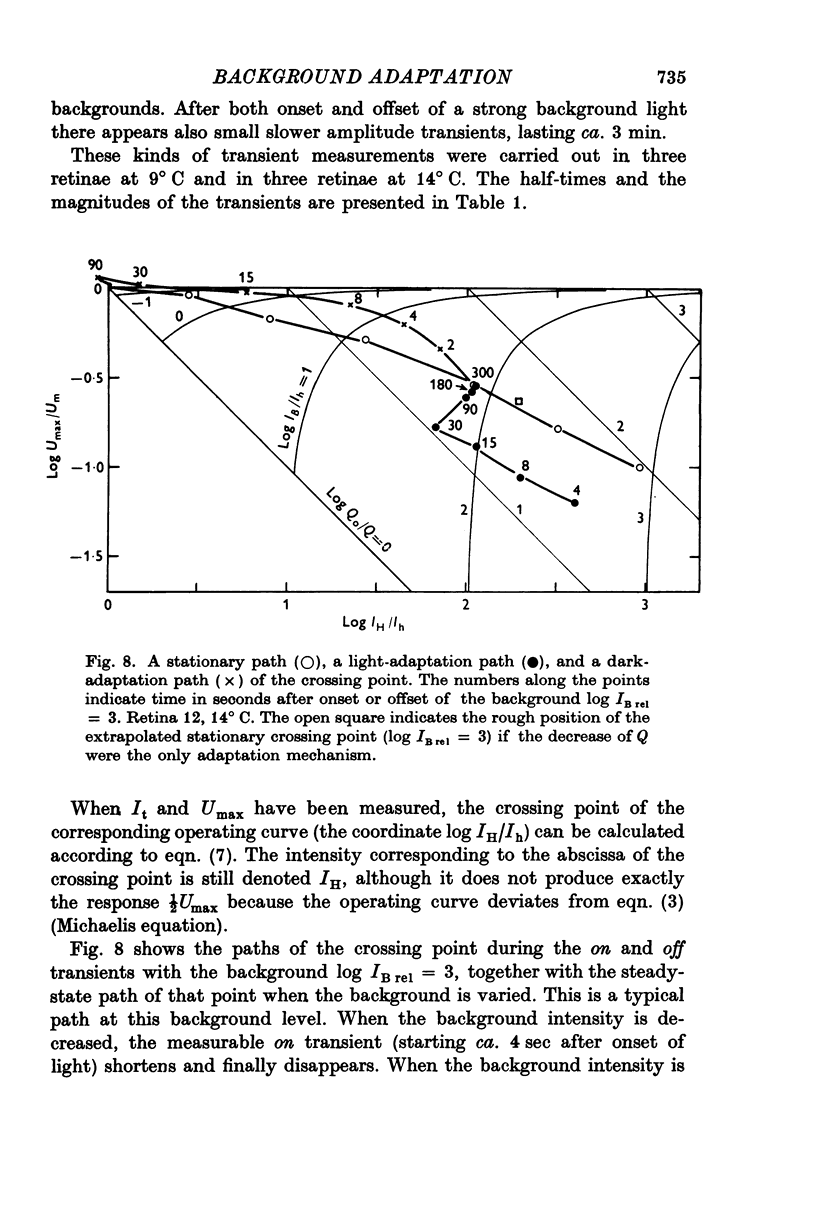
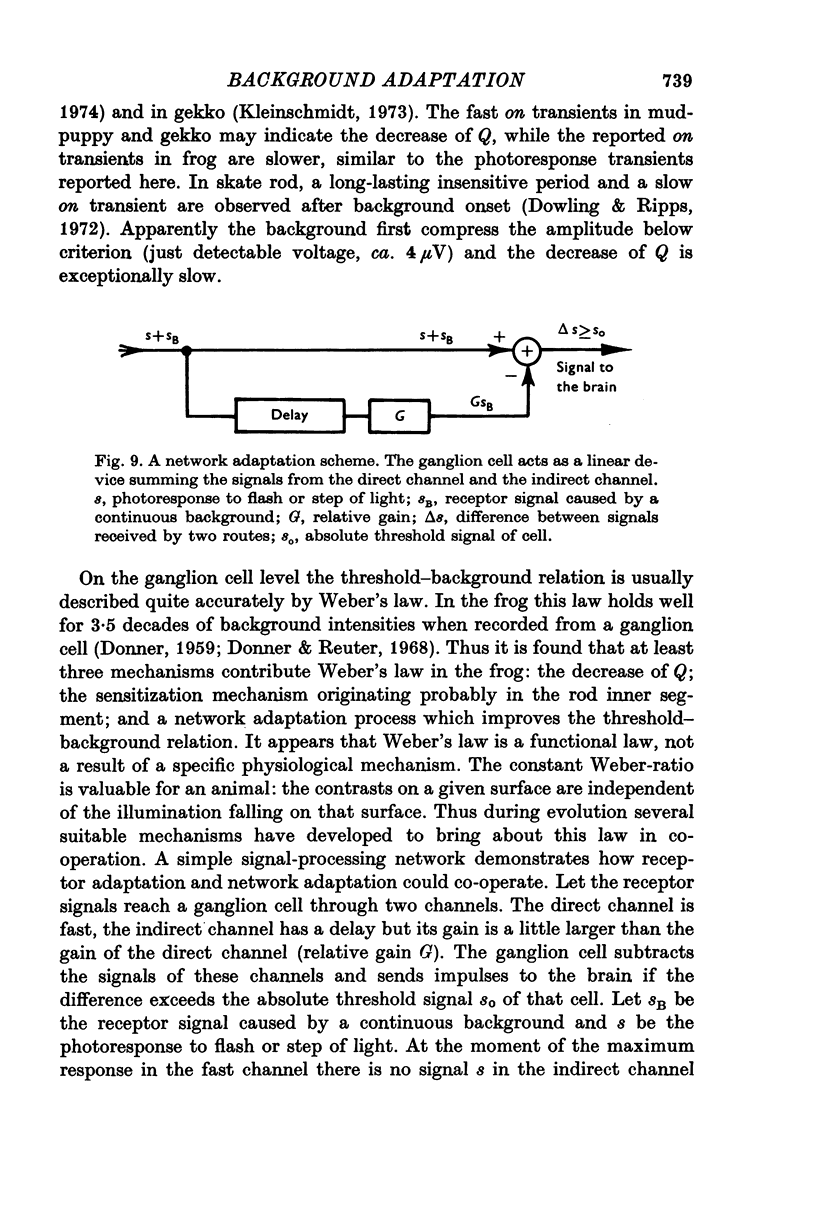
Abstract
1. Aspartate-isolated photoresponses of the red rods to flashes and steps of light have been recorded, both in the presence of and without background lights of varying strength. 2. The results are interpreted in terms of a model of rod outer segment adaptation, where the three model parameters correspond to the adaptation processes associated with the transmitter release, the transmitter background concentration and the plasma membrane leakage, respectively. 3. The stimulus-response function deviated somewhat from the Michaelis equation U/Umax=I/(I + IH). During light-adaptation the operating curve, the stimulus-response function plotted in a log-log diagram, retained approximately its shape while moving strongly to the right along the log intensity axis and to a lesser degree downwards (Umax-decrease). 4. The movement of the operating curve was such that the rods approximately obeyed Weber's law. In the cases of flash and step of light stimuli the movement of the operating curve was about the same. 5. When a moderate background light was turned on a large decrease of sensitivity was first observed. During a period 0-5-1 min the sensitivity increased towards the stationary value. After extinguishing the background light the dark sensitivity returned in 0-5-1 min and then a period of hypersensitivity lasting typically 1 min was observed. 6. The experimental results, as interpreted according to the model, indicate that light-adaptation decreases q, the number of transmitter molecules released by one bleached rhodopsin molecule. 7. There is probably an adaptation process also in the rod inner segment, which increases the sensitivty of the rod to transient stimuli.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern M., Rushton W. A., Torii S. The attenuation of rod signals by backgrounds. J Physiol. 1970 Jan;206(1):209–227. doi: 10.1113/jphysiol.1970.sp009007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONNER K. O. The effect of a coloured adapting field on the spectral sensitivity of frog retinal elements. J Physiol. 1959 Dec;149:318–326. doi: 10.1113/jphysiol.1959.sp006342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K. O., Hemilä S. Kinetics of long-lived rhodopsin photoproducts in the frog retina as a function of the amount bleached. Vision Res. 1975 Aug-Sep;15:985–995. doi: 10.1016/0042-6989(75)90241-2. [DOI] [PubMed] [Google Scholar]
