Abstract
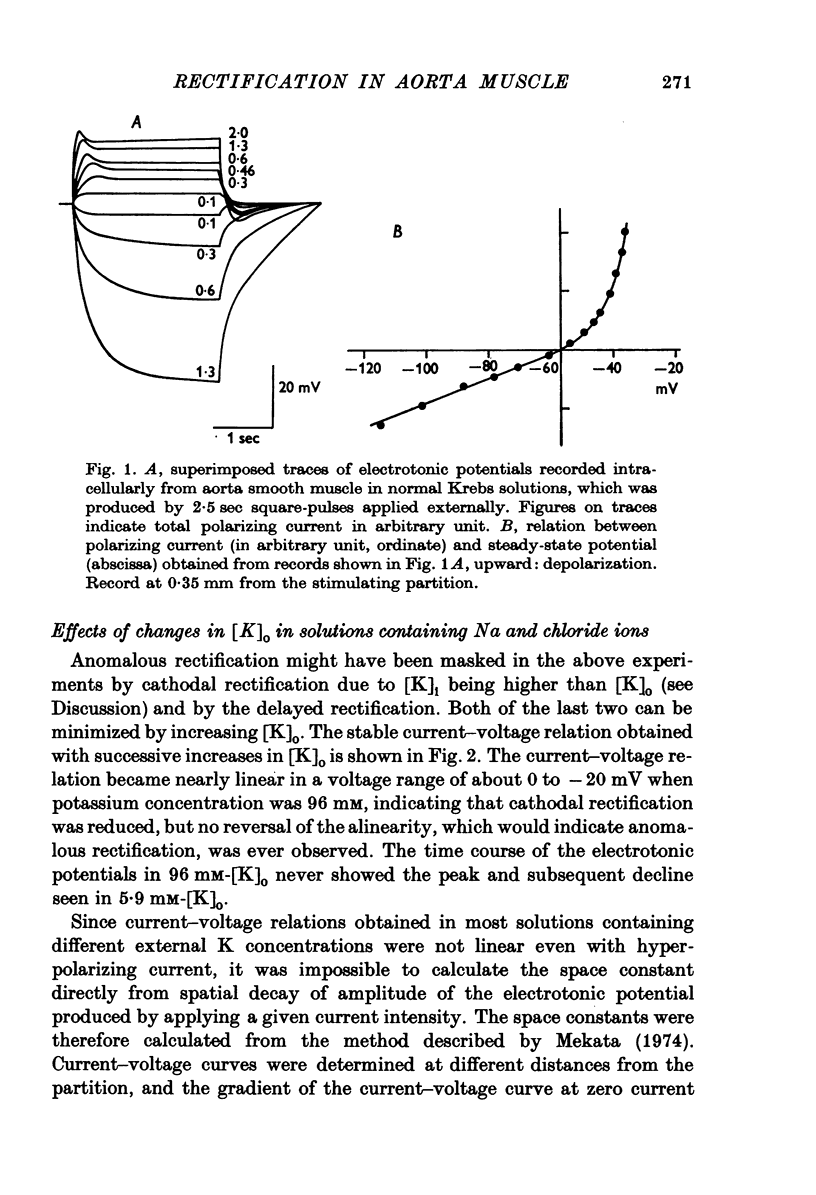
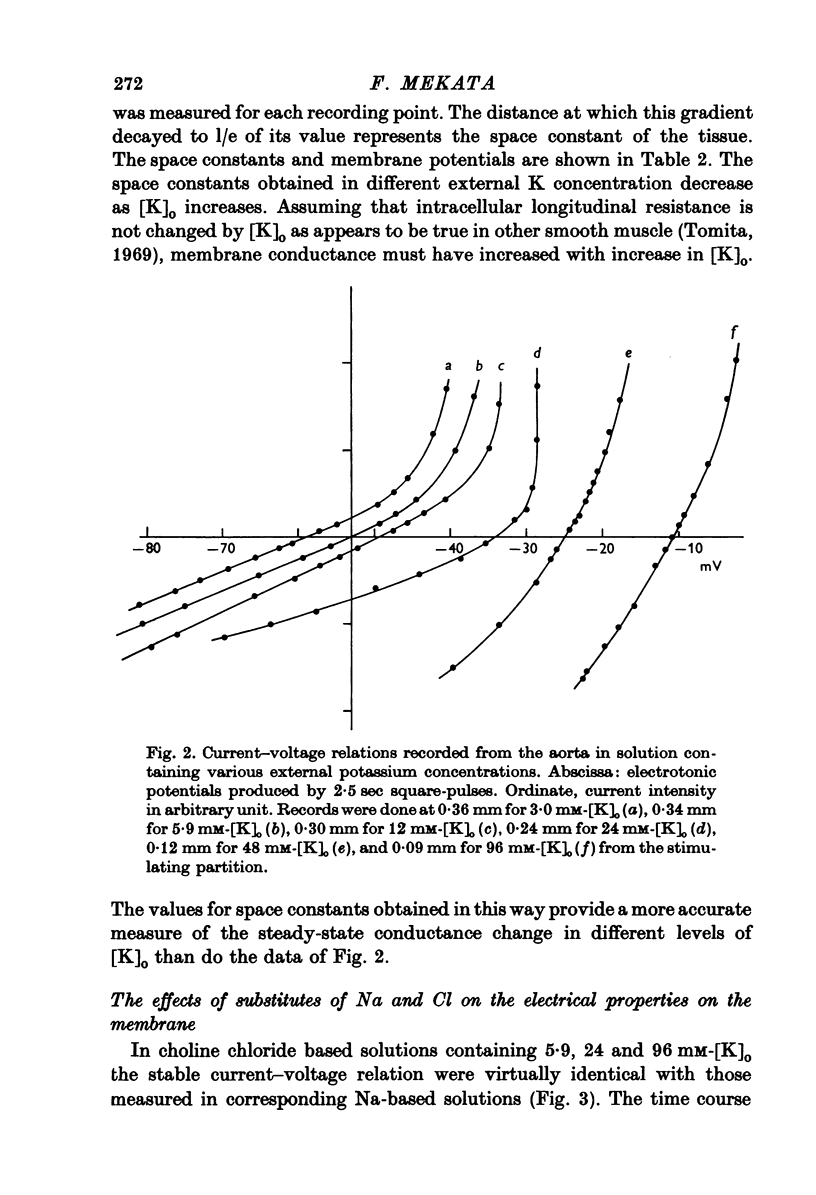
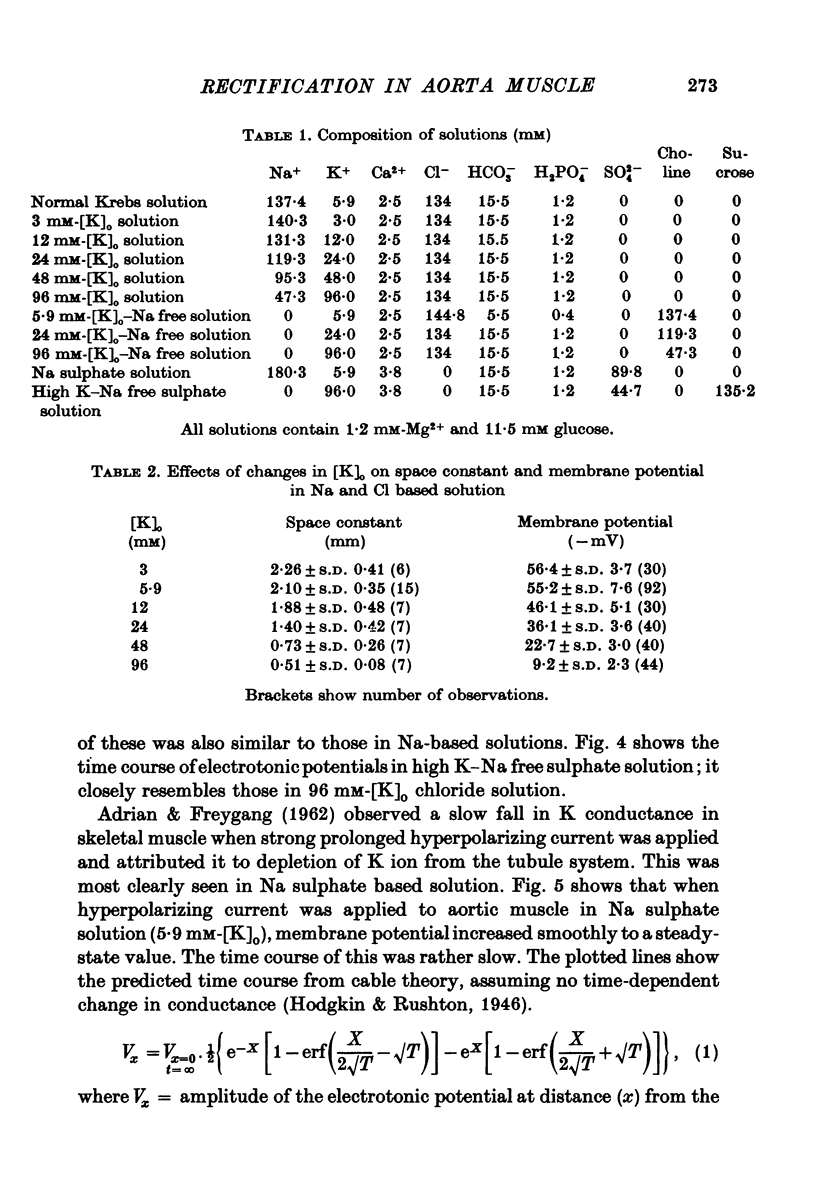
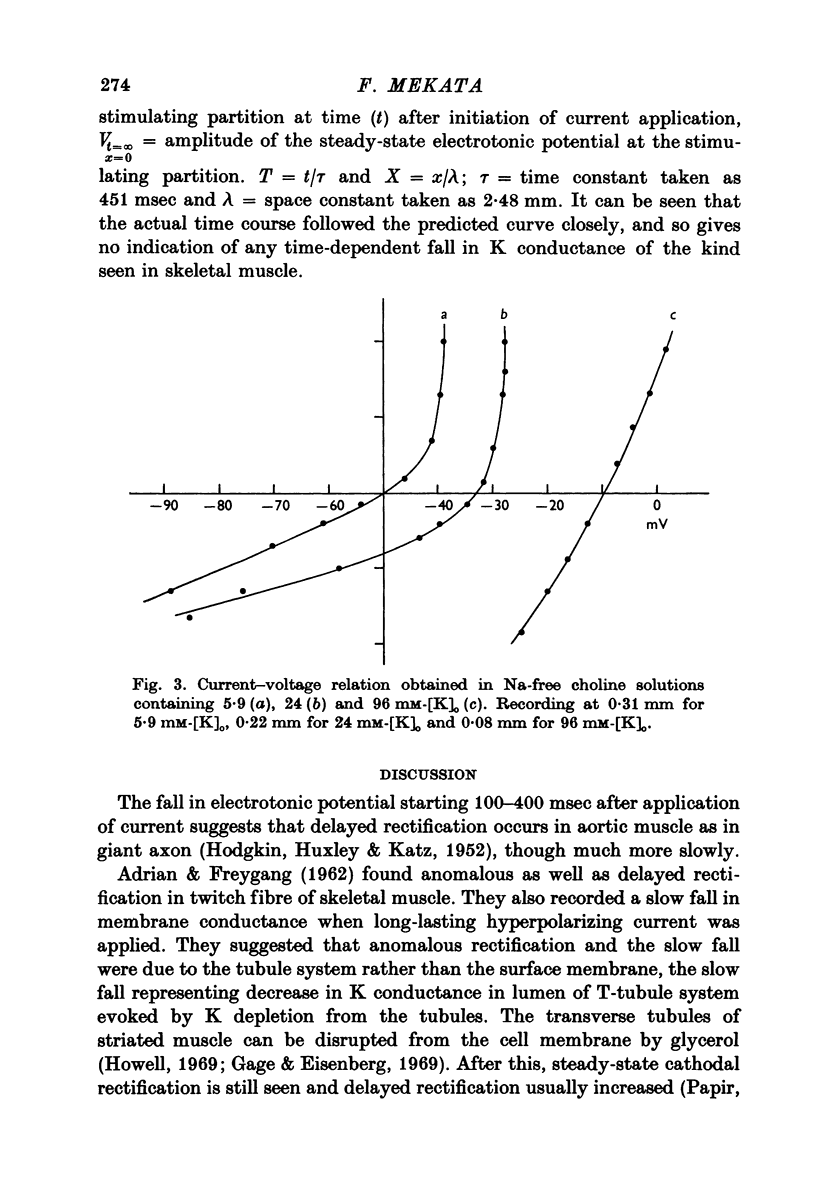
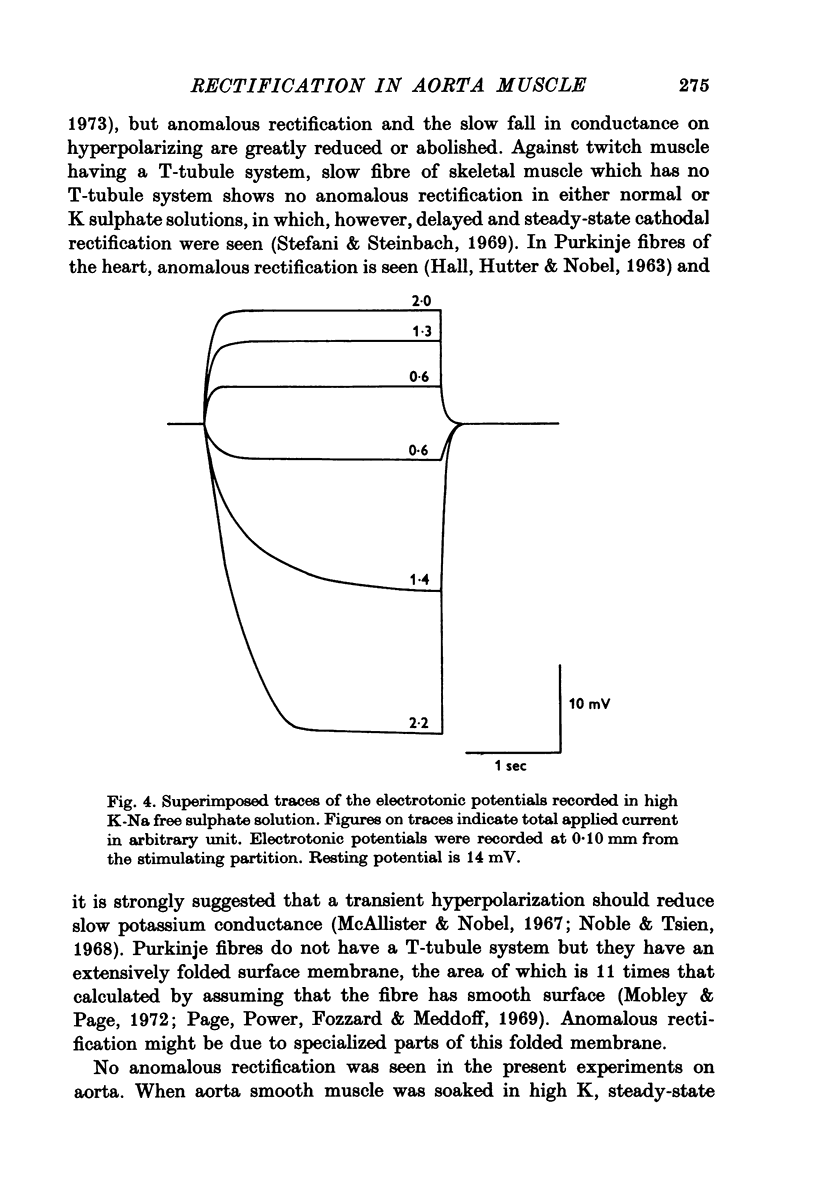
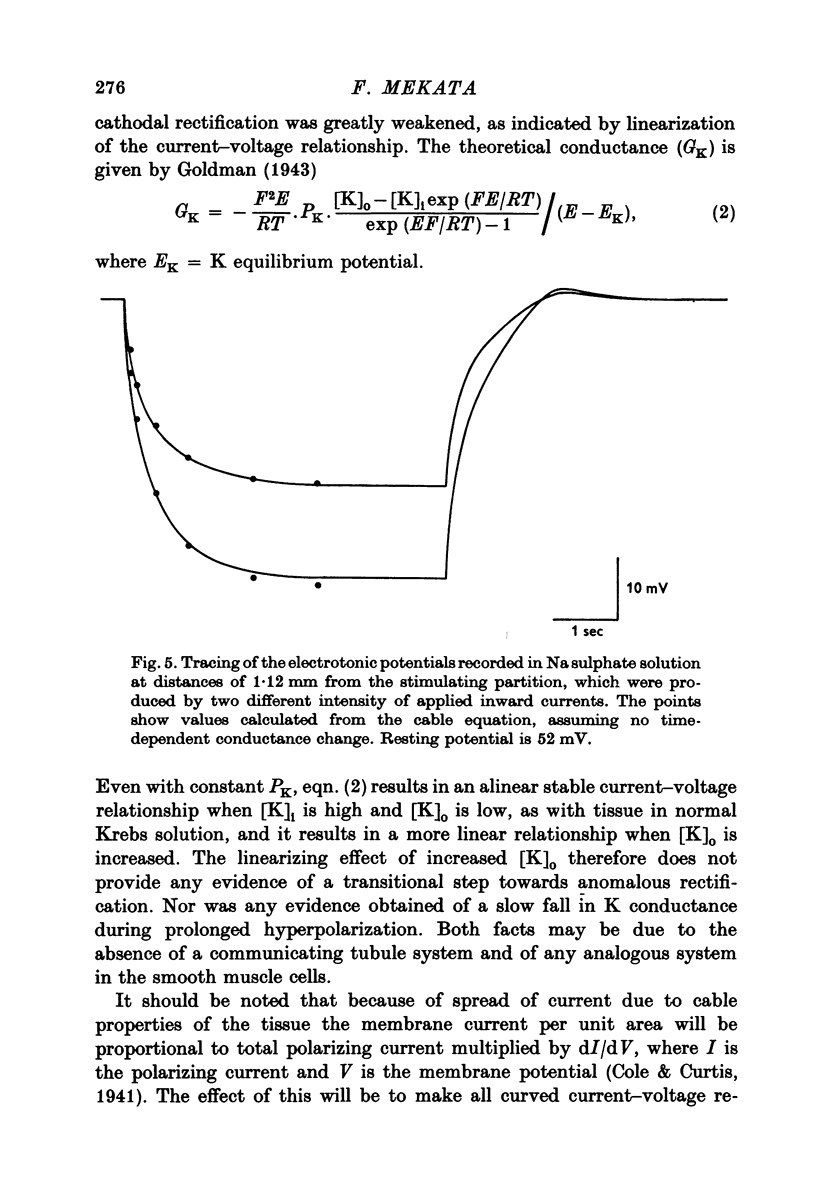
1. The current-voltage relation of the smooth muscle cell membrane of rabbit aorta was determined by the partition method. 2. No anomalous rectification was observed in any of the following solutions: normal Krebs, Na free choline, Na sulphate, and high K-Na free sulphate. 3. Delayed rectification was seen on application of depolarizing current in both normal Krebs solution and Na free choline solution. 4. High concentration of K made the steady-state current-voltage relation almost linear in a voltage range of about 0 to -20mV. This