Abstract
In Drosophila, stresses such as x-irradiation or severe heat shock can cause most epidermal cells to die by apoptosis. Yet, the remaining cells recover from such assaults and form normal adult structures, indicating that they undergo extra growth to replace the lost cells. Recent studies of cells in which the cell death pathway is blocked by expression of the caspase inhibitor P35 have raised the possibility that dying cells normally regulate this compensatory growth by serving as transient sources of mitogenic signals. Caspase-inhibited cells that initiate apoptosis do not die. Instead, they persist in an “undead” state in which they ectopically express the signaling genes decapentaplegic (dpp) and wingless (wg) and induce abnormal growth and proliferation of surrounding tissue. Here, using mutations to abolish Dpp and/or Wg signaling by such undead cells, we show that Dpp and Wg constitute opposing stimulatory and inhibitory signals that regulate this excess growth and proliferation. Strikingly, we also found that, when Wg signaling is blocked, unfettered Dpp signaling by undead cells transforms their neighbors into neoplastic tumors, provided that caspase activity is also blocked in the responding cells. This phenomenon may provide a paradigm for the formation of neoplastic tumors in mammalian tissues that are defective in executing the cell death pathway. Specifically, we suggest that stress events (exposure to chemical mutagens, viral infection, or irradiation) that initiate apoptosis in such tissues generate undead cells, and that imbalances in growth regulatory signals sent by these cells can induce the oncogenic transformation of neighboring cells.
Keywords: apoptosis, caspase inhibition, Dpp signaling, tumor transformation, Wg signaling
Programmed cell death (apoptosis) plays an important role in removing damaged or inappropriately specified cells during development (1–3). It is also involved in morphogenetic processes such as sculpting of organs or deleting unwanted cells or structures (reviewed in ref. 4). Apoptosis has to be strictly regulated: too much or too little can cause severe developmental anomalies or disease (5). In particular, one of the hallmarks of cancer is the ability of tumorous cells to evade apoptosis (5).
The executioner role in apoptosis is played by caspases, a family of cysteine proteases that cleave diverse substrates and destroy cell structure and integrity (see ref. 6 for review). Caspases are present in all cells but are kept inactive by inhibitor of apoptosis proteins (IAPs), which bind to them and prevent their function (7, 8). In Drosophila, diverse cell death stimuli, including Hox gene products (9), hormones (10), and environmental stresses (11, 12), trigger apoptosis by inducing the proapoptotic genes reaper (rpr), head involution defective (hid), grim, and sickle. These genes encode a family of related proteins that bind to and inactivate the Drosophila IAP DIAP1, thus releasing the caspases from repression (13, 14).
Apoptosis plays a significant role in a number of processes during Drosophila development, such as salivary gland histolysis (15), sculpting the larval head (9), and the removal of excess ommatidial cells (16). However, apoptosis plays little if any role during the normal development of other tissues, such as the wing imaginal disk (17, 18). Nevertheless, stress treatments like x-irradiation or heat shock induce high levels of apoptosis that can eliminate more than half of the cells in the wing disk (18, 19). Yet, despite such high levels of cell mortality, the wing disk is still capable of giving rise to adult structures of normal size and shape. This result indicates that surviving cells undergo additional rounds of proliferation to compensate for the lost cells.
Recent experiments have suggested that transient signals sent by dying cells may normally regulate this phenomenon of compensatory proliferation (18, 20, 21). By keeping apoptotic cells alive with the caspase inhibitor P35 (22), it has been observed that they induce excess growth and proliferation in neighboring nonapoptotic cells. Moreover, the signaling genes decapentaplegic (dpp) and wingless (wg) are ectopically activated in such “undead” cells, raising the possibility that the secreted Dpp and Wg proteins regulate the extra growth and proliferation. Indeed, it has been proposed that Wg signaling is necessary and, at least in some cells, also sufficient for mitogenesis by undead cells (20). The role of the Dpp pathway has not been tested, but its normal function promoting growth in the wing disk (23–25) suggests that it plays a corresponding role in the induction of extra growth by undead cells.
In this article, we investigate the properties of caspase-inhibited apoptotic cells and the role of the Wg and of Dpp signals in the mitogenic activity of these cells and in the developmental alterations they produce in surrounding tissues. We find that apoptotic stimuli such as x-rays or heat shock can cause a permanent activation of the apoptotic machinery in caspase-inhibited cells, creating clusters of undead cells that persist and induce extra growth and proliferation in surrounding tissue. By removing dpp, wg, or both gene functions in such undead cells, we show that Dpp and Wg play opposing stimulatory and inhibitory roles in controlling the response of the surrounding cells: Dpp is necessary for much or all of the mitogenic effect of undead cells, whereas Wg seems to function to prevent an excessive proliferative response. Remarkably, we find that, in the absence of Wg signaling, unfettered Dpp signaling by undead cells induces the surrounding tissue to overgrow dramatically and to acquire neoplastic, tumor-like properties that allow them to outcompete and eliminate the remaining, wild-type cells in the disk.
