Abstract
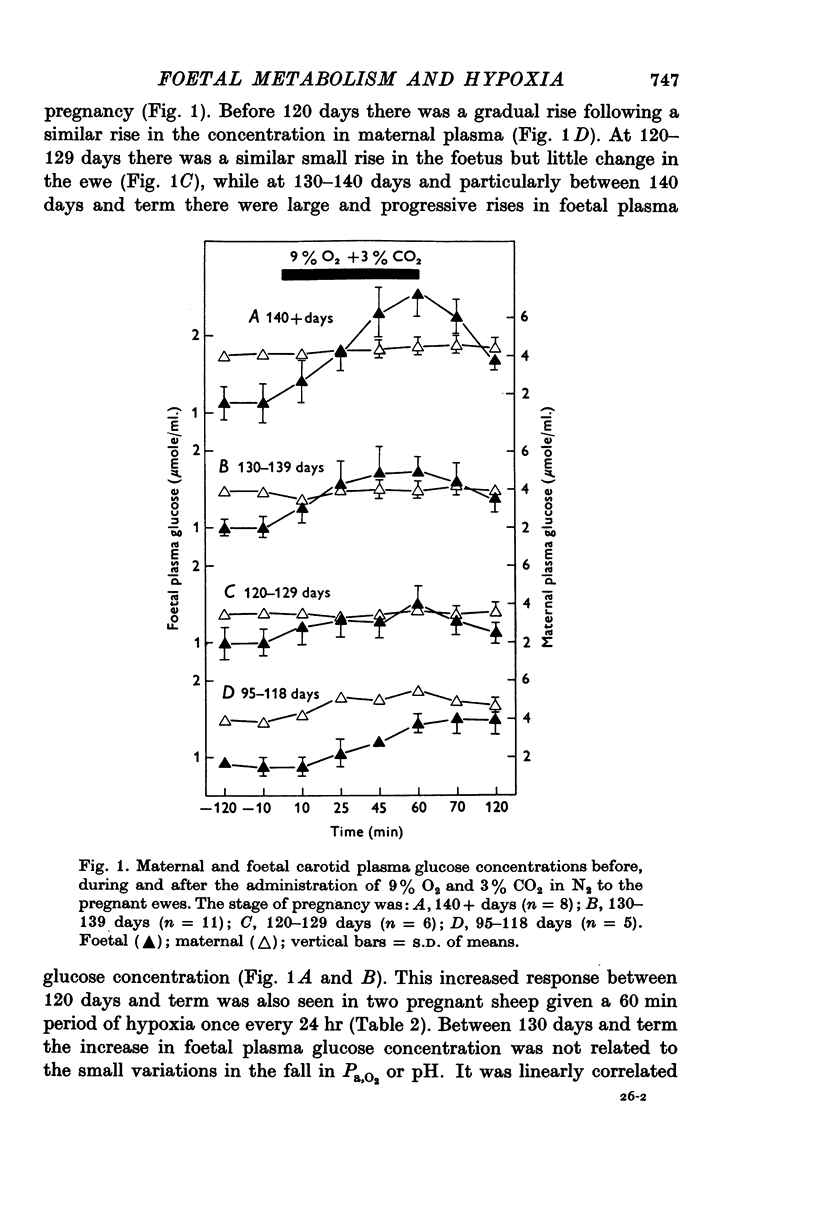
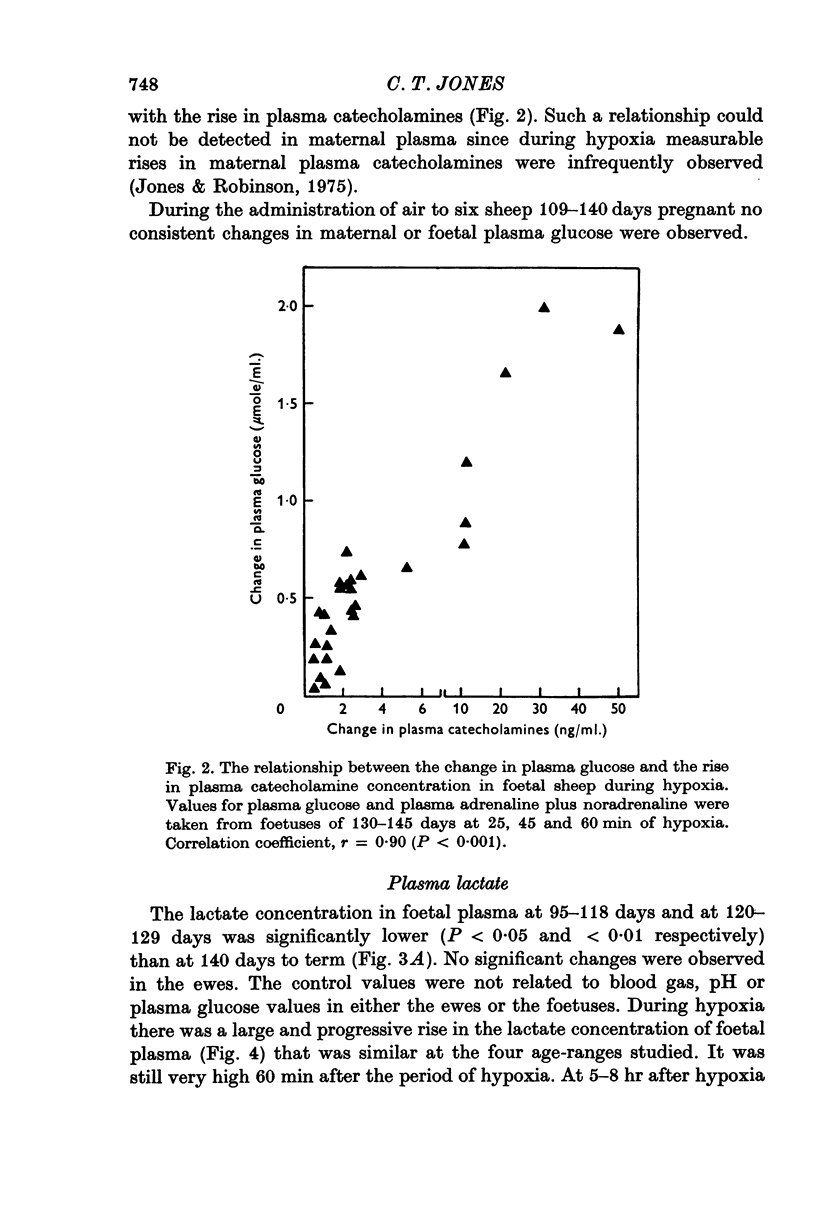
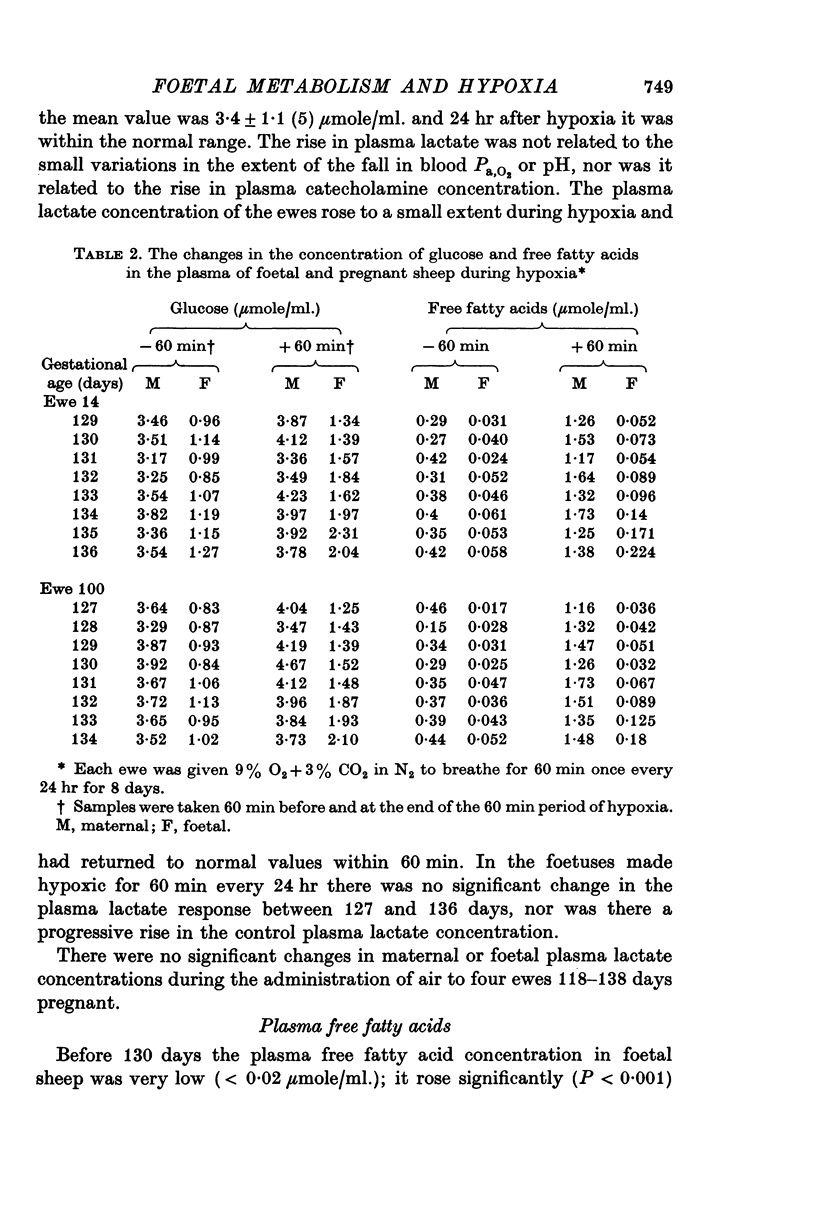
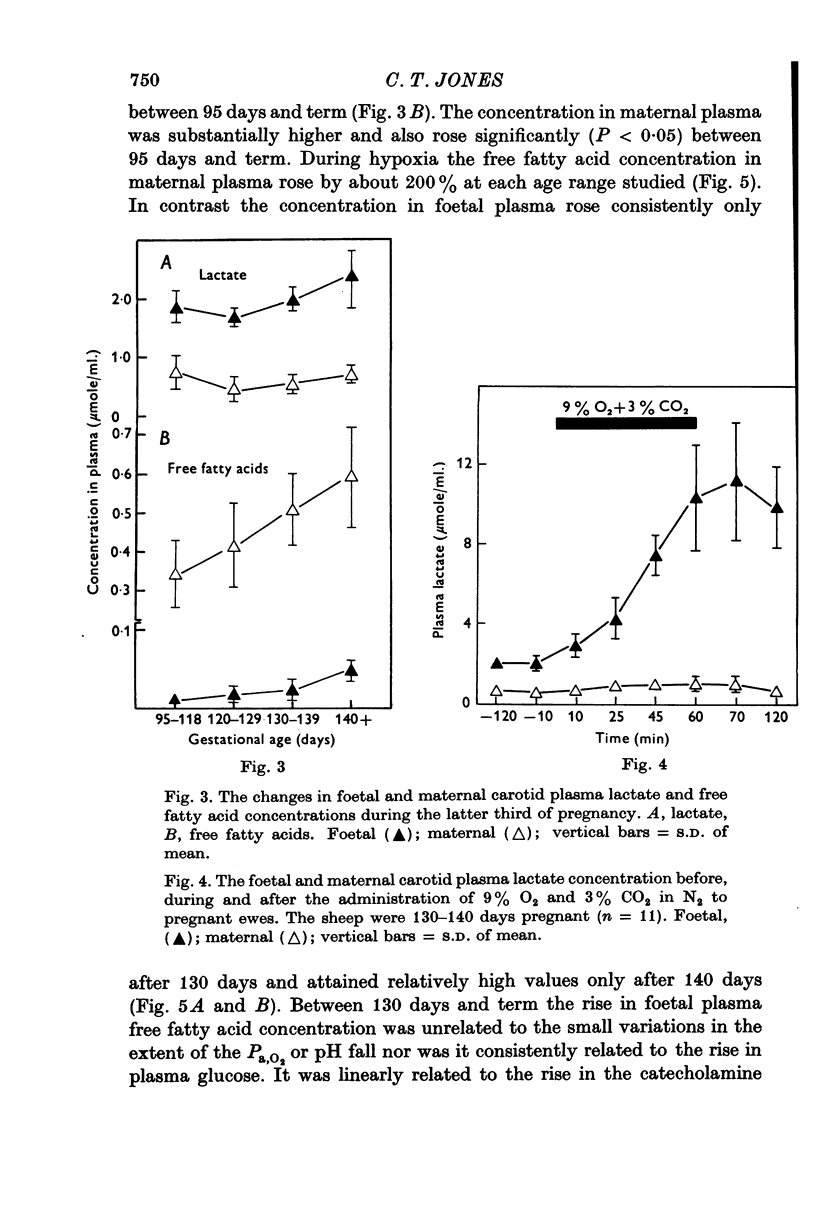
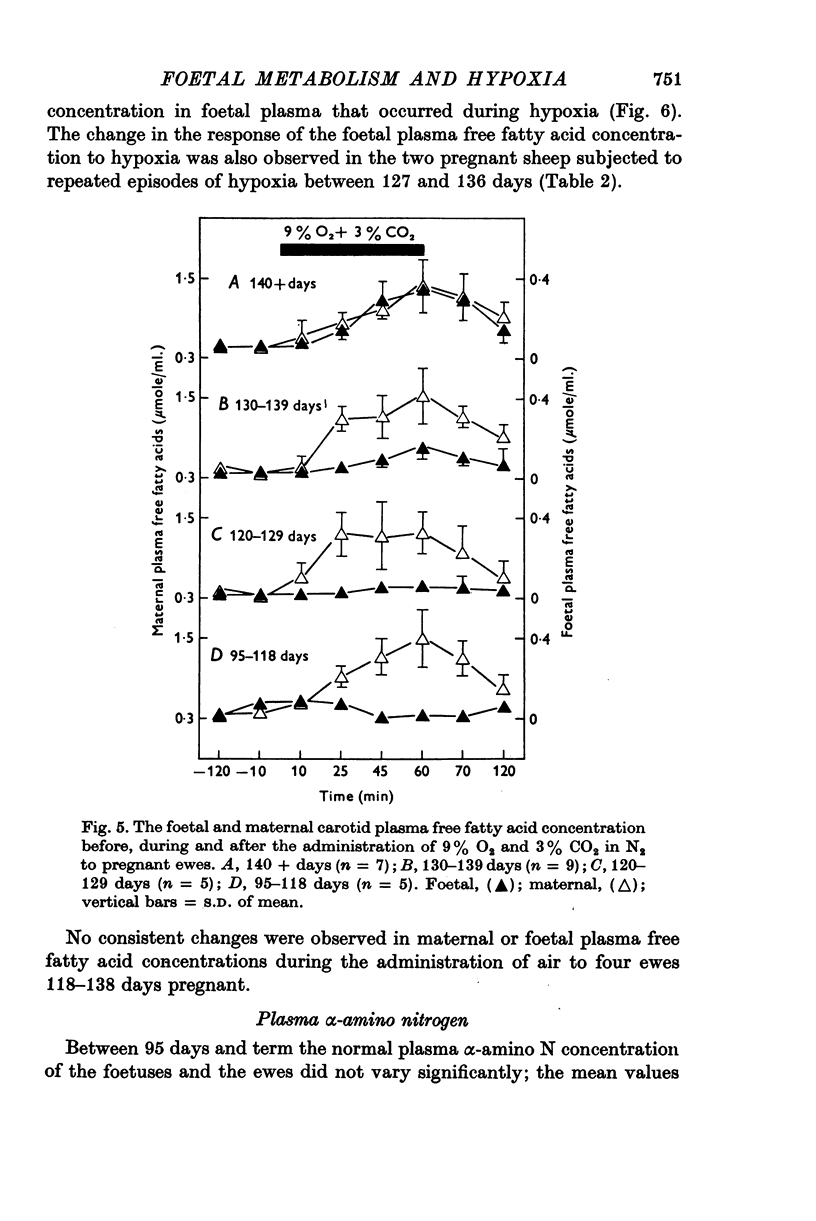
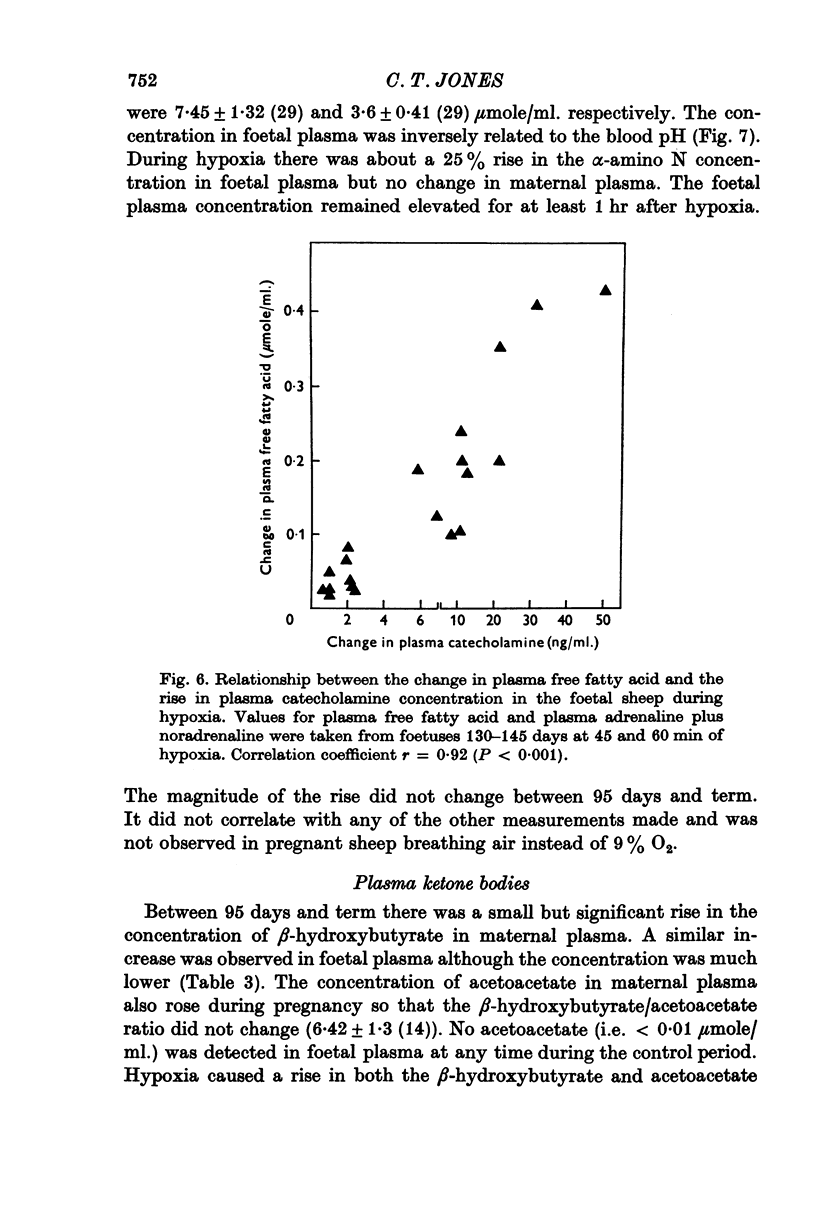
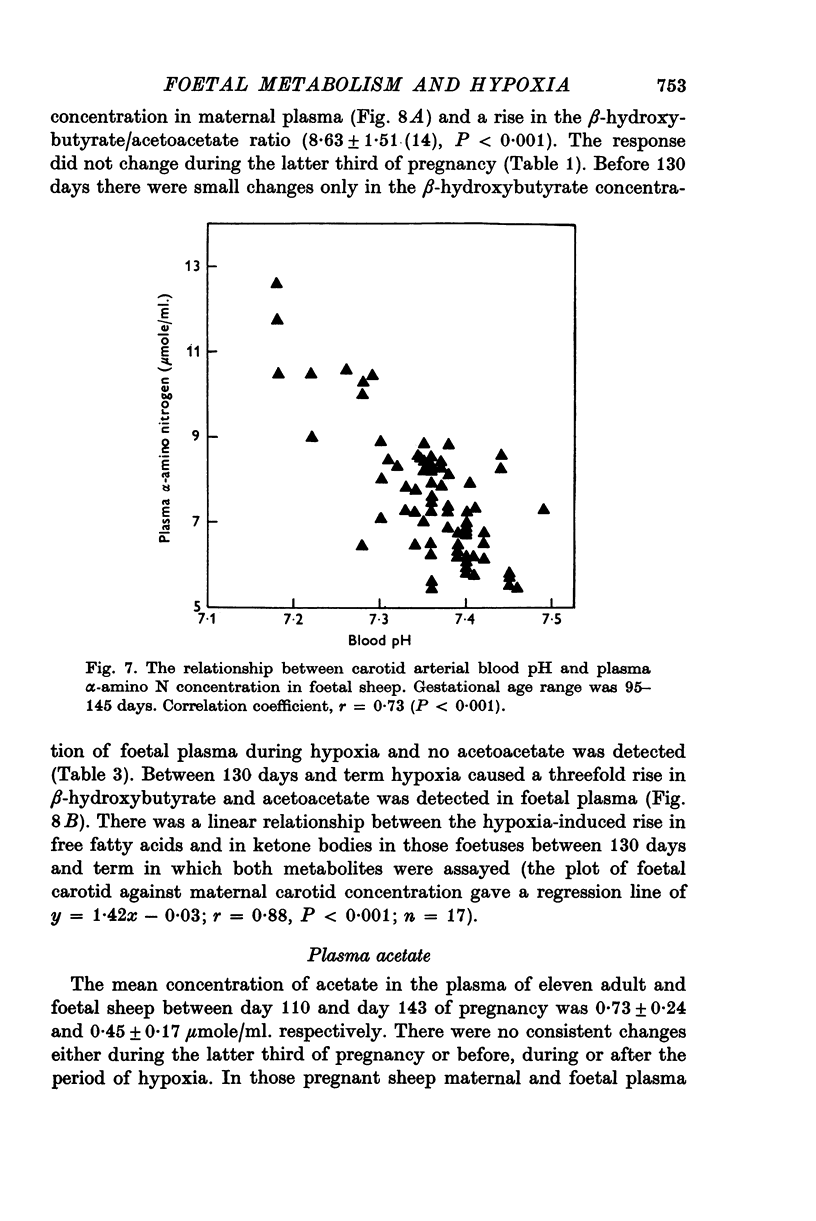
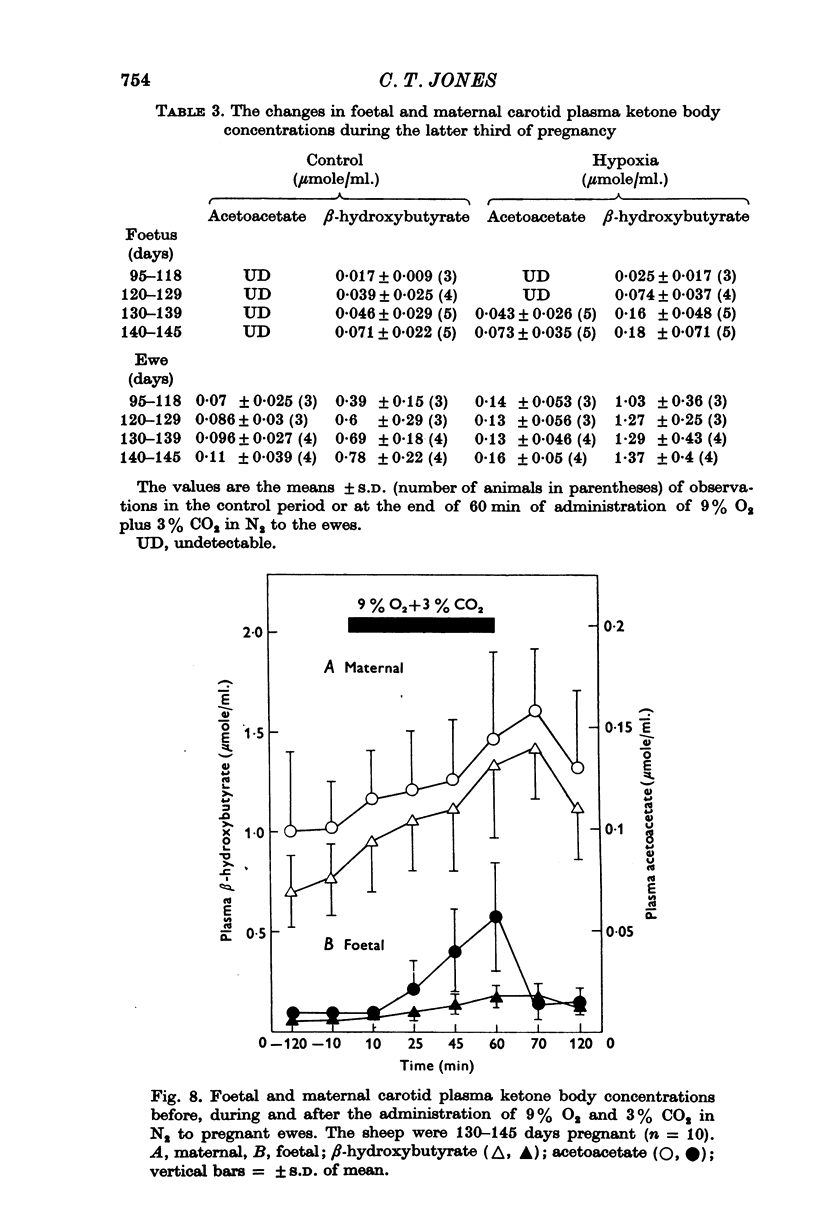
1. Foetal and maternal plasma metabolite and catecholamine concentrations have been measured in chronically catheterized sheep, 95-145 days pregnant. 2. With increasing gestational age there was rise in foetal plasma lactate, free fatty acid and ketone body concentration and in maternal plasma in free fatty acid and ketone body concentration. With the exception of alpha-amino nitrogen none of the plasma metabolites showed any correlation with foetal blood gas or pH values; alpha-amino N was inversely related to foetal blood pH. 3. Hypoxia in the foetuses was induced by causing the ewe to breathe 9% O2 with 3% CO2 in N2. This had a small effect on plasma metabolites in the ewe, mainly producing an increase in free fatty acid and ketone body concentration. 4. In the foetus hypoxia was associated with a large rise in plasma lactate and a small rise in alpha-amino N, the magnitudes of which did not change over the gestational range studied. Consistent and large increases in foetal plasma glucose, free fatty acid and ketone body concentration in response to hypoxia were seen only between 130 and 145 days. 5. In foetuses of 130-145 days the magnitude of the hypoxia-induced rise in plasma glucose and free fatty acid concentration was proportional to the plasma catecholamine concentration. 