Abstract
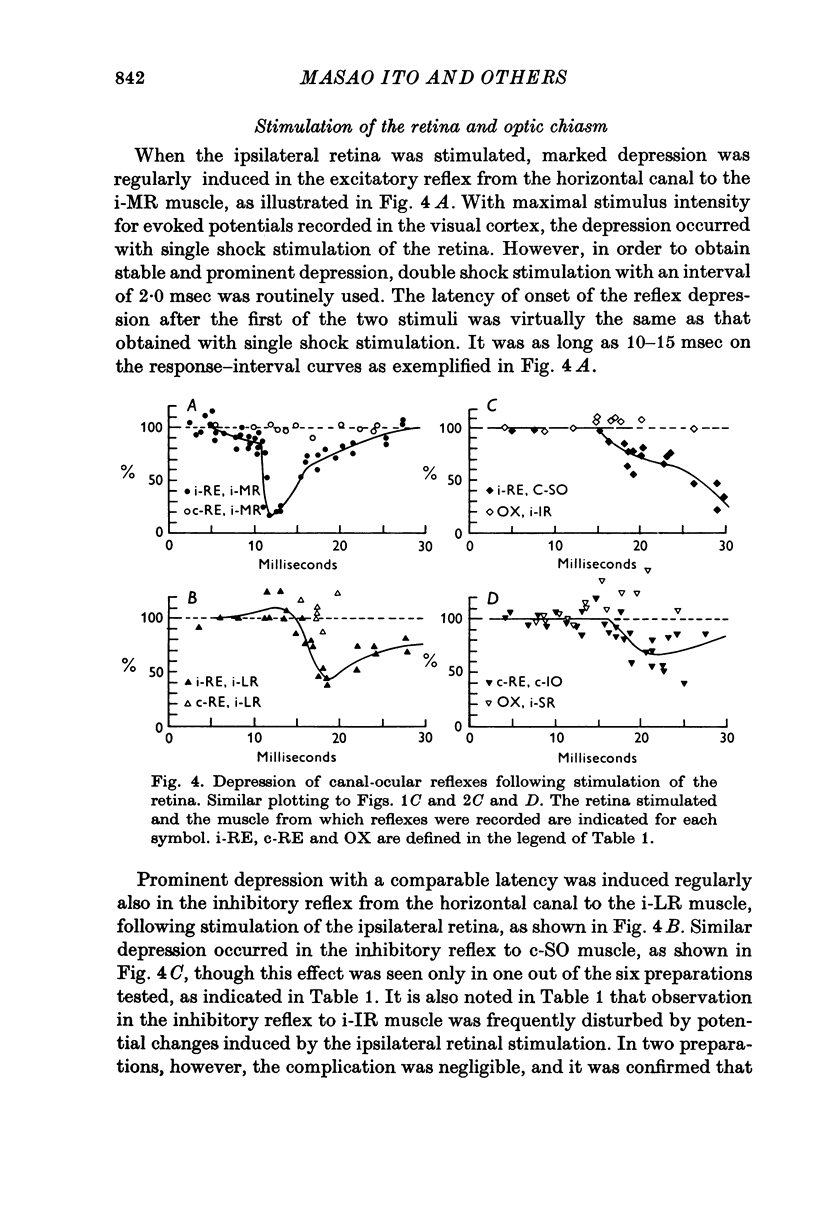
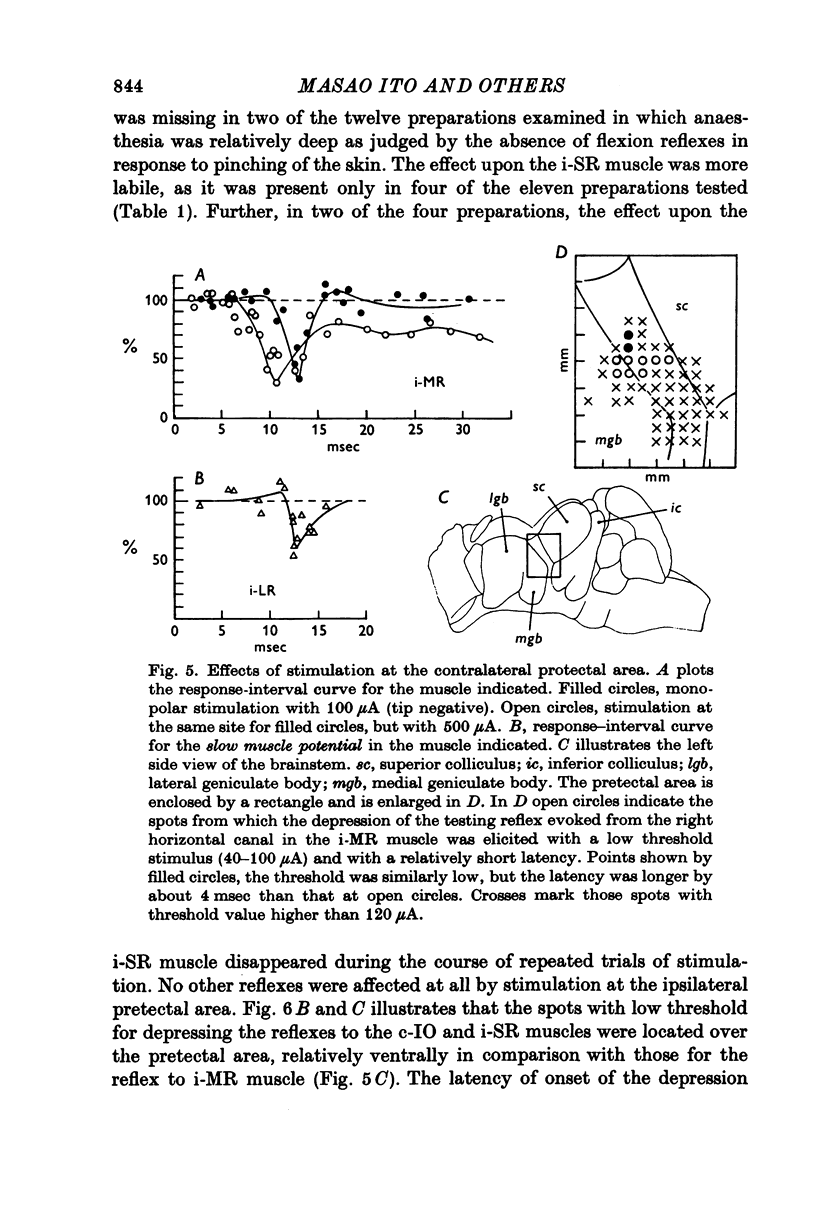
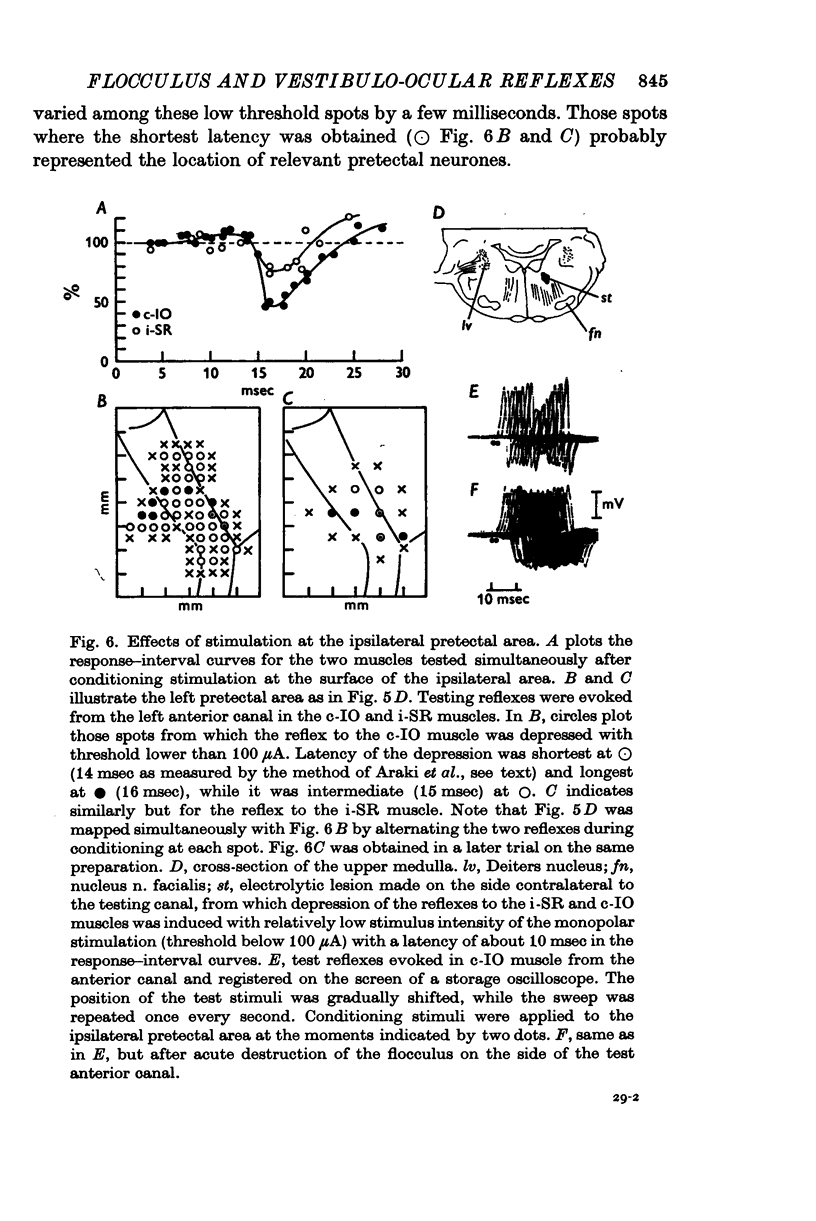
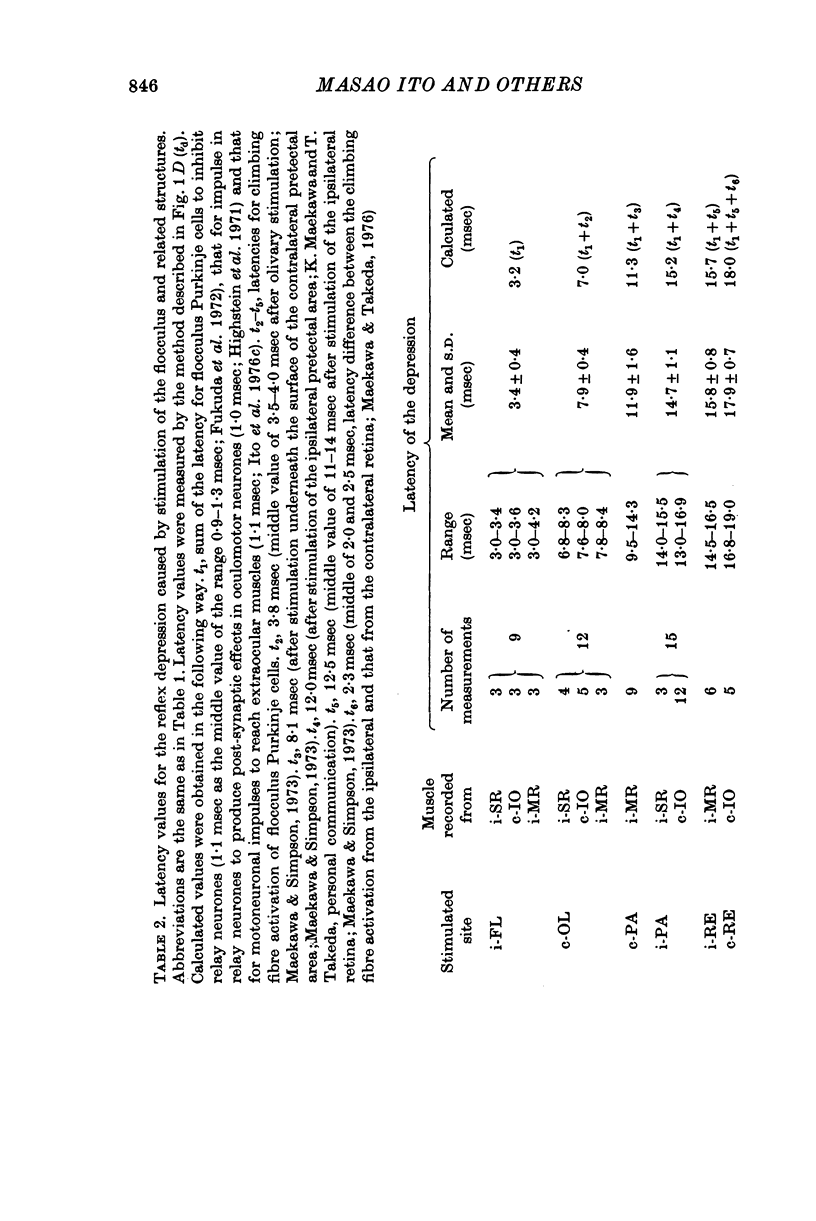
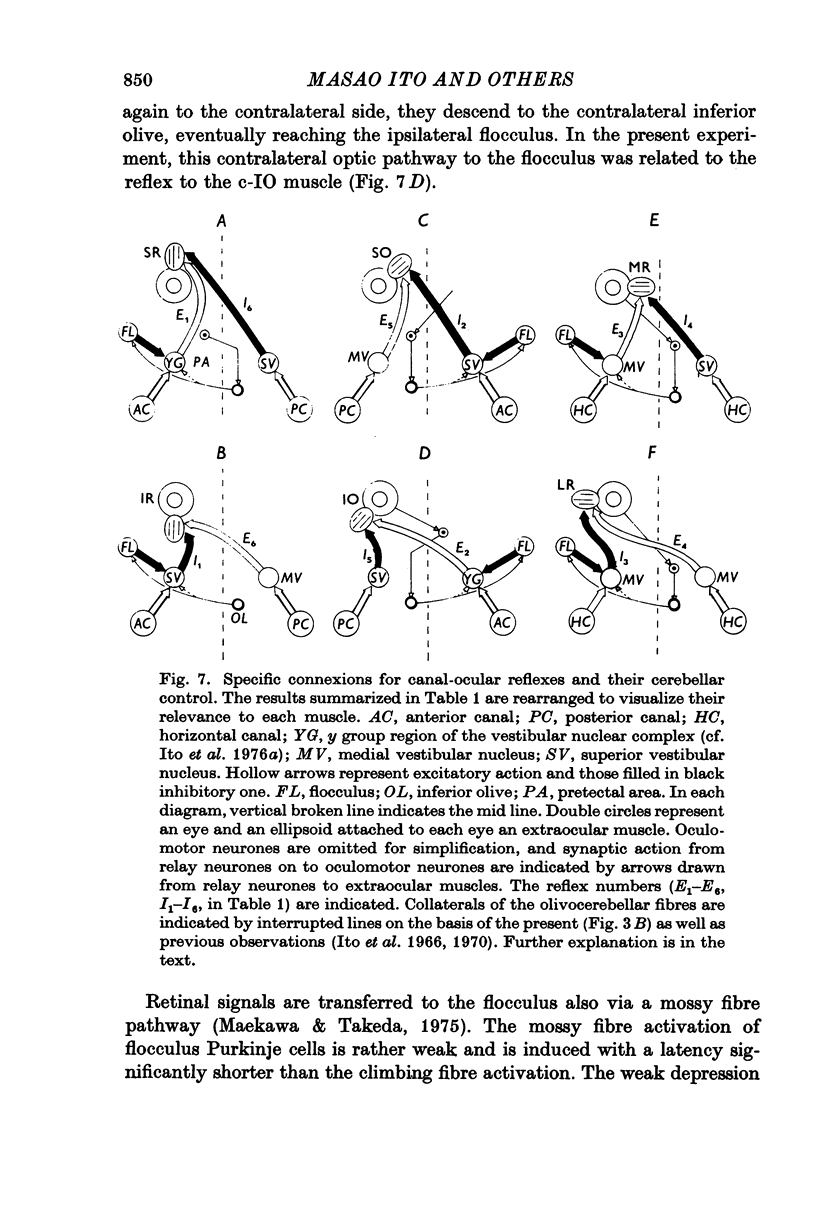
1. In anaesthetized albino rabbits, the occurrence of Purkinje cell inhibition on canal-ocular reflexes was surveyed with a reflex testing method. 2. Test reflexes were elicited by electrical stimulation of the semicircular canals. The results were appaised by recording potentials and tension from extraocular muscles. Twelve reflexes were defined in terms of the receptor canal and the effector muscle. 