Abstract
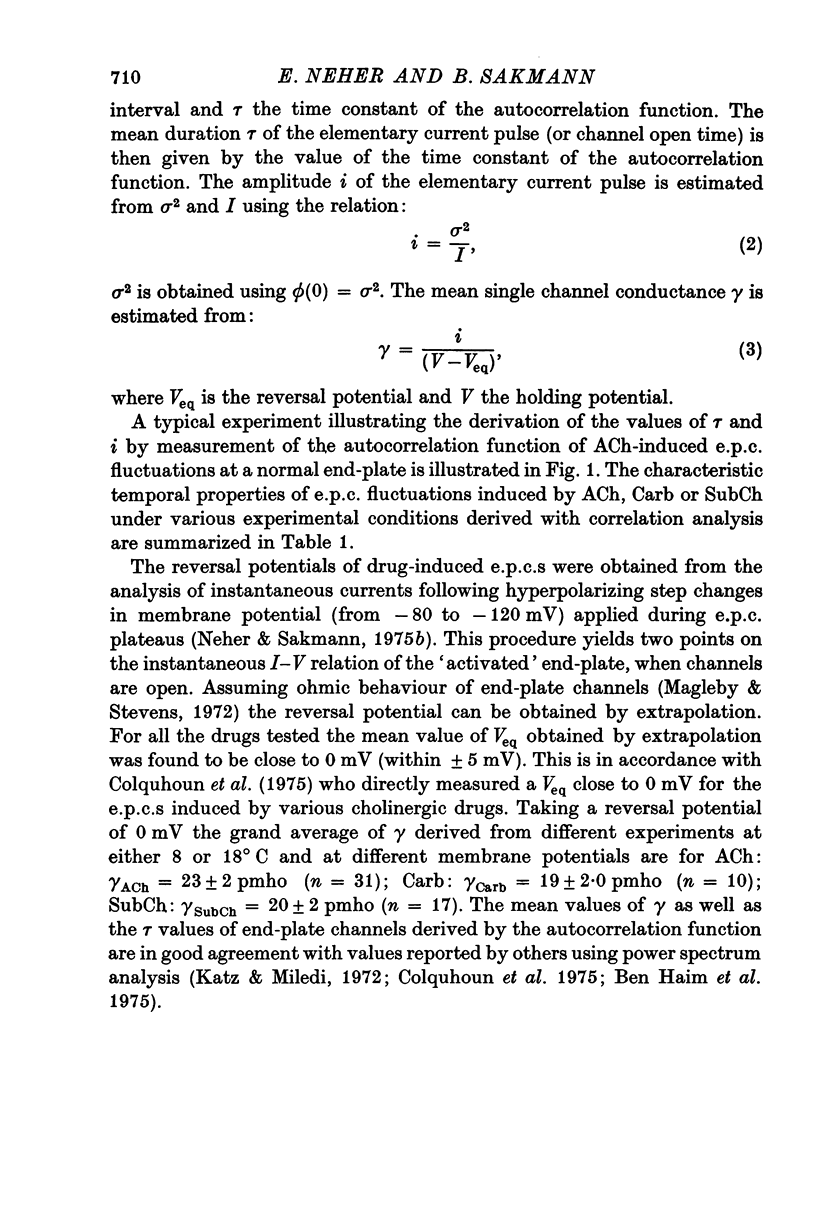
