Abstract
Neuroglobin and cytoglobin are two recently discovered members of the vertebrate globin family. Both are intracellular proteins endowed with hexacoordinated heme-Fe atoms, in their ferrous and ferric forms, and display O2 affinities comparable with that of myoglobin. Neuroglobin, which is predominantly expressed in nerve cells, is thought to protect neurons from hypoxic–ischemic injury. It is of ancient evolutionary origin, and is homologous to nerve globins of invertebrates. Cytoglobin is expressed in many different tissues, although at varying levels. It shares common ancestry with myoglobin, and can be traced to early vertebrate evolution. The physiological roles of neuroglobin and cytoglobin are not completely understood. Although supplying cells with O2 is the likely function, it is also possible that both globins act as O2-consuming enzymes or as O2 sensors. Here, we review what is currently known about neuroglobin and cytoglobin in terms of their function, tissue distribution and relatedness to the well-known hemoglobin and myoglobin. Strikingly, the data reveal that O2 metabolism in cells is more complicated than was thought before, requiring unexpected O2-binding proteins with potentially novel functional features.
Introduction
Hemoglobins (and related heme proteins, generally referred to as 'globins') are small respiratory proteins that reversibly bind O2 by means of a Fe-containing porphyrin ring. Globins have been identified in many taxa, including bacteria, plants, fungi, and animals (Hardison, 1996, 1998). Most globins augment the O2 supply to the aerobic metabolism of the respiratory chain (Wittenberg, 1970, 1992; Antonini and Brunori, 1971; Perutz, 1979, 1990; Dickerson and Geis, 1983; Bunn and Forget, 1986; Brunori, 1999; Weber and Vinogradov, 2001; Merx et al., 2002), although they can also carry out enzymatic functions (Minning et al., 1999; Ascenzi et al., 2001).
Four types of globin, differing in structure, tissue distribution and likely in function, have been discovered in man and other vertebrates: hemoglobin, myoglobin, neuroglobin and cytoglobin. The cooperative heterotetrameric hemoglobin (Hb), located in the red blood cells, serves to transport O2 in the circulatory system. The monomeric myoglobin (Mb), present mainly in the cardiac and striated muscle, acts as an O2 buffer, facilitates O2 diffusion and is involved in the removal of toxic NO (Brunori, 2001; Flögel et al., 2001). Hb and Mb have been extremely well characterized in terms of structure, function and evolution (Antonini and Brunori, 1971; Perutz, 1979, 1990; Dickerson and Geis, 1983; Bunn and Forget, 1986; Wittenberg and Wittenberg, 1989; Wittenberg, 1992; Hardison, 1996, 1998; Brunori, 1999, 2001; Ascenzi et al., 2001; Merx et al., 2002). Neuroglobin (Ngb), which is expressed predominantly in the nervous system, and cytoglobin (Cygb, also named histoglobin; Trent and Hargrove, 2002), which is present in almost all tissue types, were identified and added to the globin superfamily recently (Burmester et al., 2000, 2002; Trent and Hargrove, 2002). This review focuses on the structural, expressional and evolutionary aspects that have emerged from the analysis of the vertebrate Ngb and Cygb, and from their comparison to the well-known Hb and Mb, thereby providing new perspectives on globin family diversity in vertebrates.
Structural bases of globin action
It has long been known that Hb and Mb tertiary structure is based on a seven/eight-helix arrangement, known as the 'globin fold' (Kendrew, 1960; Perutz, 1979). The main helices within the globin fold are organized into a two-layer structure, recognized as a 'three-over-three' α-helical sandwich (Holm and Sander, 1993; Figure 1A).
Figure 1.