3. Conditioning electrical stimuli were applied to the flocculus, the inferior olive, and optic pathways at the retinae, optic chiasm, pretectal area and upper medulla. 4. The conditioning stimulation at the ipsilateral flocculus induced depression in six of the twelve canal-ocular reflexes; four of the six arose from the anterior canal and the remaining two from the horizontal canal. 5. The effect of stimulation of the contralateral inferior olive was similar to that of the ipsilateral flocculus, though less clear in two of the four reflexes from the anterior canal because of a contaminating effect. 6. The two reflexes from the horizontal canal were depressed by stimulation of the ipsilateral optic pathway which reached the ipsilateral flocculus via the contralateral pretectal area and inferior olive. 7. The four reflexes from the anterior canal were affected by stimulation of optic pathways in a different manner from each other. One was depressed from the contralateral retina via the ipsilateral pretectal area, while another was depressed from the ipsilateral retina via the contralateral pretectal area, though only occasionally. The third reflex was depressed from the ipsilateral pretectal area but not from the retina. The fourth was affected from neither the retina nor the pretectal area. 8. On the basis of latency measurements, it was concluded that the depression of canal-ocular reflexes was due to inhibition of relay neurones of the testing reflexes by flocculus Purkinje cells which were activated either directly, or indirectly through olivocerebellar climbing fibre afferents. 9. The above conclusion was supported by the observation that the depression induced by stimulation of the inferior olive and optic pathways was abolished by acute destruction of the ipsilateral flocculus. 10. The possible functional significance of the specific patterns of connexions from flocculus Purkinje cells to canal-ocular reflex pathways is discussed, and specialization among flocculus Purkinje cells in relationship with vestibulo-ocular reflexes is postulated.