6. The concentration of acetate in foetal plasma was lower than and proportional to that in the maternal plasma. Neither concentration changed significantly during hypoxia. 7. The results are discussed in relation to the ability of the foetal sheep independently to control the concentration of its plasma metabolites and to mobilize its carbon stores at times of need. They indicate that in the sheep plasma catecholamines are important regulators of plasma glucose and free fatty acid concentrations late in foetal life.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHESON G. H., DAWES G. S., MOTT J. C. Oxygen consumption and the arterial oxygen saturation in foetal and new-born lambs. J Physiol. 1957 Mar 11;135(3):623–643. doi: 10.1113/jphysiol.1957.sp005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASSALI N. S., HOLM L. W., SEHGAL N. Hemodynamic changes in fetal lamb in uteroin response to asphyxia, hypoxia, and hypercapnia. Circ Res. 1962 Sep;11:423–430. doi: 10.1161/01.res.11.3.423. [DOI] [PubMed] [Google Scholar]
- Alexander D. P., Andrews W. H., Britton H. G., Nixon D. A. Hepatic ketone body metabolism in the foetal and neonatal sheep. J Physiol. 1973 Apr;230(1):22P–23P. [PubMed] [Google Scholar]
- Alexander D. P., Britton H. G., Nixon D. A. Acetate metabolism in the isolated sheep foetus. J Physiol. 1967 May;190(2):295–307. doi: 10.1113/jphysiol.1967.sp008209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D. P., Britton H. G., Nixon D. A. Observations on the isolated foetal sheep with particular reference to the metabolism of glucose and fructose. J Physiol. 