Materials and Methods
dpp and wg Mutant Genotypes. wg activity was abolished by using the null allele wgCX4; dpp activity was abolished by using either the dppD12 allele, which eliminates dpp activity in the wing disk, or the null allele dppH61 allele in the presence of the TnJA1 mini-dpp transgene (which acts only during embryogenesis to rescue the lethality that would otherwise occur from haploinsufficiency of dpp). Both wg and dpp are located on the left arm of chromosome II, facilitating the analysis of single and double mutant genotypes.
Generation of Marked Clones. Clones of wild type, dpp–, wg–, and dpp– wg– cells expressing the viral caspase inhibitor P35 and marked by the coexpression of GFP, were generated by Flp-mediated mitotic recombination by using the mosaic analysis with a repressible cell marker (MARCM) technique (26). Larvae of the appropriate genotype (e.g., for dpp– wg– P35 clones: hsp70-flp/tub-Gal4; dppD12 wgCX4 FRT40/tub-Gal80 FRT40; UAS-GFP/UAS-p35 or hsp70-flp tub-Gal4 UAS-GFP/TnJA1; dppH61 wgCX4 FRT39/tub-Gal80 FRT39; UAS-P35/+) were heat shocked (37°C, 15 min) during the first or second instar to generate cells that are homozygous for the given dpp or wg genotype (e.g., dpp– wg–) and lack the tub-Gal80 transgene (indistinguishable results were obtained using either the FRT39 or FRT40 system). To generate clones of wg– P35 clones that coexpress rpr, a UAS-rpr transgene was used in cis with UAS-P35. The tub-Gal4 and tub-Gal80 transgenes express, respectively, the Gal4 transcription factor and its repressor Gal80, under the control of the promoter of the tubulinα1 gene, which is expressed in all imaginal disk cells; Gal80 activity dissipates within three to five cell divisions after clone induction, allowing Gal4 to drive coexpression of the UAS-P35 and UAS-GFP transgenes. In these experiments, apoptosis was induced by x-rays 24 h after clone induction, shortly before Gal80 activity dissipates.
Apoptosis Treatments. Programmed cell death was induced by either x-irradiation (1,500 R) or by severe heat shock (2.5 h at 37°C) in second or early third instar larvae. The larvae were allowed to develop subsequently until the end of larval development (72 or 96 h after the stress treatment), and the discs were assayed by using standard techniques for visualizing protein expression, BrdUrd incorporation, and caspase activity. Both stress treatments induce high levels of cell mortality in the imaginal discs (up to 70%); nevertheless, almost all larvae survive and give rise to flies of normal size and shape (18, 19). Apoptosis occurs only rarely during normal wing disk development (17, 18); therefore, the preponderance of apoptotic events observed after x-irradiation or heat shock is clearly induced in response to the stress treatment.
Immunological Reagents. Activated Drosophila effector caspase Drice was detected by using crossreacting rabbit α-human Caspase 3 antisera (26). Other antisera used were directed against Wg (Hybridoma Bank, University of Iowa, Iowa City), β-Gal (Cappel), phosphohistone 3 (Cell Signaling Technology, Beverly, MA), dMyc (27), Hid (20), and Dronc (20).
Results
Induction of Undead Cells Within Clones of Caspase-Inhibited Cells by X-Irradiation. To test whether Dpp and/or Wg function as mitogenic signals secreted by undead cells, we used x-rays (1,500 R) to induce apoptosis in preexisting clones of cells that constitutively express the caspase inhibitor P35 as well as GFP but are otherwise wild-type, or are mutant for dpp, for wg, or for both wg and dpp (henceforth referred to as P35, dpp– P35, wg– P35, and dpp– wg– P35). We then assayed whether the resulting undead cells are able to induce excess growth or proliferation of surrounding cells.
After x-irradiation, clones of all four genotypes (P35, dpp– P35, wg– P35, and dpp– wg– P35) contain clusters of distinctive cells, which may comprise between 20% and 70% of the clone: unlike the remaining cells within such clones, these cells express the proapoptotic gene hid and typically show elevated levels of the active form of the caspase Drice (Fig. 1 a and b). In P35, but otherwise wild-type clones, many of these same cells also ectopically express wg (Fig. 1a) and dpp (18, 20). Such cell clusters persist in clones of all four genotypes for the remainder of development. By contrast, no such cells persist outside of the clones, in tissue in which P35 is not expressed (Fig. 1), presumably because cells that enter apoptosis without P35 protection are rapidly eliminated. Thus, we infer that regardless of their dpp or wg genotype, irradiated P35 expressing clones contain a mixture of two types of cells: “live” cells, which never initiated apoptosis, and undead cells, which initiated, but failed to complete, the apoptotic program.
