Abstract
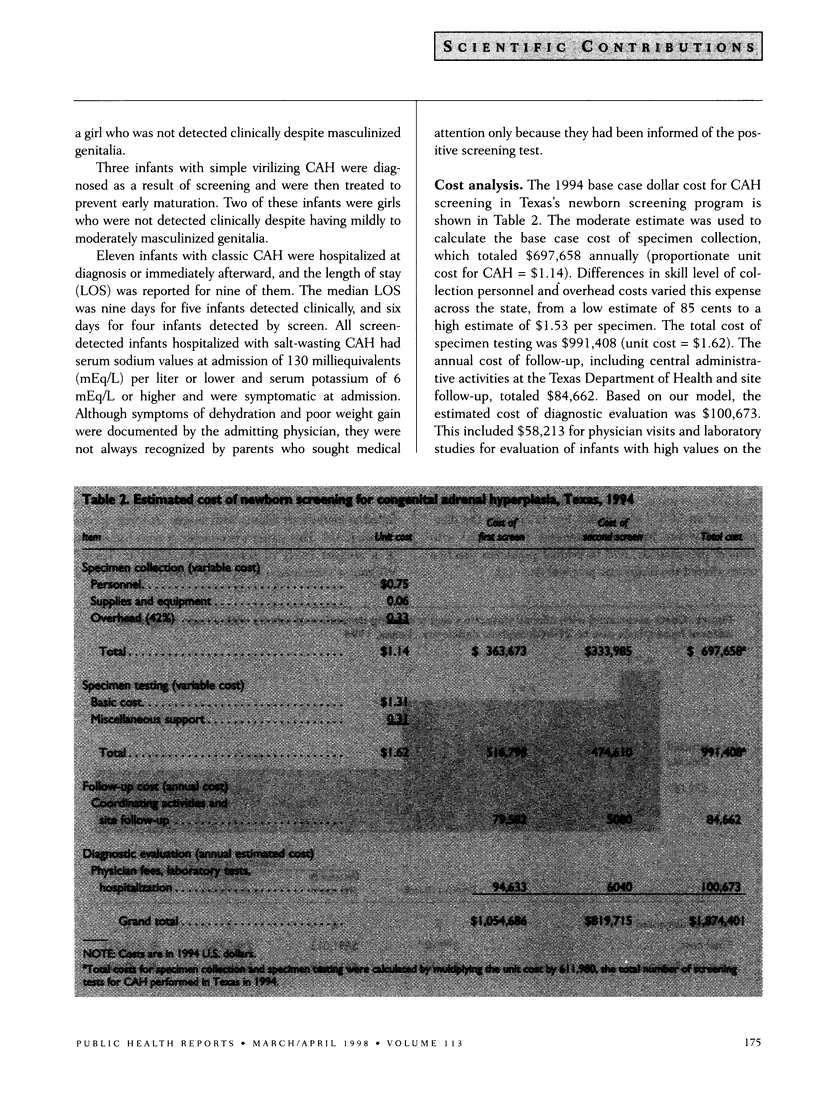
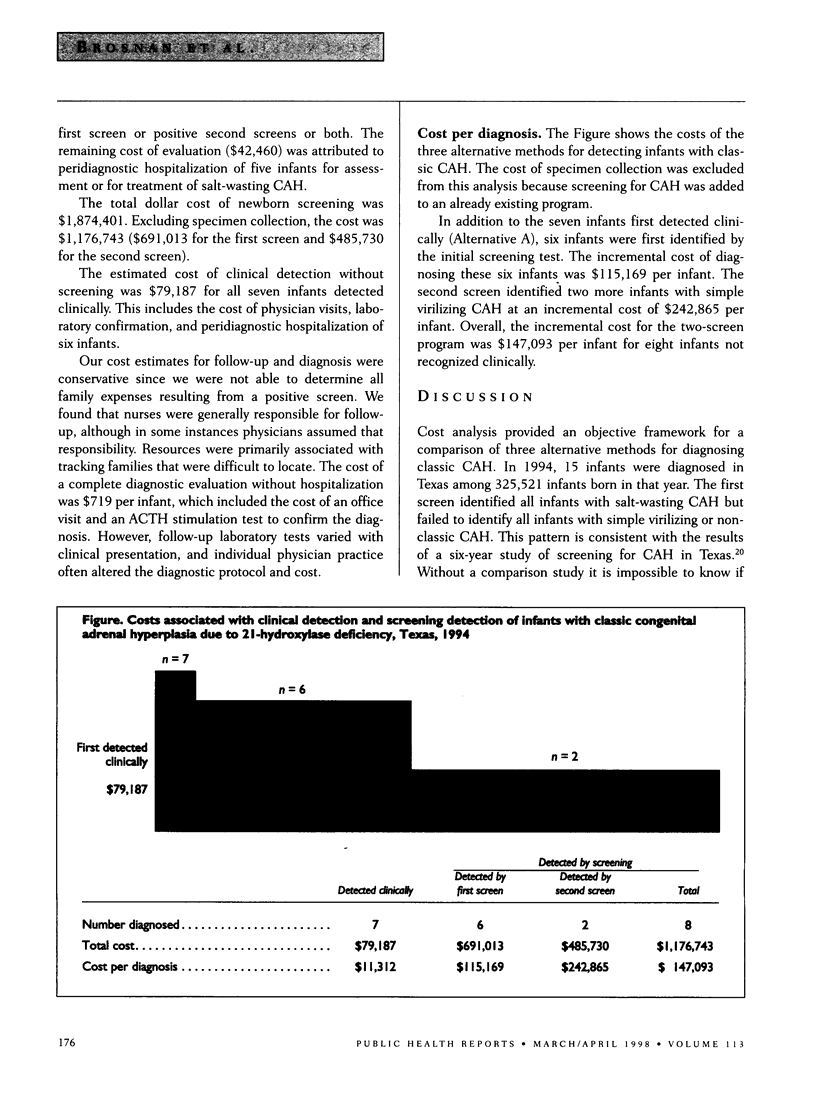
OBJECTIVE: Texas mandates a two-test newborn screening program for congenital adrenal hyperplasia (CAH): one test at birth and a second test at approximately one to two weeks after birth. The authors compared the dollar cost of detecting infants with CAH clinically and through the screening program. METHODS: The authors estimated the costs of screening newborns in 1994 for CAH, including resources used by the Texas Department of Health and the broader cost to society. RESULTS: Fifteen infants with classic CAH were diagnosed in Texas in 1994 among 325,521 infants born (1:21,701 cumulative incidence). Seven infants were detected clinically and the others were detected through screening, six on the first screen and two on the second screen. The first screen identified all previously undetected infants with severe salt-wasting CAH. The cumulative cost to diagnose the seven infants detected clinically was $79,187. The incremental costs for the screening program were $115,169 per additional infant diagnosed through the first screen and $242,865 per additional infant diagnosed through the second screen. CONCLUSIONS: If the goal is early diagnosis of infants with the severe salt-wasting form of CAH, a single screen is effective. If the goal is to detect infants with the simple virilizing form of the disorder who may benefit from early treatment, the second screen is necessary, but it is not as cost-effective as the first screen.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. B., Hoffman G. L., Fitzpatrick P., Laessig R., Maby S., Slyper A. Improved precision of newborn screening for congenital adrenal hyperplasia using weight-adjusted criteria for 17-hydroxyprogesterone levels. J Pediatr. 1997 Jan;130(1):128–133. doi: 10.1016/s0022-3476(97)70321-4. [DOI] [PubMed] [Google Scholar]
- Balsamo A., Cacciari E., Piazzi S., Cassio A., Bozza D., Pirazzoli P., Zappulla F. Congenital adrenal hyperplasia: neonatal mass screening compared with clinical diagnosis only in the Emilia-Romagna region of Italy, 1980-1995. Pediatrics. 1996 Sep;98(3 Pt 1):362–367. [PubMed] [Google Scholar]