1966 Jul;185(2):382–399. doi: 10.1113/jphysiol.1966.sp007991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D. P., Forsling M. L., Martin M. J., Nixon D. A., Ratcliffe J. G., Redstone D., Tunbridge D. The effect of maternal hypoxia on fetal pituitary hormone release in the sheep. Biol Neonate. 1972;21(3):219–228. doi: 10.1159/000240510. [DOI] [PubMed] [Google Scholar]
- BALLARD F. J., OLIVER I. T. CARBOHYDRATE METABOLISM IN LIVER FROM FOETAL AND NEONATAL SHEEP. Biochem J. 1965 Apr;95:191–200. doi: 10.1042/bj0950191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODY D. R., SHORLAND F. B. MATERNAL AND FOETAL LIPIDS OF SHEEP. Nature. 1964 May 23;202:769–769. doi: 10.1038/202769a0. [DOI] [PubMed] [Google Scholar]
- Bassett J. M., Madill D. The influence of maternal nutrition on plasma hormone and metabolite concentrations of foetal lambs. J Endocrinol. 1974 Jun;61(3):465–477. doi: 10.1677/joe.0.0610465. [DOI] [PubMed] [Google Scholar]
- Bassett J. M. Metabolic effects of catecholamines in sheep. Aust J Biol Sci. 1970 Aug;23(4):903–914. doi: 10.1071/bi9700903. [DOI] [PubMed] [Google Scholar]
- Boddy K., Dawes G. S., Fisher R., Pinter S., Robinson J. S. Foetal respiratory movements, electrocortical and cardiovascular responses to hypoxaemia and hypercapnia in sheep. J Physiol. 1974 Dec;243(3):599–618. doi: 10.1113/jphysiol.1974.sp010768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton H. G., Huggett A. S., Nixon D. A. Carbohydrate metabolism in the sheep placenta. Biochim Biophys Acta. 1967 Apr 25;136(3):426–440. doi: 10.1016/0304-4165(67)90002-5. [DOI] [PubMed] [Google Scholar]