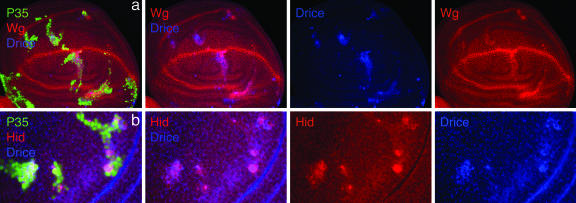
Fig. 1.
Undead cells in x-irradiated P35-expressing clones. (a) Wing disk containing several P35-expressing clones marked by coexpression of GFP (green) and stained for activated Drice (blue) and Wg (red); note that activated Drice is present in some cells within the P35 clones and that Wg is present in most or all of these Drice-positive cells. (b) Portion of a disk containing P35-expressing clones (green) stained for Hid (red) and active Drice (blue); note that some of the cells within each clone costain for Hid and Drice. For both a and b, the discs were assayed 72 h after the irradiation, indicating that undead Hid-expressing and active Drice cells within the clones retain their apoptotic nature long after the cessation of the stimulus; by contrast, no cells expressing Hid or active Drice persist outside of the clones.
The induction of such undead cells that show ectopic hid expression and Drice activity does not seem to be a specific consequence of genetic changes caused by x-irradiation, but rather a general, epigenetic response to cellular stress. In particular, we obtained similar results when apoptosis was stimulated by other means, such as severe heat shock. hh-Gal4>UAS-p35 larvae, which express P35 in all posterior compartments cells, were heat shocked (2.5 h, 37°C) during the first larval instar, and their wing discs were fixed for staining 4 days later, at the end of the third larval instar. Examination of these discs revealed clusters of undead cells that persistently express hid as well as the activated forms of Drice and the apical caspase Dronc in the posterior compartment (Fig. 5, which is published as supporting information on the PNAS web site).
Growth Stimulatory Activity of Undead Cells Depends on Dpp. As reported (18), many of the x-irradiated P35 clones that are otherwise wild-type show evidence of increased proliferation, as indicated by local elevations of BrdUrd incorporation and anti-phosphohistone 3 (PH3) staining. These local elevations are restricted to live cells within and around the P35 clones (Fig. 2a). However, undead cells (marked by elevated Hid and/or Drice expression) show only a low incidence of BrdUrd and PH3 staining, indicating that they can proliferate, but do so at an abnormally slow rate (Fig. 2b). Irradiated P35 clones are also associated with local enhancements of growth; 39 out of a total of 90 x-irradiated discs examined showed local deformations and small outpouchings of extra tissue associated with clones (Fig. 2a and Fig. 6, which is published as supporting information on the PNAS web site).
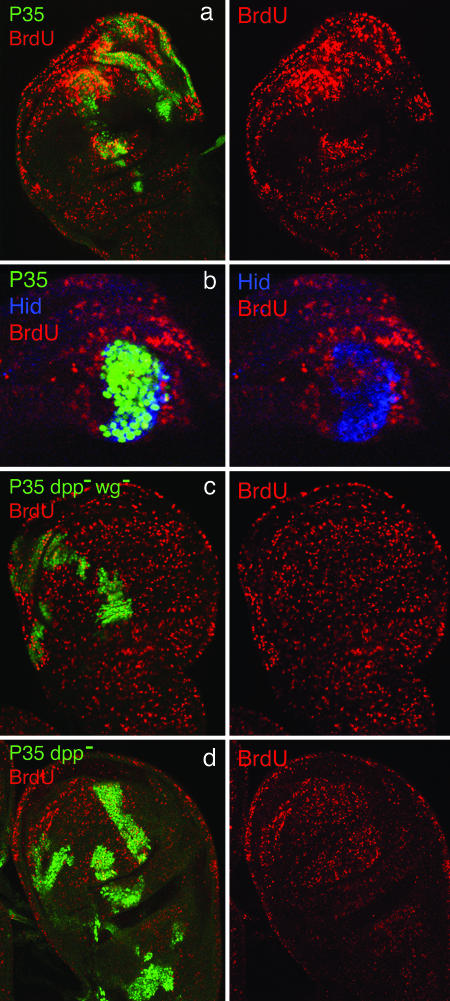
Fig. 2.