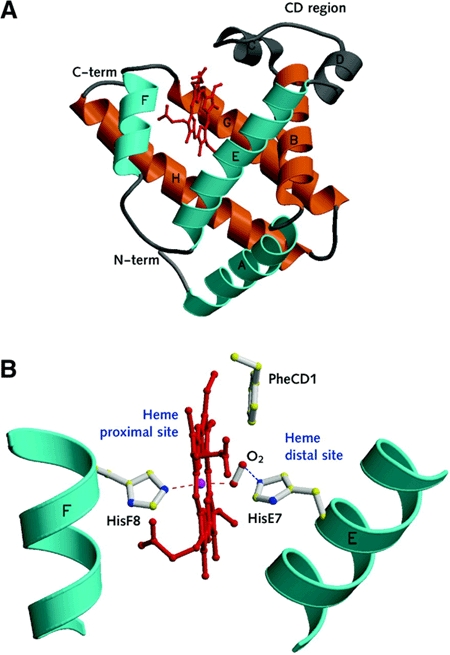
The classical globin fold. (A) A ribbon view of the typical mammalian globin fold: the 'three-over-three' α-helical sandwich is highlighted in two colours and the helices are labelled according to the conventional globin nomenclature (A, B, C.....H, starting from the N-terminus). Within each helix (as defined in sperm whale Mb, the prototype monomeric globin structure shown in the figure) residues are assigned sequential numbering. Thus, residue E7 occupies the seventh position within the E helix of the reference globin fold. To avoid discrepancies related to different protein lengths, globin amino acid sequences and structures are compared using such defined topological sites as reference (Perutz, 1979; Kapp et al., 1995). (B) A view of the heme proximal and distal sites, defined by E- and F-helices, together with the key residues PheCD1, HisE7 and HisF8 and the heme O2 ligand. The Fe coordination bonds with the proximal HisF8 residue and the O2 ligand are indicated by red dashed lines, while the hydrogen bond between O2 and the distal HisE7 residue is indicated in blue. Both figures are drawn with MOLSCRIPT (Kraulis, 1991).
To support O2 binding, the heme group (Fe-protoporphyrin IX) is hosted in a deep protein crevice (or pocket), defined sideways by the E- and F-helices (distal and proximal to the heme, respectively), and by the G- and H-helices at the dead end of the pocket (Figure 1). The heme is held in place by several non-covalent interactions with surrounding protein residues, and by a coordination bond connecting the heme-Fe atom to the 'proximal His', a truly invariant residue among globin family members, at the F8 site (Figure 1B). Thus, the heme-Fe atom is 'pentacoordinated' by four pyrrole N atoms (within the heme plane) and the proximal His NE2 atom (at the axial fifth coordination position).
Binding of O2, in Mb and Hb, occurs on the distal side of a pentacoordinated heme, where O2 establishes a sixth coordination bond to the heme Fe; the binding of O2 is generally stabilized by interaction(s) with distal residues. Often, in vertebrate globins, the main O2 stabilizing interaction is provided by a hydrogen bond donated by residue HisE7, the 'distal His' (Figure 1B). The nature and location of specific residues at the distal and proximal sites affect the heme:ligand association and dissociation kinetics to different degrees, thus defining the affinity of globin for a given biatomic species (O2, but also CO and NO). Heme hexacoordination, where the distal HisE7 residue is directly coordinated to the heme-Fe atom, is uncommon in vertebrates. In humans, hexacoordinated Hb is observed only under pathological conditions (Bunn and Forget, 1986), although it is present in some plants and bacteria under normal conditions (Hargrove et al., 2000).
Neuroglobin throughout our brain
Ngb was initially identified in the databases of uncharacterized cDNAs (expressed sequence tags, ESTs) from mouse and human brain (Burmester et al., 2000). Since then, Ngb sequences have also been recognized in rat, pufferfish and zebrafish (Awenius et al., 2001; Zhang et al., 2002), suggesting that this gene is present in a broad range of vertebrate species. Prominent Ngb mRNA and protein expression was detected in the human, mouse and rat brain. Using mRNA in situ hybridization, Ngb expression was observed in most neuronal cells of the central and peripheral nervous systems. In addition to being cell-type specific, Ngb expression also appears to vary by region in the human brain, with mRNA levels being highest in the subthalamic nucleus and lowest in the hippocampus and cerebellum. Ngb is also found in non-neural cells of the endocrine system such as the pituitary gland, the adrenal gland and the testis (Burmester et al., 2000; Reuss et al., 2002; Zhang et al., 2002).
Comparison of Ngb with vertebrate Mb and Hb sequences shows only minor similarity at the amino acid level (<25% identity; Figure 2). The structures of the mammalian and fish Ngb genes differ from those of Mbs and Hbs in that Ngbs have four exons and three introns in their coding regions, whereas the Hb and Mb genes carry only three exons and two introns (Figure 3). Shared introns are located at the conserved positions termed B12.2 (i.e. between codon positions 2 and 3 of the 12th amino acid of the globin B-helix) and G7.0. The extra intron found in the mammalian and fish Ngb genes is positioned at E11.0, and may have been acquired in the early evolution of this gene (Burmester et al., 2000).
Figure 2.