- Burd L. I., Jones M. D., Jr, Simmons M. A., Makowski E. L., Meschia G., Battaglia F. C. Placental production and foetal utilisation of lactate and pyruvate. Nature. 1975 Apr 24;254(5502):710–711. doi: 10.1038/254710a0. [DOI] [PubMed] [Google Scholar]
- COMLINE R. S., SILVER M. The release of adrenaline and noradrenaline from the adrenal glands of the foetal sheep. J Physiol. 1961 May;156:424–444. doi: 10.1113/jphysiol.1961.sp006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. G., Dawes G. S., Fishman A. P., Hyman A. I. Regional redistribution of blood flow in the mature fetal lamb. Circ Res. 1967 Aug;21(2):229–235. doi: 10.1161/01.res.21.2.229. [DOI] [PubMed] [Google Scholar]
- Char V. C., Creasy R. K. Acetate as a metabolic substrate in the fetal lamb. Am J Physiol. 1976 Feb;230(2):357–361. doi: 10.1152/ajplegacy.1976.230.2.357. [DOI] [PubMed] [Google Scholar]
- Cohn H. E., Sacks E. J., Heymann M. A., Rudolph A. M. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974 Nov 15;120(6):817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- Comline R. S., Silver M. Daily changes in foetal and maternal blood of conscious pregnant ewes, with catheters in umbilical and uterine vessels. J Physiol. 1970 Aug;209(3):567–586. doi: 10.1113/jphysiol.1970.sp009180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comline R. S., Silver M. The composition of foetal and maternal blood during parturition in the ewe. J Physiol. 