- Barden H. S., Kessel R., Schuett V. E. The costs and benefits of screening for PKU in Wisconsin. Soc Biol. 1984 Spring-Summer;31(1-2):1–17. doi: 10.1080/19485565.1984.9988558. [DOI] [PubMed] [Google Scholar]
- Barden H. S., Kessel R. The costs and benefits of screening for congenital hypothyroidism in Wisconsin. Soc Biol. 1984 Fall-Winter;31(3-4):185–200. doi: 10.1080/19485565.1984.9988574. [DOI] [PubMed] [Google Scholar]
- Briggs A., Sculpher M., Buxton M. Uncertainty in the economic evaluation of health care technologies: the role of sensitivity analysis. Health Econ. 1994 Mar-Apr;3(2):95–104. doi: 10.1002/hec.4730030206. [DOI] [PubMed] [Google Scholar]
- Finkler S. A. The distinction between cost and charges. Ann Intern Med. 1982 Jan;96(1):102–109. doi: 10.7326/0003-4819-96-1-102. [DOI] [PubMed] [Google Scholar]
- Gessner B. D., Teutsch S. M., Shaffer P. A. A cost-effectiveness evaluation of newborn hemoglobinopathy screening from the perspective of state health care systems. Early Hum Dev. 1996 Jul 19;45(3):257–275. doi: 10.1016/0378-3782(96)01761-6. [DOI] [PubMed] [Google Scholar]
- Owerbach D., Crawford Y. M., Draznin M. B. Direct analysis of CYP21B genes in 21-hydroxylase deficiency using polymerase chain reaction amplification. Mol Endocrinol. 1990 Jan;4(1):125–131. doi: 10.1210/mend-4-1-125. [DOI] [PubMed] [Google Scholar]
- Pang S., Hotchkiss J., Drash A. L., Levine L. S., New M. I. Microfilter paper method for 17 alpha-hydroxyprogesterone radioimmunoassay: its application for rapid screening for congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1977 Nov;45(5):1003–1008. doi: 10.1210/jcem-45-5-1003. [DOI] [PubMed] [Google Scholar]
- Sorenson J. R., Levy H. L., Mangione T. W., Sepe S. J. Parental response to repeat testing of infants with 'false-positive' results in a newborn screening program. Pediatrics. 1984 Feb;73(2):183–187. [PubMed] [Google Scholar]
- Tluczek A., Mischler E. H., Farrell P. M., Fost N., Peterson N. M., Carey P., Bruns W. T., McCarthy C. Parents' knowledge of neonatal screening and response to false-positive cystic fibrosis testing. J Dev Behav Pediatr. 1992 Jun;13(3):181–186. [PubMed] [Google Scholar]