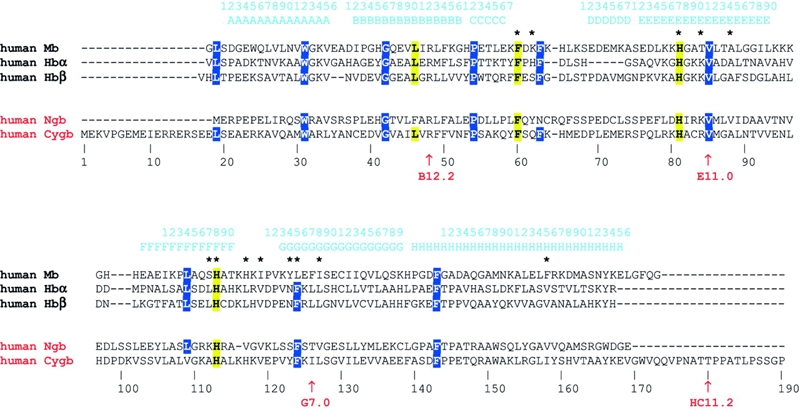
Multiple amino acid sequence alignment of human Mb, Hb (α and β chains), Ngb and Cygb. The stretch of sequence encoding α-helices and the globin topological numbering (referred to human Mb) are shown above the sequence. Key distal sites and heme binding residues are highlighted in yellow, and structurally relevant residues are marked in blue. The asterisks identify residues in contact with the heme in human Mb. Residue numbering refers to human Cygb. The intron positions are marked by arrows, and labeled below the aligned sequences.
Figure 3.
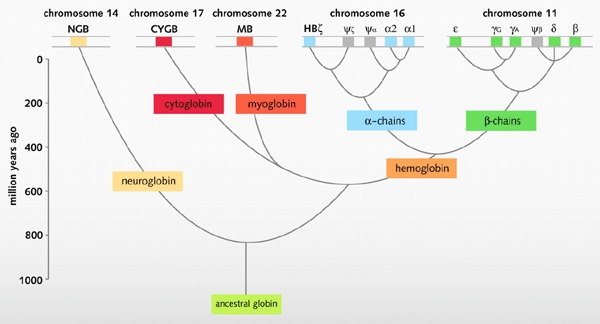
An evolutionary model of human globins. The different locations of globin genes in human chromosomes are reported at the top of the figure, distinguishing between the functional genes (in color) and the pseudogenes (in grey). Figure modified from Burmester et al. (2002).
Ngb is also similar to the nerve-specific globin of the annelid Aphrodite aculeata (27% residue identities; Dewilde et al., 1996), and phylogenetic analyses show that vertebrate Ngb is homologous to invertebrate nerve globins (Burmester et al., 2000), suggesting a common ancient evolutionary origin of these proteins. It is thus conceivable that Ngb-like globins are widely distributed in the nervous systems of many animal phyla.
Sequence conservation among the Ngbs of different species is much higher than that between the orthologous Hbs and Mbs (Burmester et al., 2000). For example, human and mouse Ngb sequences, both comprising 151 amino acids, are 94% identical. The Ngb amino acid sequence (Figure 2) fits well into a globin alignment based on the conserved α-helices and on the structural motifs of the classical globin fold (Burmester et al., 2000; Awenius et al., 2001). Among the most evident conserved features, the distal and proximal His residues, as well as the Phe residue immediately following the C helix (PheCD1), are invariant in Hb, Mb and Ngb. Additionally, an overall conservation of several apolar residues that are expected to build the heme crevice suggests that vertebrate NGB displays the classical three-over-three α-helical sandwich fold characteristic of vertebrate globins. Crystals of human Ngb have recently been reported (Pesce et al., 2002) and the three-dimensional structure is expected to be solved soon.
Neuroglobin hexacoordinated heme and oxygen affinity
Spectroscopic studies have shown Ngb to be the first example of a hexacoordinated globin in vertebrates, where residue HisE7 is the sixth heme-Fe ligand both in the ferrous deoxygenated (Fe2+) and ferric (Fe3+) forms. O2 and CO displace the endogenous protein ligand, leading to the reversibly oxygenated and carbonylated derivatives, respectively. Kinetics and thermodynamics of O2 and CO binding to Ngb may be depicted by a minimum three-state mechanism (equation 1), encompassing the dissociation of the endogenous HisE7 ligand as the rate-limiting step, i.e. [koff(H)]:
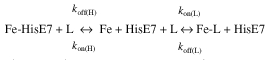 |
1 |
where L is either O2 or CO (Burmester et al., 2000; Couture et al., 2001; Dewilde et al., 2001; Trent et al., 2001).