1972 Apr;222(1):233–256. doi: 10.1113/jphysiol.1972.sp009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES G. S., MOTT J. C., SHELLEY H. J. The importance of cardiac glycogen for the maintenance of life in foetal lambs and newborn animals during anoxia. J Physiol. 1959 Jun 11;146(3):516–538. doi: 10.1113/jphysiol.1959.sp006208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Duncan S. L., Lewis B. V., Merlet C. L., Owen-Thomas J. B., Reeves J. T. Hypoxaemia and aortic chemoreceptor function in foetal lambs. J Physiol. 1969 Mar;201(1):105–116. doi: 10.1113/jphysiol.1969.sp008745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Fox H. E., Leduc B. M., Liggins G. C., Richards R. T. Respiratory movements and rapid eye movement sleep in the foetal lamb. J Physiol. 1972 Jan;220(1):119–143. doi: 10.1113/jphysiol.1972.sp009698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes G. S., Lewis B. V., Milligan J. E., Roach M. R., Talner N. S. Vasomotor responses in the hind limbs of foetal and new-born lambs to asphyxia and aortic chemoreceptor stimulation. J Physiol. 1968 Mar;195(1):55–81. doi: 10.1113/jphysiol.1968.sp008446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncombe W. G. The colorimetric micro-determination of long-chain fatty acids. Biochem J. 1963 Jul;88(1):7–10. doi: 10.1042/bj0880007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson J. L., Hudson D. G., Hull D. Evidence of increased fatty acid transfer across the placenta during a maternal fast in rabbits. Biol Neonate. 1975;27(1-2):50–55. doi: 10.1159/000240758. [DOI] [PubMed] [Google Scholar]