Full text
PDF



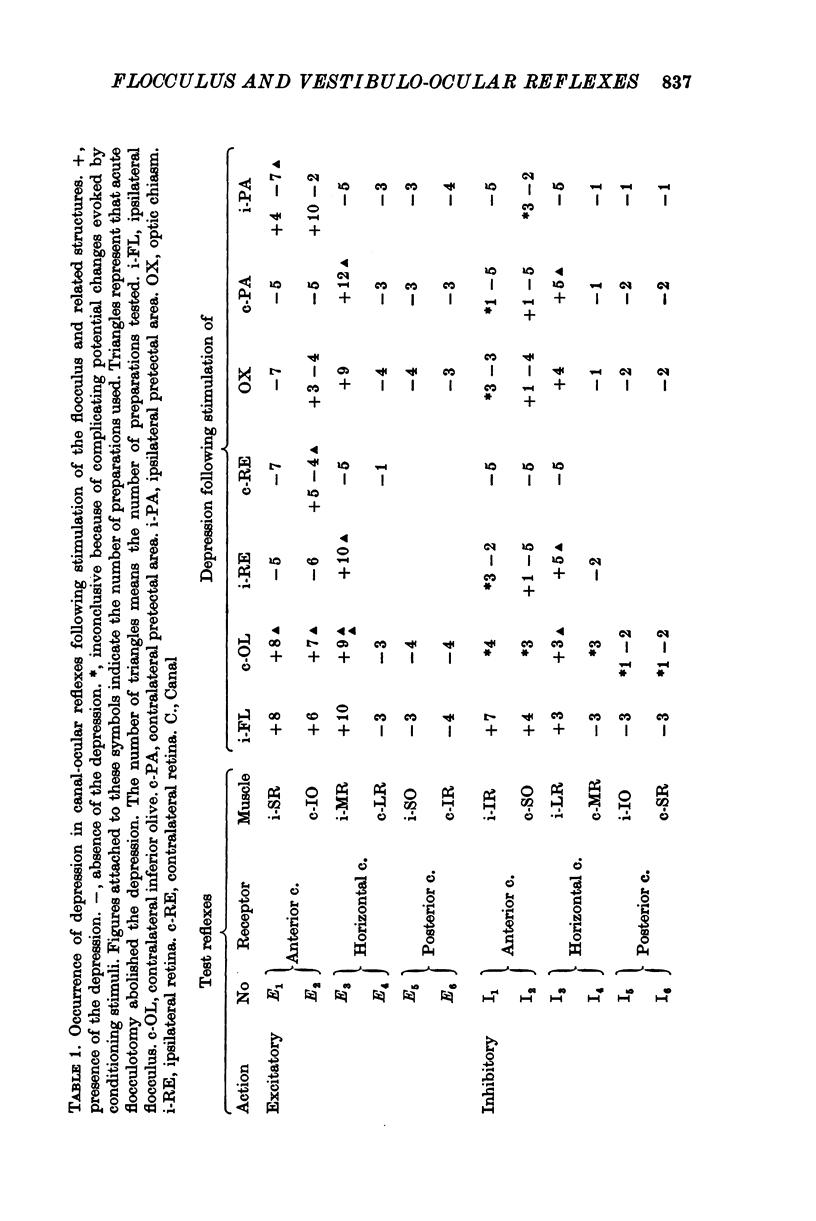
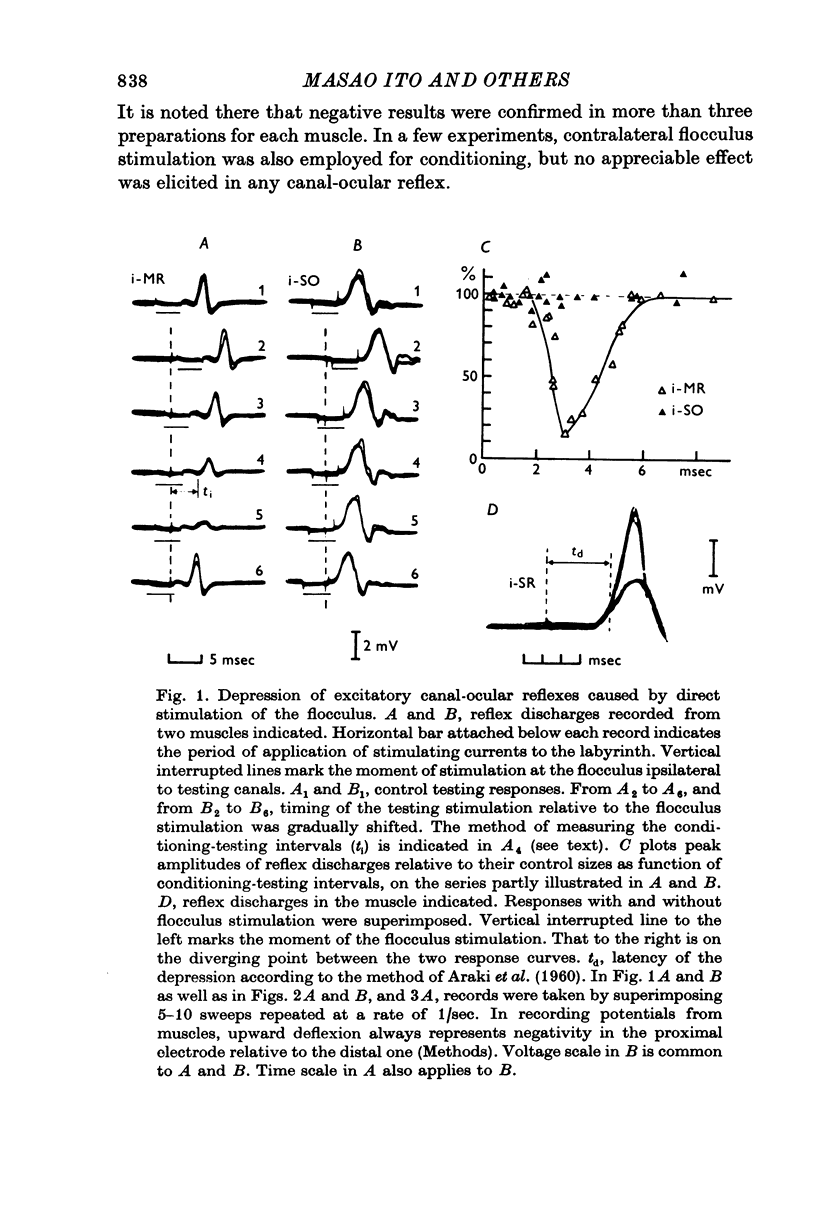
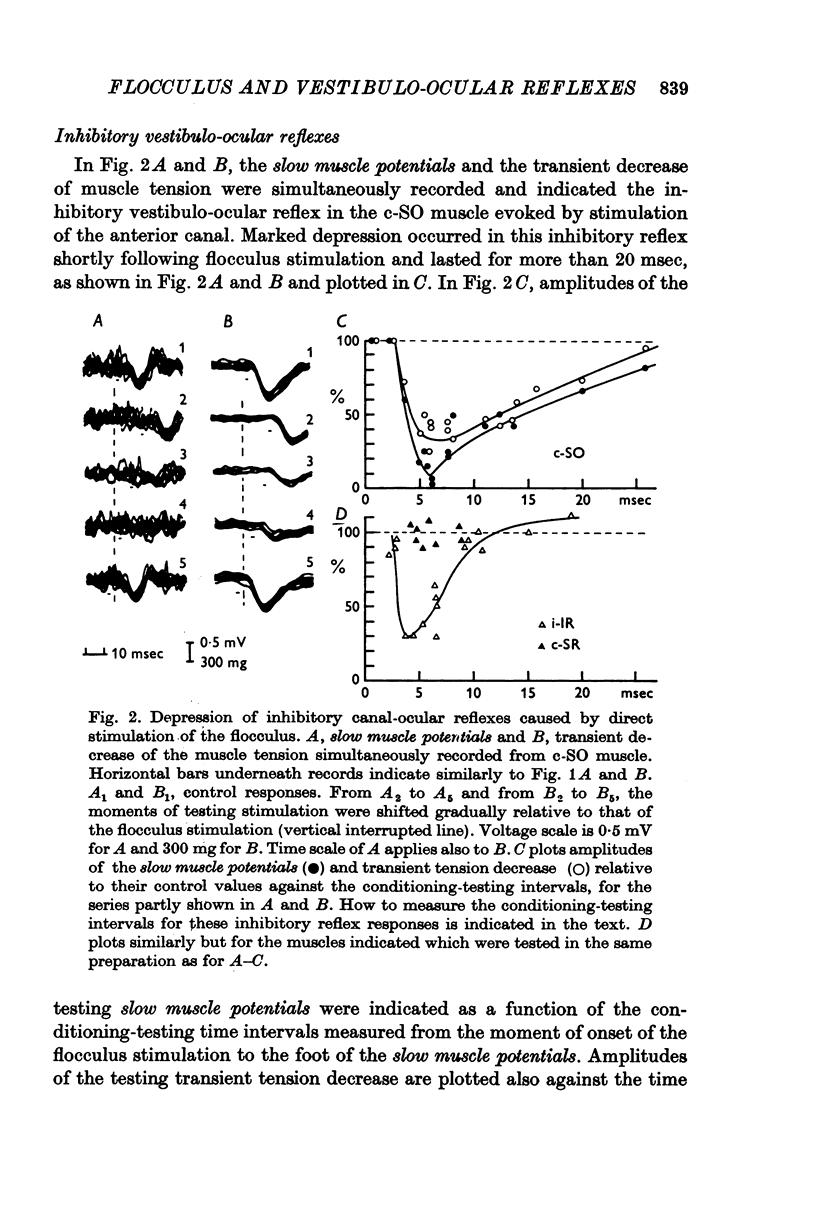

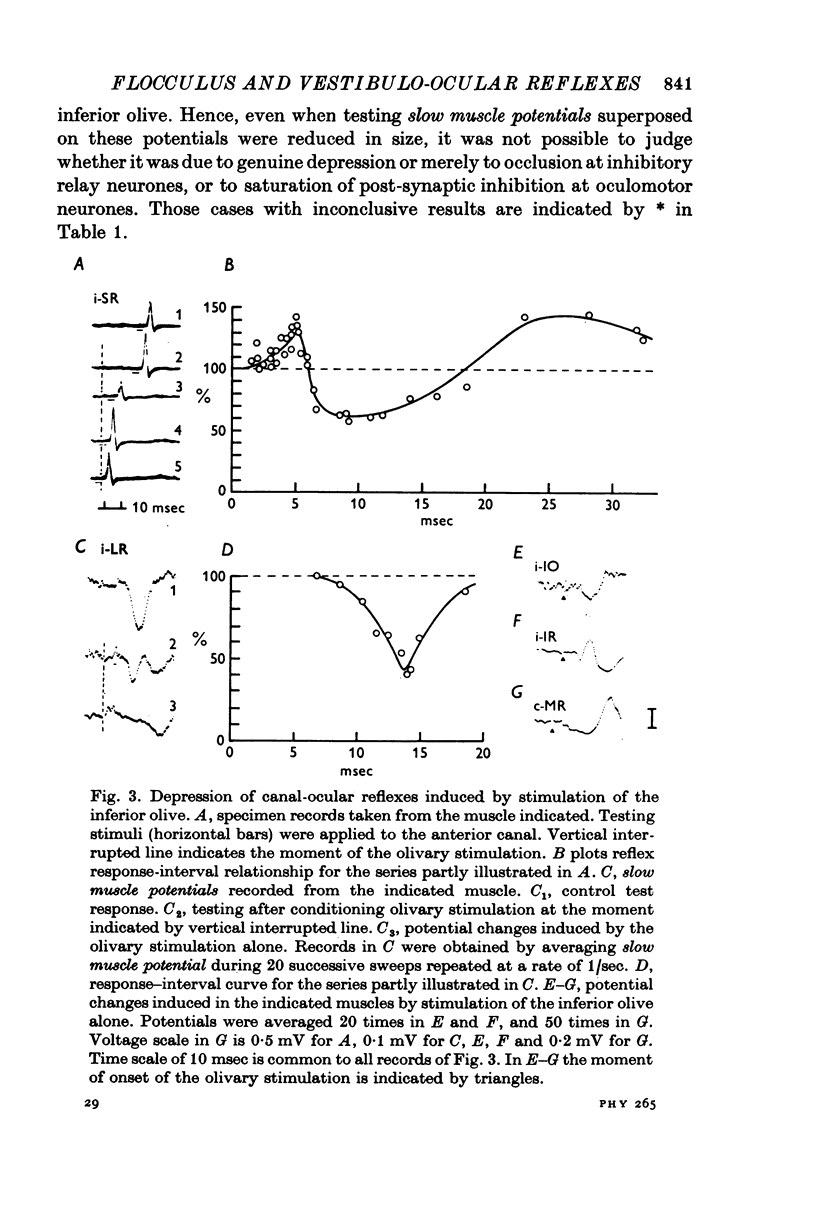








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., EOCLES J. C., ITO M. Correlation of the inhibitory post-synaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. J Physiol. 1960 Dec;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angaut P., Brodal A. The projection of the "vestibulocerebellum" onto the vestibular nuclei in the cat. Arch Ital Biol. 1967 Nov;105(4):441–479. [PubMed] [Google Scholar]
- Baker R., Precht W., Llinás R. Cerebellar modulatory action on the vestibulo-trochlear pathway in the cat. Exp Brain Res. 1972;15(4):364–385. doi: 10.1007/BF00234124. [DOI] [PubMed] [Google Scholar]
- COHEN B., SUZUKI J. I., BENDER M. B. EYE MOVEMENTS FROM SEMICIRCULAR CANAL NERVE STIMULATION IN THE CAT. Ann Otol Rhinol Laryngol. 1964 Mar;73:153–169. doi: 10.1177/000348946407300116. [DOI] [PubMed] [Google Scholar]