Kinetic studies have shown high values of the second order association rate constant [kon(L)] and low values of the dissociation rate constant [koff(L)] for both O2 and CO, indicating a high intrinsic affinity (i.e. high K(L) values) for these ligands (Table 1 and Supplementary data, available at EMBO reports Online). Resonance Raman spectroscopic studies suggest that the high stability of the Ngb–O2 and −CO complexes reflect polar interactions between the heme-bound ligand and the protein matrix, stronger than those observed in Hb and Mb (Couture et al., 2001). Despite conflicting results on the value of the rate constant for HisE7 dissociation from the heme-Fe atom ([koff(H)] values range between 4.5 and 8.2 × 103 s−1), the values of the apparent equilibrium constant, K′(L), for oxygenation and carbonylation of Ngb appear to be lower than those of the intrinsic affinity constant, K(L) (see Supplementary data). However, because of competition with the endogenous HisE7 ligand for the sixth coordination position of the heme-Fe atom, the apparent O2 and CO affinities for Ngb (expressed by K′(L)) are similar to those observed for Mb and isolated α- and β-chains of Hb (see Supplementary data; Burmester et al., 2000; Dewilde et al., 2001; Trent et al., 2001).
Table 1.
Values of kinetic and thermodynamic parameters for the binding of o2 and of the endogenous ligand HisE7 to ferrous human NGB and CYGB
| Globin | Ligand | kona | koff (S−1) | K′(L)b(M−1) | P50′c(torr) | p50*d(torr) |
| NGBe | O2 | 2.5 × 108 M−1 s−1 | 0.80 | 7.0 × 105 | 0.79 | 1 |
| NGBf | O2 | 1.3 × 108 M−1 S−1 | 0.30 | 2.0 × 108 | 2.8 × 10−3 | – |
| CYGBg | O2 | 3.0 × 107 M−1 s−1 | 0.35 | 1.0 × 106 | 0.55 | – |
| NGBe | HisE7 | 2.0 × 103 s−1 | 4.5 | – | – | – |
| NGBf | HisE7 | 9.8 × 103 s−1 | 8.2 × 103 | – | – | – |
| CYGBg | HisE7 | 4.3 × 102 s−1 | 5 | – | – | – |
aValues of K′(on) differ in their units depending on whether the heme-Fe ligand is endogenous (i.e. HisE7, s−1) or exogenous (i.e. O2 or CO, M−1 s−1).
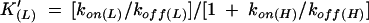 |
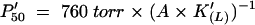 |
dThe value of P50* was determined from equilibrium experiments.
eData from Burmester et al. (2000); Dewilde et al. (2001).
Infrared spectroscopy reveals pronounced heterogeneity in the distal heme pocket, with multiple bound conformations and multiple CO docking sites among which the ligand can migrate. Geminate ligand binding (i.e. rapid rebinding of a heme-photodissociated CO molecule from the distal site, or a protein matrix location, to the heme-Fe atom) is extremely fast, suggesting a very small enthalpy barrier at the heme Fe. The distal pocket heterogeneity may arise from local conformational disorder in the CD region and the PheB10 residue (Kriegl et al., 2002), as seen in the tertiary structure of the hexacoordinated rice Hb (Hargrove et al., 2000).
The 'universal' cytoglobin
Like Ngb, Cygb was found in the EST databases of man, mouse and zebrafish (Burmester et al., 2002; Trent and Hargrove, 2002). The rat homologue had been previously identified in a proteomics approach that showed upregulation of a heme-containing protein in the stellate cells of fibrotic liver; the spotted protein was termed 'stellate cell activated protein', or STAP (Kawada et al., 2001). Cytoglobin was subsequently renamed in view of its expression in many human tissues (Burmester et al., 2002).
Phylogenetic analyses suggest that Cygb and Mb form a common clade within the tree of the vertebrate globins (Burmester et al., 2002), these two genes having separated from one another more than 450 million years ago. This notion is supported by the observation that the chromosomal regions encompassing the human CYGB (17q25) and MB genes (22q12) (Figure 3) represent long paralogous stretches of genomic DNA, which are thought to have originated by an ancient duplication event (McLysaght et al., 2002). In this evolutionary scenario, Mb would be a musclespecific version of an intracellular globin that features broad tissue distribution and general function.