Excess growth and cell proliferation induced by undead P35-expressing cells depends on Dpp signaling. (a) X-irradiated wing disk with several clones of P35-expressing, but otherwise wild-type, cells (green), stained for BrdUrd incorporation (red). BrdUrd incorporation is abnormally elevated in the clones and in the surrounding regions, and some of the clones are associated with local outgrowths or other deformities of the disk. (b) Portion of an x-irradiated disk with a single P35-expressing clone stained for BrdUrd incorporation and Hid expression (blue). Abnormally high levels of BrdUrd incorporation can be seen outside of the clone as well as in cells within, except in the Hid-expressing cells, which show little or no incorporation. (c and d) Discs containing dpp– wg– P35 and dpp– P35 clones, respectively, stained for BrdUrd incorporation; in the absence of dpp activity, these clones do not induce excess growth or cell proliferation.
Unlike P35 clones, neither dpp– P35 nor dpp– wg– P35 clones seem to be associated with extra proliferation or growth after stress. A total of 85 x-irradiated discs were analyzed for BrdUrd incorporation, and, in all cases, BrdUrd levels were similar inside and outside the clones (Fig. 2 c and d), except for the very low levels in the undead cells within the clones. Similarly, none of the clones in these discs were associated with local outgrowths or deformations. The conclusion from these experiments is that the ability of undead cells to induce additional proliferation depends principally or solely on their capacity to send the Dpp signal. This effect on proliferation is consistent with the role of the Dpp pathway as a major factor controlling growth in the wing disk (24, 25). The results also indicate that Wg signaling by undead cells has no effect on growth or proliferation in the absence of Dpp signaling, because undead cells of both dpp– and dpp– wg– genotypes seem to be equally inert.
We note that Ryoo et al. (20) have reported experiments they interpret as evidence for a requirement for Wg in the induction of compensatory proliferation by apoptotic cells. In their experiments, they conditionally abrogated the response to Wg signaling by a temporally restricted expression of a dominant negative form of the transcription factor TCF that blocks Wg signal transduction in cells that also expressed hid and P35 in the posterior compartment. Under these conditions they observed a severe reduction of BrdUrd incorporation. In addition, they also found that activating the Wg transduction pathway by expressing a constitutively active form of Armadillo protein led to enhanced BrdUrd labeling, again implicating Wg signaling in promoting mitogenesis. Although these observations are consistent with a general requirement for Wg signaling in wing growth, as observed (28), they do not specifically test whether undead cells depend on Wg signaling to induce overproliferation in their neighbors. In experiments described below, we do so by asking whether undead cells that are unable to send Wg signal can still stimulate growth and proliferation in surrounding cells. These experiments indicate, unexpectedly, that the loss of Wg signaling actually stimulates the mitogenic effect of undead cells, implicating Wg as a growth inhibitor.
Induction of Neoplastic Tumors by Undead wg– Cells. Because the preceding results suggested that undead cells induce growth and proliferation in neighboring cells chiefly or exclusively through Dpp, we anticipated that undead cells in stressed wg– P35 clones would induce local elevations in growth and proliferation, similar to those caused by undead cells in stressed P35 clones. To our surprise, we found that stressed wg– P35 clones are associated with a strikingly different phenotype: they form masses of overproliferating cells that lose the original monolayer organization characteristic of the wing disk epithelium and instead behave like neoplastic tumors (Fig. 3b). In contrast, no such overgrowth or overproliferation was observed in corresponding control discs containing unstressed wg– P35 clones (n = 51; Fig. 3a), or, as described above, in stressed wg– P35 clones that are also mutant for dpp. Thus, it seems that Dpp sent by undead cells triggers the tumorous response, but that it can do so only if these cells are blocked from sending Wg.
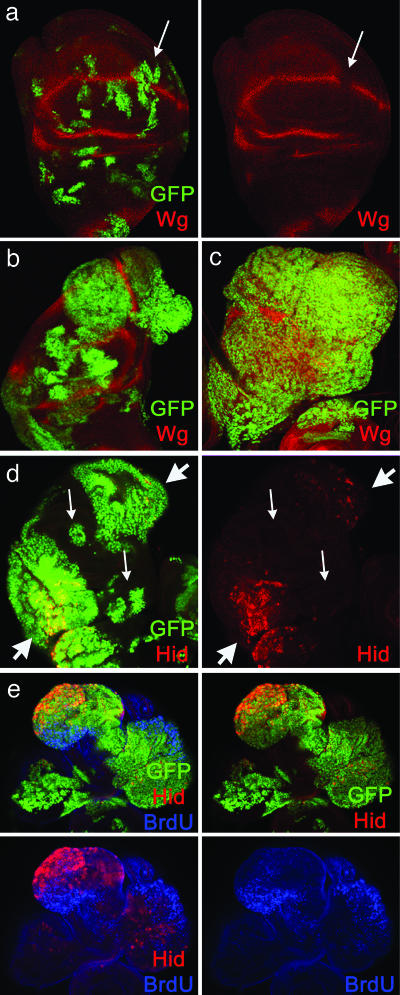
Fig. 3.