- Edwards A. V., Silver M. Comparison of the hyperglycaemic and glycogenolytic responses to catecholamines with those to stimulation of the hepatic sympathetic innervation in the dog. J Physiol. 1972 Jun;223(2):571–593. doi: 10.1113/jphysiol.1972.sp009863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Reed N., Saperstein R. The isolation and metabolism of brown fat cells. J Biol Chem. 1967 Apr 25;242(8):1887–1894. [PubMed] [Google Scholar]
- Gemmell R. T., Bell A. W., Alexander G. Morphology of adipose cells in lambs at birth and during subsequent transition of brown to white adipose tissue in cold and in warm conditons. Am J Anat. 1972 Feb;133(2):143–164. doi: 10.1002/aja.1001330203. [DOI] [PubMed] [Google Scholar]
- HINKS M., MASTERS C. J. DEVELOPMENTAL CHANGES IN RUMINANT LACTATE DEHYDROGENASE. Biochemistry. 1964 Nov;3:1789–1791. doi: 10.1021/bi00899a035. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Heim T., Hull D. The blood flow and oxygen consumption of brown adipose tissue in the new-born rabbit. J Physiol. 1966 Sep;186(1):42–55. doi: 10.1113/jphysiol.1966.sp008019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hird F. J., Weidemann M. J. Ketone-body synthesis in relation to age of lambs. Biochem J. 1964 Nov;93(2):423–430. doi: 10.1042/bj0930423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James E., Meschia G., Battaglia F. C. A-V differences of free fatty acids and glycerol in the ovine umbilical circulation. Proc Soc Exp Biol Med. 1971 Dec;138(3):823–826. doi: 10.3181/00379727-138-35999. [DOI] [PubMed] [Google Scholar]