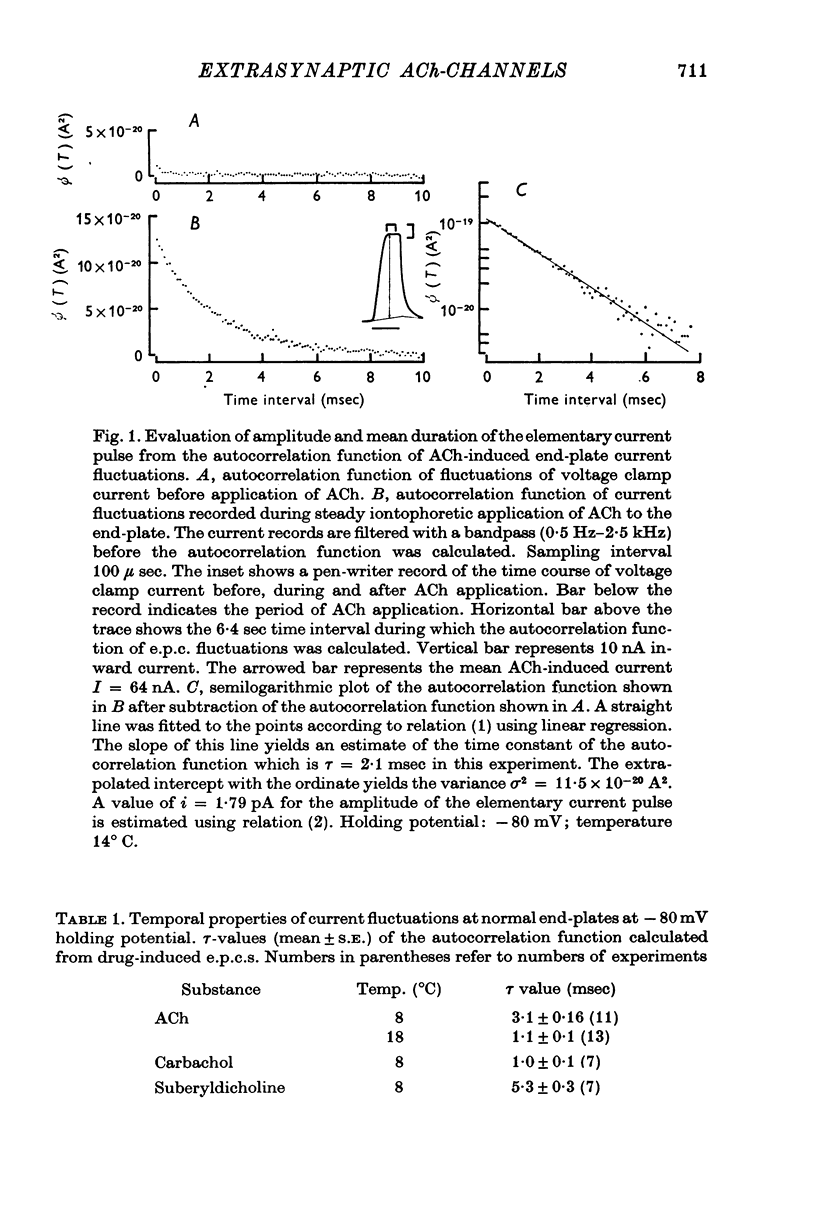