All Cygb genes described so far contain the globin-typical introns at positions B12.2 and G7.0. In addition, the mammalian Cygb has an intron downstream of the H helix (HC11.2), close to the 3′ end of the coding region. The lengths of the mammalian (190 amino acids) and fish (174 amino acids) Cygbs exceed those of vertebrate Mbs and Hbs, which typically comprise 140 to 150 residues (Figure 2; Burmester et al., 2002; Trent and Hargrove, 2002). The differences relative to classical globins are mainly located in the unusually long N- and C-terminal regions, which occasionally have been observed in some invertebrate and primitive vertebrate respiratory globins (Bolognesi et al., 1997; Neuwald et al., 1997; Hardison, 1998). In the mammalian Cygb, the presence of the long N-terminus may be partly explained by a short duplication of the 5′ gene coding region; the C-terminal extension results mainly from the recruitment of an additional short 3′ exon (Burmester et al., 2002). This exon is not present in fish and seems to have been acquired later in tetrapod evolution.
Sequence conservation among the mammalian Cygbs is extremely high for vertebrate globins: mouse and human Cygb differ by only 4% of their amino acids. No extended sequence insertions appear to perturb the core region of Cygb, where structural features of the classical globin fold can be recognized. The key residues, PheCD1, HisE7 and HisF8, which are important for structure and function in respiratory globins, are conserved in Cygbs. As in the Ngb case, amino acid sequence analysis suggests that the classical three-over-three α-helical sandwich fold is likely adopted by Cygb (Figure 2; Burmester et al., 2002; Trent and Hargrove, 2002).
Cygb also belongs to the newly discovered class of vertebrate hexacoordinated Hbs (Burmester et al., 2002; Trent and Hargrove, 2002). Kinetics and thermodynamics for O2 and CO binding to Cygb follow the minimum threestate mechanism proposed for Ngb oxygenation and carbonylation (see equation 1). However, values of [kon(L)] for O2 and CO binding to Cygb are lower than those reported for ligand binding to Ngb, whereas the values of [koff(L)] for ligand dissociation from Ngb and Cygb are similar. Overall, this again results in ligand affinities (P50′ of 0.55 and 3.4 × 10−2 torr, for O2 and CO, respectively) that are comparable to those displayed by Mb (P50* of 0.65 and 1.7 × 10−2 torr, for O2 and CO, respectively; Table 1 and Supplementary data; Trent and Hargrove et al., 2002).
Possible function(s) of neuroglobin and cytoglobin
The physiological roles of pentacoordinated vertebrate globins are well defined. The transport of O2 by Hb, as well as the Mb-facilitated diffusion and storage of O2, are among the best characterized protein mechanisms (Wittenberg, 1970, 1992; Antonini and Brunori, 1971; Perutz, 1979, 1990; Dickerson and Geis, 1983; Bunn and Forget, 1986; Wittenberg and Wittenberg, 1989; Hardison, 1996, 1998; Brunori, 1999; Merx et al., 2002). However, the situation is less clear in the case of the less abundant hexacoordinated globins, Ngb and Cygb, and several functions are conceivable.
Ngb and Cygb may have a Mb-like function in O2 metabolism, facilitating O2 diffusion to the mitochondria (Burmester et al., 2000; Moens and Dewilde, 2000). In the case of Ngb, this hypothesis is supported by its ability to rescue neuronal cells from hypoxic–ischemic insults, and by its enhanced expression under hypoxic conditions in vitro as well as in focal cerebral ischemia in vivo (Sun et al., 2001). Moreover, the survival of neuronal cells in culture after hypoxia is reduced upon inhibition of Ngb expression with an antisense oligodeoxynucleotide, and enhanced by Ngb overexpression. The mechanism of hypoxic induction is still unclear, but several lines of evidence suggest that the stress response may be mediated by the hypoxia-inducible transcription factor HIF-1 (Sun et al., 2001). Also, the hypoxic induction of Ngb is blocked by an inhibitor of MEK (mitogen-activated protein kinase/extracellular signal-regulated kinase), and Ngb expression can be induced by heme, although heme availability and hypoxia-induced pathways are controlled through different mechanisms (Zhu et al., 2002). Values of the kinetic parameters for O2 binding (Table 1) and the local concentration of Ngb within neuronal and endocrine cells (Reuss et al., 2002) are in keeping with a Ngb role as O2 supplier.