Tumorous transformations associated with x-irradiated wg– P35 clones. (a) Control (nonirradiated) disk containing wg– P35 clones labeled for P35 (GFP) and Wg (red). The clones appear normal, except that they cannot synthesize the Wg protein (arrow). (b–e) X-irradiated discs containing wg– P35 clones associated with tumors (stained for Wg (red; b and c), Hid (red; d and e), and BrdUrd incorporation (blue, e). (b) Disk containing several overgrowing clones that disrupt the normal morphology. (c) Disk in which the clones have fused and cover almost the totality of the disk. (d) Disk containing two overproliferating clones that express Hid (thick arrows) and several nonoverproliferating ones that do not express Hid (thin arrows). (e) Disk showing intense BrdUrd incorporation inside tumorous clones, except in the apoptotic cells that express Hid.
Clones of wg– P35 cells in irradiated discs fall into two distinct classes. Clones in the first class do not contain undead (Hid-expressing) cells and grow normally. Clones in the second class contain clusters of undead cells and overgrow dramatically, forming disorganized masses of tissue that often bulge out from the main body of the disk (Fig. 3 b, d, and e). These overproliferating clones tend to fuse, forming large single patches that cover the greater part of the disk (Fig. 3c), and in many cases (37/137) they seem to constitute the entire disk. BrdUrd incorporation and anti-phosphohistone 3 staining show that P35 wg– clones that include undead cells proliferate at a much higher rate than the surrounding tissue (Fig. 3e). However, undead (Hid-expressing) cells within these clones divide only rarely (Fig. 3e), indicating that it is the remaining, live cells within the clones that overgrow.
Tumorous Cells Induced by Undead wg– Cells Up-Regulate dMyc and Eliminate Surrounding Tissue by Acting as Supercompetitors. The ability of overproliferating wg– P35 cells to take over entire discs indicates not only that they multiply at abnormally elevated rates, but also that they eliminate surrounding cells. We examined Drice activity in 63 irradiated discs carrying overgrowing clones to assess whether such clones induce apoptosis in neighboring cells. In all cases, we observed elevated levels of Drice activity, particularly in wild-type cells abutting the borders of overproliferating clones or located in islands within these clones (Fig. 4 a and b). No such Drice activity was observed in nonirradiated discs containing clones of wg– P35 cells or in association with nonovergrowing clones in irradiated discs (Fig. 4a).
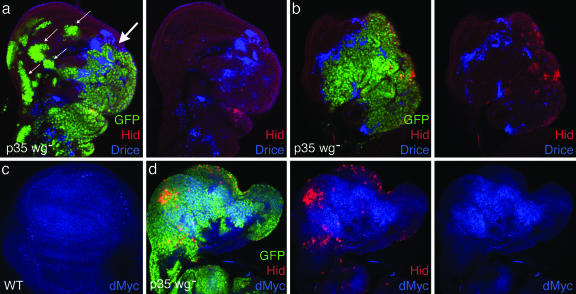
Fig. 4.
Tumorous wg– P35 clones exhibit elevated dMyc expression and induce apoptosis in surrounding wild-type cells. (a) X-irradiated wing disk containing a large tumor of wg– P35 cells (green; thick arrow), which includes undead (Hid-expressing; red) cells and induces Drice activity (blue) in neighboring wild-type cells, as well as in a few islands of wild-type cells remaining within the tumor; note also the presence of nonproliferative clones that do not express Hid (thin arrows) or induce Drice activity. (b) Irradiated disk exhibiting a more advanced stage in the elimination of wild-type cells; the majority of the disk is covered by a single wg– P35 tumor that contains Hid-expressing cells and has induced apoptosis in neighboring wild-type cells. (c) dMyc expression (blue) in a wild-type disk; expression levels are generally low, except for a modest elevation in most of the “wing pouch.” (d) Irradiated disk containing a large wg– P35 tumor that occupies most of the disk; dMyc expression (blue) is elevated in most cells of the tumor, but not in those that express Hid (red).
The behavior of wg– P35 cells resembles that of “supercompetitor” cells that contain elevated activity of the dMyc gene (29, 30) and that are able to eliminate wild-type cells by cell competition (31). Indeed, we find that many cells within overgrowing wg– P35 clones show elevated levels of dMyc protein (Fig. 4d). Interestingly, these elevated dMyc levels were observed only in wg– P35 clones that contain a mixture of live and undead cells, and only in the live cells within these clones (Fig. 4d), suggesting that they are caused by signals emanating from undead cells. The failure of x-irradiated dpp– wg– P35 clones to overgrow implicates Dpp as one such signal.