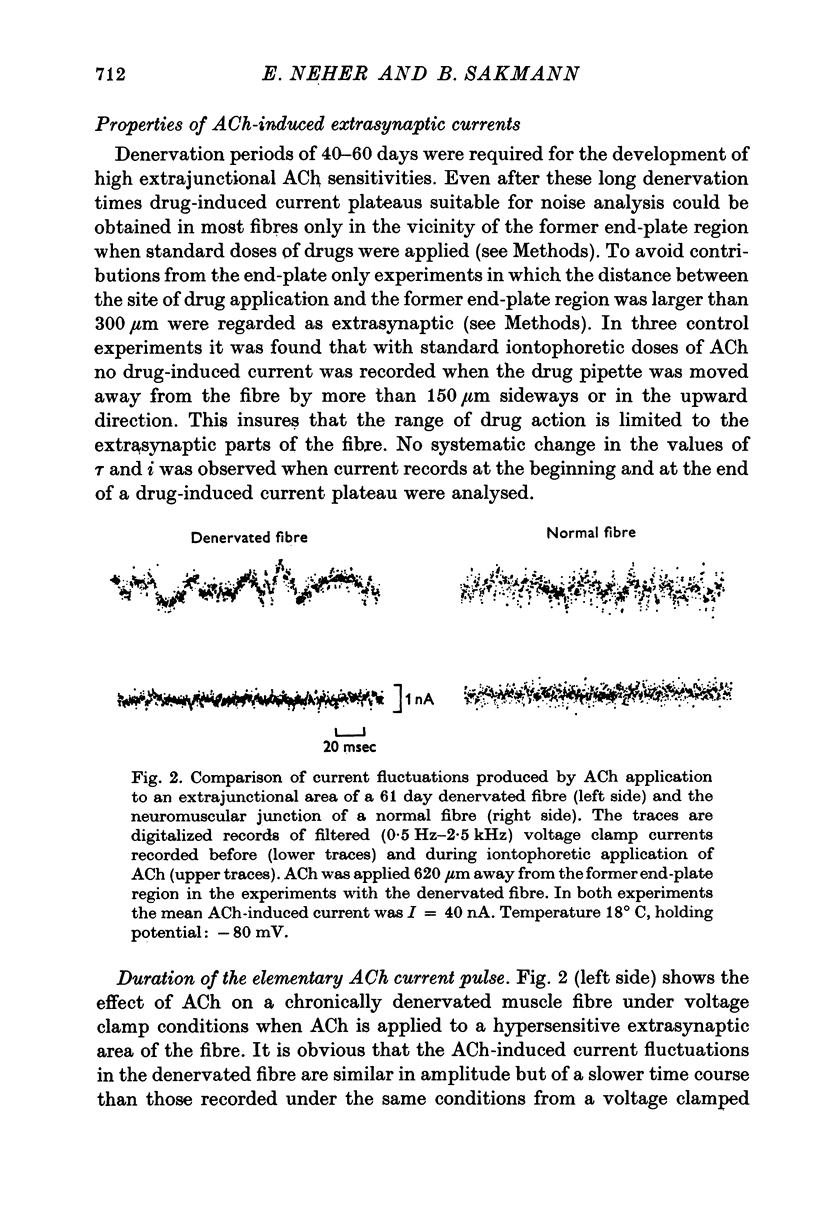