A second role of Ngb and Cygb may be as NADH oxidases, which facilitate the glycolytic production of ATP under semi-anaerobic conditions. Such a function has been proposed for the hexacoordinated non-symbiotic Hbs of plants, e.g. maize (Sowa et al., 1998). Ngb and Cygb may also function as O2 sensors, participating in a signal transduction pathway that modulates the activities of regulatory proteins in response to changes in O2 concentration. Indeed, it has been suggested that large conformational changes occur in the heme pocket of hexacoordinated globins upon ligand binding (Hargrove et al., 2000; Kriegl et al., 2002) and these might very well trigger signals in hypothetical downstream regulators.
Finally, Ngb and Cygb may carry out yet undocumented enzymatic activities. In analogy with Mb (Ascenzi et al., 2001; Brunori, 2001; Flögel et al., 2001), flavohemoglobins (Liu et al., 2000), truncated Hbs (Ouellet et al., 2002) and Ascaris Hb (Minning et al., 1999), Ngb and Cygb might be involved in NO/O2 chemistry. However, no obvious correlation between NO production and Ngb expression has been observed at the cellular level (Reuss et al., 2002). Cygb also displays a catalase activity (Kawada et al., 2001), whose physiological relevance is, however, unclear since many different globins marginally support such an activity.
Conclusions
The functions of Hb and Mb in O2 supply are thought to be well understood, although recent investigations have shed light on surprising additional functions (Ascenzi et al., 2001; Brunori, 2001; Flögel et al., 2001). The recent discovery of two novel types of globins, Ngb and Cygb, displaying heme hexacoordination has a substantial impact on our understanding of O2 metabolism in man and other vertebrates. The vastly different expression patterns of the four globin types (Hb, Mb, Ngb and Cygb) strongly suggest diverse roles. Furthermore, it is conceivable that Ngb and Cygb will cast new light on the ancestral function of vertebrate globins in general (Cygb), and within the nervous system in particular (Ngb). They may also show the well-known Hb and Mb to be highly specialized heme-proteins that evolved in (in)vertebrates in response to the demands of the circulatory system and of muscle, respectively, thereby enabling these organisms to develop larger and more efficient body plans.
Supplementary Material
Values of kinetic and thermodynamic parameters for the binding of exogenous and endogenous ligands to ferrous human globins and mouse (m) Ngb.
Acknowledgments
We are grateful to Professor M. Brunori, University of Rome 'La Sapienza' for providing helpful criticism. The financial support from the National Research Council of Italy (Genetica Molecolare and Genomica Funzionale Projects), from the European Commission Project 'Neuroglobin', and from the Deutsche Forschungsgemeinschaft (Bu956/5, Ha2103/3-2), is fully acknowledged. S.D. is a post-doc of the FWO (Fund for Scientific Research-Flanders).
References
- Antonini E. and Brunori M. (1971) Hemoglobin and Myoglobin in their Reactions with Ligands. North Holland Publishing Co., Amsterdam, The Netherlands. [Google Scholar]
- Ascenzi P., Salvati L. and Brunori M. (2001) Does myoglobin protect Trypanosoma cruzi from the antiparasitic effects of nitric oxide? FEBS Lett., 501, 103–105. [DOI] [PubMed] [Google Scholar]
- Awenius C., Hankeln T. and Burmester T. (2001) Neuroglobins from the zebrafish Danio rerio and the pufferfish Tetraodon nigroviridis. Biochem. Biophys. Res. Commun., 287, 418–421. [DOI] [PubMed] [Google Scholar]
- Bolognesi M., Bordo D., Rizzi M., Tarricone C. and Ascenzi P. (1997) Nonvertebrate hemoglobins: structural bases for reactivity. Prog. Biophys. Mol. Biol., 68, 29–68. [DOI] [PubMed] [Google Scholar]
- Brunori M. (1999) Hemoglobin is an honorary enzyme. Trends Biochem. Sci., 24, 158–161. [DOI] [PubMed] [Google Scholar]