Only Caspase-Inhibited, P35-Expressing Cells Seem Susceptible to Tumorous Transformation by Undead wg– Cells. All of the cells constituting the dramatic outgrowths associated with wg– P35 clones express GFP, indicating that the tumorous tissue is formed exclusively by wg– P35 cells. This finding is intriguing, because it would be expected that at least some wild-type cells outside the clones would also receive the Dpp signal and therefore should also overproliferate. The fact that they do not suggests that the tumorous transformation depends on the receiving cells expressing P35. This idea is supported by an experiment in which we coexpressed the coding sequence of the proapoptotic gene rpr together with P35 in wg– P35 clones. In control discs in which we induced clones of GFP-labeled wg– cells that express rpr without P35, few if any rpr-expressing cells were observed after 72 h, and the rare, exceptional cells were pycnotic; therefore, under these conditions, rpr activity seems to cause the death of all cells within such clones. Accordingly, in rpr-expressing wg– P35 clones, we would expect that most or all cells would be undead, in contrast to our previous experiments in which irradiation induces apoptosis in only some but not other cells, generating a mixture of live and undead cells. We examined 66 discs with an average of six rpr-expressing wg– P35 clones per disk and found that most or all of the cells within these clones seem to be undead because they express hid, show greatly reduced BrdUrd incorporation, and grow slowly, forming abnormally small clones. Significantly, none of these clones were associated with large, neoplastic overgrowths, although we found several cases of small, local outgrowths induced nearby (Fig. 7, which is published as supporting information on the PNAS web site). Thus, the tumorous transformation is observed only in stressed wg– P35 clones, which consist of a mixture of live and undead cells, and not in rpr expressing wg– P35 clones in which all cells appear undead. We conclude that undead wg– P35 cells cannot transform neighboring wild-type cells into tumors; instead, only live wg– P35 cells within wg– P35 clones seem to be susceptible.
Discussion
Cells that initiate apoptosis in response to x-irradiation normally disappear rapidly, but, as shown here and previously (18), they can persist indefinitely with the help of the caspase inhibitor P35 and maintain characteristics of the apoptotic program, such as Hid, Dronc, and Drice activities and ectopic wg and dpp expression. The same is also true of cells that initiate apoptosis in response to severe heat shock, a stress that is unlikely to change the integrity of the genome, in contrast to x-irradiation (Fig. 5). Because undead cells can divide, albeit at a much lower rate than live ones, this epigenetic condition seems to be inherited through cell division. Thus, the developmental aberrations induced by undead cells in surrounding tissue are likely caused by their continuing to send mitogenic signals that might normally be sent only transiently by dying cells.
Our results indicate that the ability of undead cells to induce growth and proliferation depends on their being able to send Dpp signal, a finding that fits with the proposal that Dpp normally regulates cell growth and proliferation in the wing disk (23–25). Conversely, the excessive growth induced by undead cells within wg– P35 clones indicates that Wg acts to inhibit the mitogenic action of Dpp, a role consistent with the finding (32, 33) that Wg functions as a growth repressor during some phases of wing development.
We propose that Dpp and Wg regulate the abnormal growth induced by undead cells by exerting opposite stimulatory and inhibitory effects: Dpp promotes cell division whereas Wg inhibits the response to Dpp, thus constraining the production of new cells and limiting the extent of overgrowth. In normal circumstances in which caspase activity is not blocked by P35 expression, apoptotic cells disappear rapidly, and the transient production of Dpp and Wg may play a role in restoring, but not exceeding, the missing cells. When the apoptotic cells are kept alive with P35, the persistent production of Dpp and Wg and their slightly imbalanced effects cause local overproliferation and outgrowth, which are most clearly observed when an entire compartment is affected (18). If they cannot send the Dpp signal (e.g., because they are mutant for dpp), there is no proliferative stimulus, but if they can send Dpp but not Wg, the growth promoting function of Dpp acts unimpeded and induces a dramatic over-production of tissue.
This model accounts at least in part for the remarkable capacity of undead cells within wg– P35 clones to induce tumors: absence of the proposed inhibitory action of Wg would remove a constraint on the growth-promoting action of Dpp. The lack of wg activity itself may also be a growth-promoting factor, because there is evidence that wg normally acts as a dMyc repressor in cells flanking the prospective wing margin (27); the increased dMyc levels in the absence of wg activity promote cell cycle progression and growth (33). However, the tumorous behavior of stressed wg– P35 clones cannot be explained simply by the uninhibited action of Dpp emitted by undead cells or the consequent elevation of dMyc activity in the surrounding cells, because neither ectopic Dpp signaling nor overexpression of dMyc is sufficient to cause neoplastic transformation in the imaginal discs (29, 30). We suggest that undead cells send additional signals that act together with Dpp to induce the neoplastic transformation.