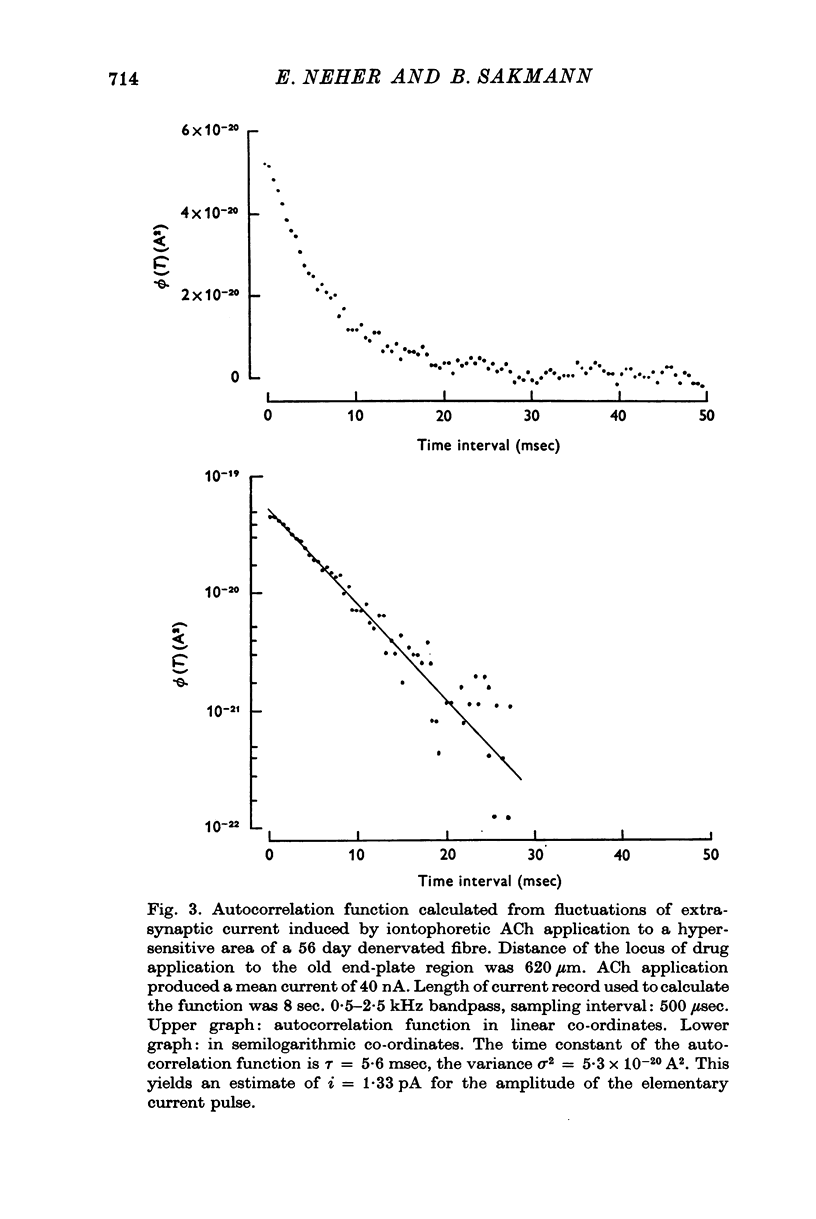
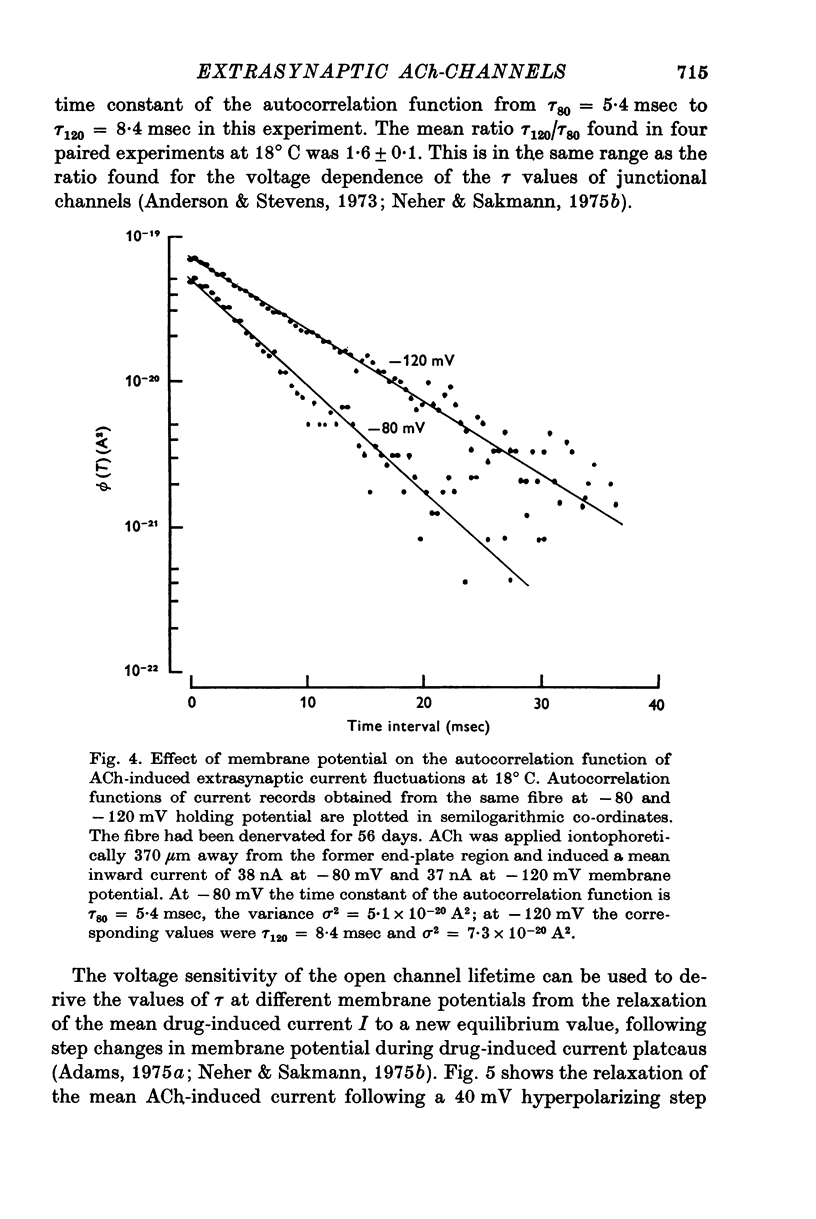