- Brunori M. (2001) Nitric oxide moves myoglobin centre stage. Trends Biochem. Sci., 26, 209–210. [DOI] [PubMed] [Google Scholar]
- Bunn H.F. and Forget B.G. (1986) Hemoglobin: Molecular, Genetic and Clinical Aspects. Saunders, W.B., Philadelphia, PA. [Google Scholar]
- Burmester T., Weich B., Reinhardt S. and Hankeln T. (2000) A vertebrate globin expressed in the brain. Nature, 407, 520–523. [DOI] [PubMed] [Google Scholar]
- Burmester T., Ebner B., Weich B. and Hankeln T. (2002) Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol. Biol. Evol., 19, 416–421. [DOI] [PubMed] [Google Scholar]
- Couture M., Burmester T., Hankeln T. and Rousseau D.L. (2001) The heme environment of mouse neuroglobin: evidence for the presence of two conformations of the heme pocket. J. Biol. Chem., 276, 36377–36382. [DOI] [PubMed] [Google Scholar]
- Dewilde S., Blaxter M., Van Hauwaert M.L., Vanfleteren J., Esmans E.L., Marden M., Griffon N. and Moens L. (1996) Globin and globin gene structure of the nerve myoglobin of Aphrodite aculeata. J. Biol. Chem., 271, 19865–19870. [DOI] [PubMed] [Google Scholar]
- Dewilde S., Kiger L., Burmester T., Hankeln T., Baudin-Creuza V., Aerts T., Marden M.C., Caubergs R. and Moens L. (2001) Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J. Biol. Chem., 276, 38949–38955. [DOI] [PubMed] [Google Scholar]
- Dickerson R.E. and Geis I. (1983) Hemoglobin: Structure, Function, Evolution, and Pathology. Benjamin/Cummings, Menlo Park, CA. [Google Scholar]
- Flögel U., Merx M.W., Gödecke A., Decking U.K. and Schrader J. (2001) Myoglobin: A scavenger of bioactive NO. Proc. Natl Acad. Sci. USA, 98, 735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison R.C. (1996) A brief history of hemoglobins: plant, animal, protist, and bacteria. Proc. Natl Acad. Sci. USA, 93, 5675–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison R.C. (1998) Hemoglobins from bacteria to man: evolution of different patterns of gene expression. J. Exp. Biol., 201, 1099–1117. [DOI] [PubMed] [Google Scholar]
- Hargrove M.S., Brucker E.A., Stec B., Sarath G., Arredondo-Peter R., Klucas R.V., Olson J.S. and Phillips G.N. Jr (2000) Crystal structure of a nonsymbiotic plant hemoglobin. Structure, 8, 1005–1014. [DOI] [PubMed] [Google Scholar]
- Holm L. and Sander C. (1993) Structural alignment of globins, phycocyanins and colicin A. FEBS Lett., 315, 301–306. [DOI] [PubMed] [Google Scholar]
- Kapp O.H., Moens L., Vanfleteren J., Trotman C.N.A., Suzuki T. and Vinogradov S.N. (1995) Alignment of 700 globin sequences: extent of amino acid substitution and its correlation with variation in volume. Protein Sci., 4, 2179–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada N., Kristensen D.B., Asahina K., Nakatani K., Minamiyama Y., Seki S. and Yoshizato K. (2001) Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J. Biol. Chem., 276, 25318–25323. [DOI] [PubMed] [Google Scholar]
- Kendrew J.C., Dickerson R.E., Strandberg B.E., Hart R.G., Davies D.R., Phillips D.C. and Shore V.C. (1960) Structure of myoglobin. A three dimensional Fourier synthesis at 2 Å resolution. Nature, 185, 422–427. [DOI] [PubMed] [Google Scholar]
- Kraulis P.J. (1991) MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr., 24, 946–950. [Google Scholar]
- Kriegl J.M., Bhattacharyya A.J., Nienhaus K., Deng P., Minkow O. and Nienhaus G.U. (2002) Ligand binding and protein dynamics in neuroglobin. Proc. Natl Acad. Sci. USA, 99, 7992–7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Zeng M., Hausladen A., Heitman J. and Stamler J.S. (2000) Protection from nitrosative stress by yeast flavohemoglobin. Proc. Natl Acad. Sci. USA, 97, 4672–4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLysaght A., Hokamp K. and Wolfe K.H. (2002) Extensive genomic duplication during early chordate evolution. Nat. Genet., 31, 200–204. [DOI] [PubMed] [Google Scholar]
- Merx M.W., Flögel U., Stumpe T., Gödecke A., Decking U.K. and Schrader J. (2002) Myoglobin facilitates oxygen diffusion. FASEB J., 15, 1077–1079. [DOI] [PubMed] [Google Scholar]