Another prerequisite for the induction of tumors by undead cells seems to be that the responding cells must also be unable to execute the cell death pathway. We speculate that unregulated growth within wg– P35 clones may create new cellular stresses both inside and outside the clones that induce secondary apoptotic events. Apoptotic cells outside the clones would be rapidly eliminated, but those within would be protected by P35 and join a growing population of undead cells that become new sources of Dpp and other tumor-inducing factors. The populations of both undead and live cells within the clone would thus expand at the expense of the surrounding wild-type tissue, eventually eliminating all of the cells that do not express P35. Circumstantial evidence in favor of this view is the large number of undead (Hid-expressing) cells found in discs overgrown by wg– P35 clones (Fig. 8, which is published as supporting information on the PNAS web site). Given that undead cells proliferate at low rate, it seems likely that at least some if not most of the undead cells in wg– P35 tumors will have arisen by secondary apoptotic events, rather than by descent from the initial founder population of stress-induced, undead cells.
Such a mechanism can account for the dramatic expansion of wg– P35 clones at the expense of surrounding wild-type tissue, once they are seeded by the initial induction of undead cells. However, it does not explain why only P35-expressing cells, and not neighboring wild-type cells, develop neoplastic properties such as the failure to maintain a normal epithelial morphology. This difference in behavior raises the possibility that P35 expression may have additional consequences, aside from the direct block of the cell death pathway, that predispose cells to neoplastic transformation.
Our results have potential implications for models of tumor transformation in mammals. It is normally argued (5) that oncogenesis is a multistep process that requires a number of successive somatic mutations, but there are also indications that, in some instances, the transformation of cancer cells is associated with epigenetic phenomena, that is, heritable changes in gene function not caused by somatic mutations (reviewed in ref. 34). Here, we provide an example in which cell populations that cannot execute the cell death pathway are predisposed to oncogenic transformation by just such an epigenetic event, namely the induction of undead cells in response to cellular stress. In Drosophila, the ability of such undead cells to induce neighboring cells to become tumorous seems to depend on their sending an abnormal balance of growth regulatory signals that up-regulate activity of the proto-oncogene dMyc in neighboring cells. As a consequence, the responding cells behave as supercompetitors that overproliferate and eventually eliminate surrounding wild-type cells (31). These findings suggest a mechanism for generating neoplastic tumors in caspase-inhibited cells.
Evading apoptosis is widely recognized as a hallmark of cancer cells (5). There is also evidence that caspase activity is inhibited in some aggressive human cancers (35). Our findings may therefore provide a paradigm for the formation of neoplastic tumors in tissues that are unable to die.
Supplementary Material
Acknowledgments
We thank Ernesto Sánchez-Herrero, Hermann Steller, and Laura Johnston for comments on the manuscript; Rosa González, Angélica Cantarero, Atsuko Adachi, and Xiao-Jing Qiu for their help; and Hermann Steller (The Rockefeller University, New York) and Eduardo Moreno [Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid] for antibodies. The work in Madrid was funded by the Ministerio de Educación y Ciencia. G.S. is an investigator of the Howard Hughes Medical Institute.
Author contributions: A.P.-G., F.A.M., G.S., and G.M. designed research; A.P.-G., F.A.M., and G.S. performed research; A.P.-G., F.A.M., G.S., and G.M. analyzed data; and G.S. and G.M. wrote the paper.
Conflict of interest statement: No conflicts declared.