- Carpenter M. B., Stein B. M., Peter P. Primary vestibulocerebellar fibers in the monkey: distribution of fibers arising from distinctive cell groups of the vestibular ganglia. Am J Anat. 1972 Oct;135(2):221–249. doi: 10.1002/aja.1001350209. [DOI] [PubMed] [Google Scholar]
- Fukuda J., Highstein S. M., Ito M. Cerebellar inhibitory control of the vestibulo-ocular reflex investigated in rabbit 3rd nucleus. Exp Brain Res. 1972 Apr 27;14(5):511–526. doi: 10.1007/BF00236593. [DOI] [PubMed] [Google Scholar]
- Highstein S. M., Ito M., Tsuchiya T. Synaptic linkage in the vestibulo-ocular reflex pathway of rabbit. Exp Brain Res. 1971;113(3):306–326. doi: 10.1007/BF00234952. [DOI] [PubMed] [Google Scholar]
- Highstein S. M. The organization of the vestibulo-oculomotor and trochlear reflex pathways in the rabbit. Exp Brain Res. 1973;17(3):285–300. doi: 10.1007/BF00234667. [DOI] [PubMed] [Google Scholar]
- Ito M., Highstein S. M., Fukuda J. Cerebellar inhibition of the vestibulo-ocular reflex in rabbit and cat and its blockage by picrotoxin. Brain Res. 1970 Feb 3;17(3):524–526. doi: 10.1016/0006-8993(70)90261-1. [DOI] [PubMed] [Google Scholar]