effect, and steady-state cathodal rectification which was seen in physiological solution, could be explained qualitatively by constant field theory without involving channels capable of anomalous rectification. 5. A slow decrease in K conductance, during application of large and long-lasting hyperpolarizing currents, which occurs in skeletal muscle and is attributed to the tubule system, was never observed in the arteries either in Krebs, Na-free choline, or Na sulphate solution.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole K. S., Curtis H. J. MEMBRANE POTENTIAL OF THE SQUID GIANT AXON DURING CURRENT FLOW. J Gen Physiol. 1941 Mar 20;24(4):551–563. doi: 10.1085/jgp.24.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL A. E., HUTTER O. F., NOBLE D. Current-voltage relations of Purkinje fibres in sodium-deficient solutions. J Physiol. 1963 Apr;166:225–240. doi: 10.1113/jphysiol.1963.sp007102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell J. N. A lesion of the transverse tubules of skeletal muscle. J Physiol. 1969 May;201(3):515–533. doi: 10.1113/jphysiol.1969.sp008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Ca concentration and flux in Ca-deprived arteries. J Physiol. 1972 Jul;224(1):35–59. doi: 10.1113/jphysiol.1972.sp009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Mekata F. Biophysical properties of the longitudinal smooth muscle of the guinea-pig rectum. J Physiol. 1971 Feb;212(3):667–683. doi: 10.1113/jphysiol.1971.sp009349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. E., Noble D. The effect of subthreshold potentials on the membrane current in cardiac Purkinje fibres. J Physiol. 