References
- 1.Vaux, D. L. & Korsmeyer, S. J. (1999) Cell 96, 245–254. [DOI] [PubMed] [Google Scholar]
- 2.Danial, N. N. & Korsmeyer, S. J. (2004) Cell 116, 205–219. [DOI] [PubMed] [Google Scholar]
- 3.Moreno, E., Basler, K. & Morata, G. (2002) Nature 416, 755–759. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson, M. D., Neil, M. & Raff. M. C. (1997) Cell 88, 347–354. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan, D. & Weinberg, R. A. (2000) Cell 100, 57–70. [DOI] [PubMed] [Google Scholar]
- 6.Riedl, S. J. & Shi, Y. (2004) Nat. Rev. Mol. Cell Biol. 5, 897–907. [DOI] [PubMed] [Google Scholar]
- 7.Goyal, L., McCall, K., Agapite, J., Hartwieg, E. & Steller, H. (2000) EMBO J. 19, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang, S. L., Hawkins, C. J., Yoo, S. J., Muller, H. A. & Hay, B. A. (1999) Cell 98, 453–463. [DOI] [PubMed] [Google Scholar]
- 9.Lohmann, I., McGinnis, N., Bodmer, M. & McGinnis, W. (2002) Cell 110, 457–466. [DOI] [PubMed] [Google Scholar]
- 10.Jiang, C., Baehrecke, E. H. & Thummel, C. S. (1997) Development (Cambridge, U.K.) 124, 4673–4683. [DOI] [PubMed] [Google Scholar]
- 11.Brodsky, M. H., Nordstrom, W., Tsang, G., Kwan, E., Rubin, G. M. & Abrams, J. M. (2000) Cell 101, 103–113. [DOI] [PubMed] [Google Scholar]
- 12.Ollmann, M., Young, L. M., Di Como, C. J., Karim, F., Belvin, M., Robertson, S., Whittaker, K., Demsky, M., Fisher, W. W., Buchman, A., et al. (2000) Cell 101, 91–101. [DOI] [PubMed] [Google Scholar]
- 13.Ryoo, H. D., Bergmann, A., Gonen, H., Ciechanover, A. & Steller, H. (2002) Nat. Cell Biol. 4, 432–438. [DOI] [PubMed] [Google Scholar]
- 14.Yoo, S. J., Huh, J. R., Muro, I., Yu, H., Wang, L., Wang, S. L., Feldman, R. M., Clem, R. J., Muller, H. A. & Hay, B. A. (2002) Nat. Cell Biol. 4, 416–424. [DOI] [PubMed] [Google Scholar]
- 15.Yin V. P. & Thummel C. S. (2005) Semin. Cell Dev. Biol. 16, 237–243. [DOI] [PubMed] [Google Scholar]
- 16.Wolff. T. & Ready, D. F. (1991) Development (Cambridge, U.K.) 113, 825–839. [DOI] [PubMed] [Google Scholar]
- 17.Milan, M., Campuzano, S. & Garcia-Bellido, A. (1997) Proc. Natl. Acad. Sci. USA 94, 5691–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Garijo, A., Martin, F. A. & Morata, G. (2004) Development (Cambridge, U.K.) 131, 5591–5598. [DOI] [PubMed] [Google Scholar]
- 19.Haynie, J. L. & Bryant, P. J. (1977) Wilhelm Rouxs Arch. 183, 85–100. [DOI] [PubMed] [Google Scholar]
- 20.Ryoo, H. D., Gorenc, T. & Steller, H. (2004) Dev. Cell 7, 491–501. [DOI] [PubMed] [Google Scholar]
- 21.Huh, J. R., Guo, M. & Hay, B. A. (2004) Curr. Biol. 14, 1262–1266. [DOI] [PubMed] [Google Scholar]
- 22.Hay, B. A., Wolff, T. & Rubin, G. M. (1994) Development (Cambridge, U.K.) 120, 2121–2219. [DOI] [PubMed] [Google Scholar]
- 23.Burke, R. & Basler, K. (1996) Development (Cambridge, U.K.) 122, 2261–2269. [DOI] [PubMed] [Google Scholar]
- 24.Martín-Castellanos, C. & Edgar, B. A. A. (2002) Development (Cambridge, U.K.) 129, 1003–1013. [DOI] [PubMed] [Google Scholar]
- 25.Martin, F. A., Perez-Garijo, A., Moreno, E. & Morata, G. (2004) Development (Cambridge, U.K.) 131, 4921–4930. [DOI] [PubMed] [Google Scholar]
- 26.Lee, T. & Luo, L. (1999) Neuron 22, 451–461. [DOI] [PubMed] [Google Scholar]
- 27.Johnston, L. A. Prober, D. A., Edgar, B. A., Eisenman, R. N. & Gallant, P. (1999) Cell 98, 779–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz-Benjumea, F. & Cohen, S. M. (1995) Development (Cambridge, U.K.) 121, 4215–4225. [DOI] [PubMed] [Google Scholar]
- 29.de la Cova, C., Abril, M., Bellosta, P., Gallant, P. & Johnston, L. A. (2004) Cell 117, 107–116. [DOI] [PubMed] [Google Scholar]
- 30.Moreno, E. & Basler, K. (2004) Cell 117, 117–129. [DOI] [PubMed] [Google Scholar]
- 31.Morata, G. & Ripoll, P. (1975) Dev. Biol. 42, 211–221. [DOI] [PubMed] [Google Scholar]
- 32.Johnston, L. A. & Sanders, A. L. (2003) Nat. Cell Biol. 5, 827–833. [DOI] [PubMed] [Google Scholar]
- 33.Duman-Schell, M., Johnston, L. & Du, W. (2004) Proc. Natl. Acad. Sci USA 101, 3857–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esteller, M. (2005) Annu. Rev. Pharmacol. Toxicol. 45, 629–656. [DOI] [PubMed] [Google Scholar]
- 35.Fennell, D. A. (2005) Clin. Cancer Res. 11, 2097–2105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.