- Minning D.M., Gow A.J., Bonaventura J., Braun R., Dewhirst M., Goldberg D.E. and Stamler J.S. (1999) Ascaris haemoglobin is a nitric oxide-activated 'deoxygenase'. Nature, 401, 497–502. [DOI] [PubMed] [Google Scholar]
- Moens L. and Dewilde S. (2000) Globins in the brain. Nature, 407, 461–462. [DOI] [PubMed] [Google Scholar]
- Neuwald A.F., Liu J.S., Lipman D.J. and Lawrence C.E. (1997) Extracting protein alignment models from the sequence database. Nucleic Acids Res., 25, 1665–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet H., Ouellet Y., Richard C., Labarre M., Wittenberg B., Wittenberg J. and Guertin M. (2002) Truncated hemoglobin HbN protects Mycobacterium bovis from nitric oxide. Proc. Natl Acad. Sci. USA, 99, 5902–5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M.F. (1979) Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu. Rev. Biochem., 48, 327–386. [DOI] [PubMed] [Google Scholar]
- Perutz M.F. (1990) Mechanisms regulating the reactions of human hemoglobin with oxygen and carbon monoxide. Annu. Rev. Physiol., 52, 1–25. [DOI] [PubMed] [Google Scholar]
- Pesce A., Nardini M., Dewilde S., Ascenzi P., Burmester T., Hankeln T., Moens L. and Bolognesi M. (2002) Human neuroglobin: crystals and preliminary X-ray diffraction analysis. Acta Crystallogr. D, 58, 1848–1850. [DOI] [PubMed] [Google Scholar]
- Reuss S., Saaler-Reinhardt S., Weich B., Wystub S., Reuss M., Burmester T. and Hankeln T. (2002) Expression analysis of neuroglobin mRNA in rodent tissues. Neurosci., in press. [DOI] [PubMed] [Google Scholar]
- Sowa A.W., Duff S.M.G, Guy P.A. and Hill R.D. (1998) Altering hemoglobin levels changes energy status in maize cells under hypoxia. Proc. Natl Acad. Sci. USA, 95, 10317–10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer B.A., Sligar S.G., Olson J.S. and Phillips G.N. Jr (1994) Mechanisms of ligand recognition in myoglobin. Chem. Rev., 94, 699–714. [Google Scholar]
- Sun Y., Jin K., Mao X.O., Zhu Y. and Greenberg D.A. (2001) Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc. Natl Acad. Sci. USA, 98, 15306–15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent J.T. III and Hargrove M.S. (2002) A ubiquitously expressed human hexacoordinate hemoglobin. J. Biol. Chem., 277, 19538–19545. [DOI] [PubMed] [Google Scholar]
- Trent J.T. III, Watts R.A. and Hargrove M.S. (2001) Human neuroglobin, a hexacoordinate hemoglobin that reversibly binds oxygen. J. Biol. Chem., 276, 30106–30110. [DOI] [PubMed] [Google Scholar]
- Weber R. and Vinogradov S.N. (2001) Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol. Rev., 81, 569–628. [DOI] [PubMed] [Google Scholar]
- Whitaker T.L., Berry M.B., Ho E.L., Hargrove M.S., Phillips G.N. Jr, Komiyama N.H., Nagai K. and Olson J.S. (1995) The D-helix in myoglobin and in the β subunit of hemoglobin is required for the retention of heme. Biochemistry, 34, 8221–8226. [DOI] [PubMed] [Google Scholar]
- Wittenberg B.A. and Wittenberg J.B. (1989) Transport of oxygen in muscle. Annu. Rev. Physiol., 51, 857–878. [DOI] [PubMed] [Google Scholar]
- Wittenberg J.B. (1970) Myoglobin-facilitated oxygen diffusion: role of myoglobin in oxygen entry into muscle. Physiol. Rev., 50, 559–636. [DOI] [PubMed] [Google Scholar]
- Wittenberg J.B. (1992) Functions of cytoplasmatic hemoglobins and myohemerythrin. Adv. Comp. Environ. Physiol., 13, 60–85. [Google Scholar]
- Zhang C. et al. (2002) Full-length cDNA cloning of human neuroglobin and tissue expression of rat neuroglobin. Biochem. Biophys. Res. Commun., 290, 1411–1419. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Sun Y., Jin K. and Greenberg D.A. (2002) Hemin induces neuroglobin expression in neural cells. Blood, 100, 2494–2498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values of kinetic and thermodynamic parameters for the binding of exogenous and endogenous ligands to ferrous human globins and mouse (m) Ngb.