- Ito M. Neural design of the cerebellar motor control system. Brain Res. 1972 May 12;40(1):81–84. doi: 10.1016/0006-8993(72)90110-2. [DOI] [PubMed] [Google Scholar]
- Ito M., Nisimaru N., Yamamoto M. Inhibitory interaction between the vestibulo-ocular reflexes arising from semicircular canals of rabbits. Exp Brain Res. 1976 Aug 27;26(1):89–103. doi: 10.1007/BF00235251. [DOI] [PubMed] [Google Scholar]
- Ito M., Nisimaru N., Yamamoto M. Pathways for the vestibulo-ocular reflex excitation arising from semicircular canals of rabbits. Exp Brain Res. 1976 Jan 26;24:257–271. doi: 10.1007/BF00235014. [DOI] [PubMed] [Google Scholar]
- Ito M., Nisimaru N., Yamamoto M. Postsynaptic inhibition of oculomotor neurons involved in vestibulo-ocular reflexes arising from semicircular canals of rabbits. Exp Brain Res. 1976 Jan 26;24:273–283. doi: 10.1007/BF00235015. [DOI] [PubMed] [Google Scholar]
- Ito M., Nisimaru N., Yamamoto M. Specific neural connections for the cerebellar control of vestibulo-ocular reflexes. Brain Res. 1973 Sep 28;60(1):238–243. doi: 10.1016/0006-8993(73)90863-9. [DOI] [PubMed] [Google Scholar]
- Ito M., Obata K., Ochi R. The origin of cerebellar-induced inhibition of Deiters neurones. II. Temporal correlation between the trans-synaptic activation of Purkinje cells and the inhibition of Dieters neurones. Exp Brain Res. 1966;2(4):350–364. doi: 10.1007/BF00234780. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K., Kawai N., Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10(1):64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Kyojimaekawa, Toshiakitakeda, Maekawa K., Takeda T. Mossy fiber responses evoked in the cerebellar flocculus of rabbits by stimulation of the optic pathway. Brain Res. 1975 Nov 21;98(3):590–595. doi: 10.1016/0006-8993(75)90376-5. [DOI] [PubMed] [Google Scholar]
- Maekawa K., Simpson J. I. Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual system. J Neurophysiol. 1973 Jul;36(4):649–666. doi: 10.1152/jn.1973.36.4.649. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J. The elementary vestibulo-ocular reflex arc. J Neurophysiol. 1950 Nov;13(6):395–407. doi: 10.1152/jn.1950.13.6.395. [DOI] [PubMed] [Google Scholar]