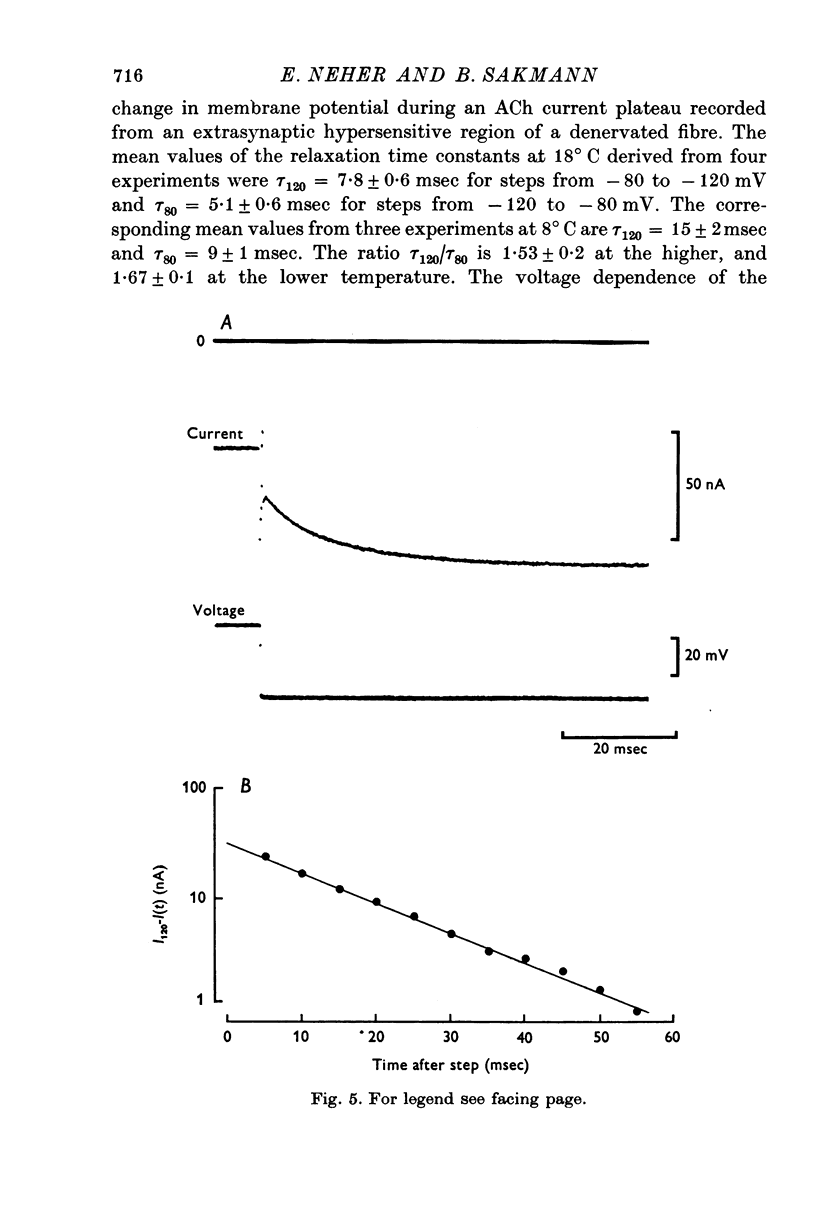