- Donner K. O., Reuter T. The dark-adaptation of single units in the frog's retina and its relation to the regeneration of rhodopsin. Vision Res. 1965 Dec;5(11):615–632. doi: 10.1016/0042-6989(65)90035-0. [DOI] [PubMed] [Google Scholar]
- Donner K. O., Reuter T. Visual adaptation of the rhodopsin rods in the frogs retina. J Physiol. 1968 Nov;199(1):59–87. doi: 10.1113/jphysiol.1968.sp008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Adaptation in skate photoreceptors. J Gen Physiol. 1972 Dec;60(6):698–719. doi: 10.1085/jgp.60.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUKAWA T., HANAWA I. Effects of some common cations on electroretinogram of the toad. Jpn J Physiol. 1955 Dec 15;5(4):289–300. doi: 10.2170/jjphysiol.5.289. [DOI] [PubMed] [Google Scholar]
- Frank R. N. Electroretinographic response from the green rods of the isolated, perfused frog retina. Vision Res. 1970 Nov;10(11):1101–1107. doi: 10.1016/0042-6989(70)90027-1. [DOI] [PubMed] [Google Scholar]
- Hood D., Hock P. A. Light adaptation of the receptors: increment threshold functions for the frog's rods and cones. Vision Res. 1975 May;15(5):545–553. doi: 10.1016/0042-6989(75)90301-6. [DOI] [PubMed] [Google Scholar]
- Murakami M., Kaneko A. Differentiation of P 3 subcomponents in cold-blooded vertebrate retinas. Vision Res. 1966 Dec;6(12):627–636. doi: 10.1016/0042-6989(66)90074-5. [DOI] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter T., Virtanen K. Border and colour coding in the retina of the frog. Nature. 1972 Sep 29;239(5370):260–263. doi: 10.1038/239260a0. [DOI] [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. I. General properties of the response. Vision Res. 1969 Dec;9(12):1435–1442. doi: 10.1016/0042-6989(69)90059-5. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Anno H., Tomita T. The rod response in the frog and studies by intracellular recording. Vision Res. 1970 Nov;10(11):1093–1100. doi: 10.1016/0042-6989(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative hyperpolarization in rods. J Physiol. 1975 Jan;244(1):53–81. doi: 10.1113/jphysiol.1975.sp010784. [DOI] [PMC free article] [PubMed] [Google Scholar]