1967 May;190(2):381–387. doi: 10.1113/jphysiol.1967.sp008216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Current spread in the smooth muscle of the rabbit aorta. J Physiol. 1974 Oct;242(1):143–155. doi: 10.1113/jphysiol.1974.sp010698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley B. A., Page E. The surface area of sheep cardiac Purkinje fibres. J Physiol. 1972 Feb;220(3):547–563. doi: 10.1113/jphysiol.1972.sp009722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Stein R. B. The threshold conditions for initiation of action potentials by excitable cells. J Physiol. 1966 Nov;187(1):129–162. doi: 10.1113/jphysiol.1966.sp008079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E., Power B., Fozzard H. A., Meddoff D. A. Sarcolemmal evaginations with knob-like or stalked projections in Purkinje fibers of the sheep's heart. J Ultrastruct Res. 1969 Aug;28(3):288–300. doi: 10.1016/s0022-5320(69)90086-0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Devine C. E., Somlyo A. V., North S. R. Sarcoplasmic reticulum and the temperature-dependent contraction of smooth muscle in calcium-free solutions. J Cell Biol. 1971 Dec;51(3):722–741. doi: 10.1083/jcb.51.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Steinbach A. B. Resting potential and electrical properties of frog slow muscle fibres. Effect of different external solutions. J Physiol. 1969 Aug;203(2):383–401. doi: 10.1113/jphysiol.1969.sp008869. [DOI] [PMC free article] [PubMed] [Google Scholar]