- Jones C. T., Ashton I. K. Lipid biosynthesis in liver slices of the foetal guinea pig. Biochem J. 1976 Jan 15;154(1):149–158. doi: 10.1042/bj1540149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. T. Lipid metabolism and mobilization in the guinea pig during pregnancy. Biochem J. 1976 May 15;156(2):357–365. doi: 10.1042/bj1560357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. T., Robinson R. O. Plasma catecholamines in foetal and adult sheep. J Physiol. 1975 Jun;248(1):15–33. doi: 10.1113/jphysiol.1975.sp010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. T., Rurak D. The distribution and clearances of hormones and metabolites in the circulation of the foetal sheep. Q J Exp Physiol Cogn Med Sci. 1976 Oct;61(4):287–295. doi: 10.1113/expphysiol.1976.sp002360. [DOI] [PubMed] [Google Scholar]
- Jost A., Picon L. Hormonal control of fetal development and metabolism. Adv Metab Disord. 1970;4:123–184. doi: 10.1016/b978-0-12-027304-1.50010-7. [DOI] [PubMed] [Google Scholar]
- Leat W. M. Fatty acid composition of the plasma lipids of newborn and maternal ruminants. Biochem J. 1966 Feb;98(2):598–603. doi: 10.1042/bj0980598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann L. I. Effects of hypoxia on umbilical circulation and fetal metabolism. Am J Physiol. 1970 May;218(5):1453–1458. doi: 10.1152/ajplegacy.1970.218.5.1453. [DOI] [PubMed] [Google Scholar]
- Mellor D. J., Slater J. S. Daily changes in amniotic and allantoic fluid during the last three months of pregnancy in conscious, unstressed ewes, with catheters in their foetal fluid sacs. J Physiol. 1971 Sep;217(3):573–604. doi: 10.1113/jphysiol.1971.sp009587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble R. C., Steele W., Moore J. H. The plasma lipids of the ewe during pregnancy and lactation. Res Vet Sci. 1971 Jan;12(1):47–53. doi: 10.1007/BF02558517. [DOI] [PubMed] [Google Scholar]
- PUGH P. D., SCARISBRICK R. Acetate uptake by the foetal sheep. J Physiol. 1955 Sep 28;129(3):67P–67P. doi: 10.1113/jphysiol.1955.sp005387. [DOI] [PubMed] [Google Scholar]
- Reid R. L. The physiopathology of undernourishment in pregnant sheep, with particular reference to pregnancy toxemia. Adv Vet Sci. 1968;12:163–238. [PubMed] [Google Scholar]
- Roux J. F., Yoshioka T. Lipid metabolism in the fetus during development. Clin Obstet Gynecol. 1970 Sep;13(3):595–620. doi: 10.1097/00003081-197009000-00009. [DOI] [PubMed] [Google Scholar]
- Shelley H. J. Blood sugars and tissue carbohydrate in foetal and infant lambs and rhesus monkeys. J Physiol. 1960 Oct;153(3):527–552. doi: 10.1113/jphysiol.1960.sp006553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinebourne E. A., Vapaavuori E. K., Williams R. L., Heymann M. A., Rudolph A. M. Development of baroreflex activity in unanesthetized fetal and neonatal lambs. Circ Res. 1972 Nov;31(5):710–718. doi: 10.1161/01.res.31.5.710. [DOI] [PubMed] [Google Scholar]
- WIDDOWSON E. M. Chemical composition of newly born mammals. Nature. 1950 Oct 14;166(4224):626–628. doi: 10.1038/166626a0. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensvoort P. The development of adipose tissue in sheep foetuses. Pathol Vet. 1967;4(1):69–78. doi: 10.1177/030098586700400107. [DOI] [PubMed] [Google Scholar]