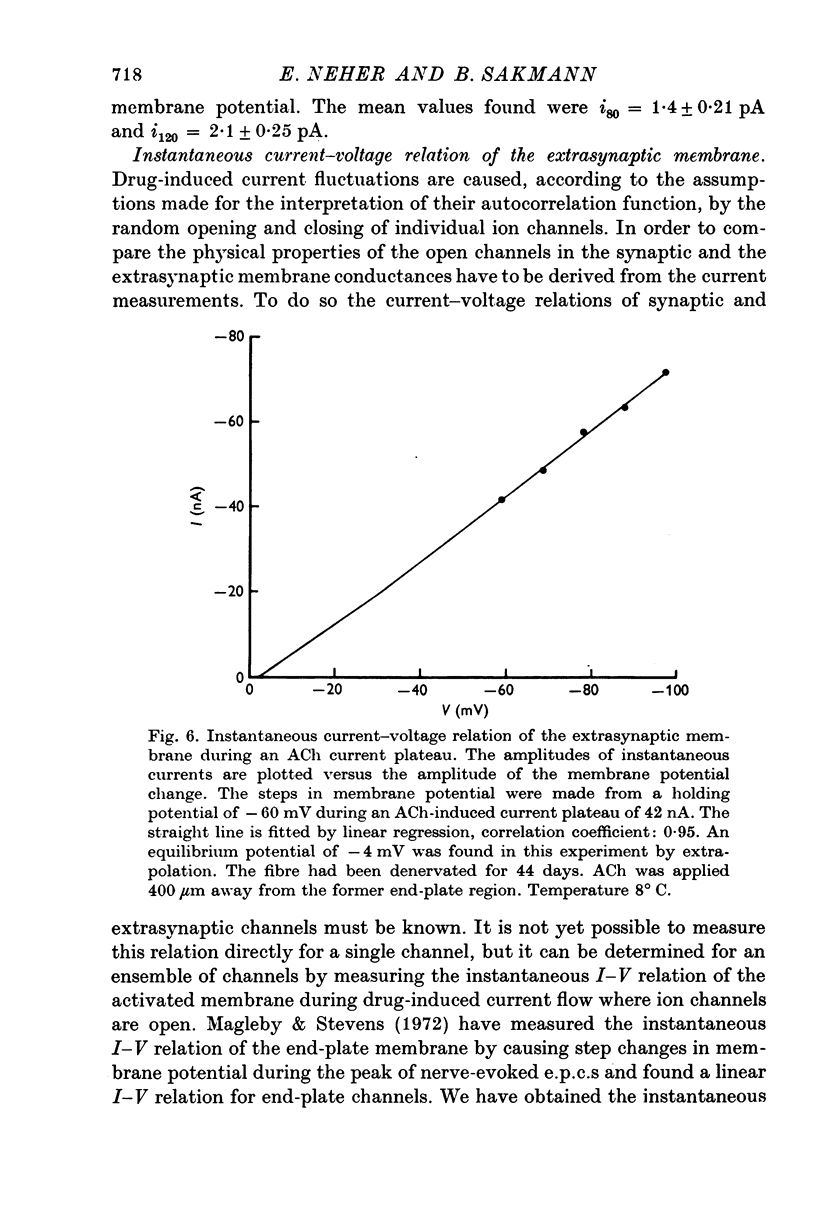
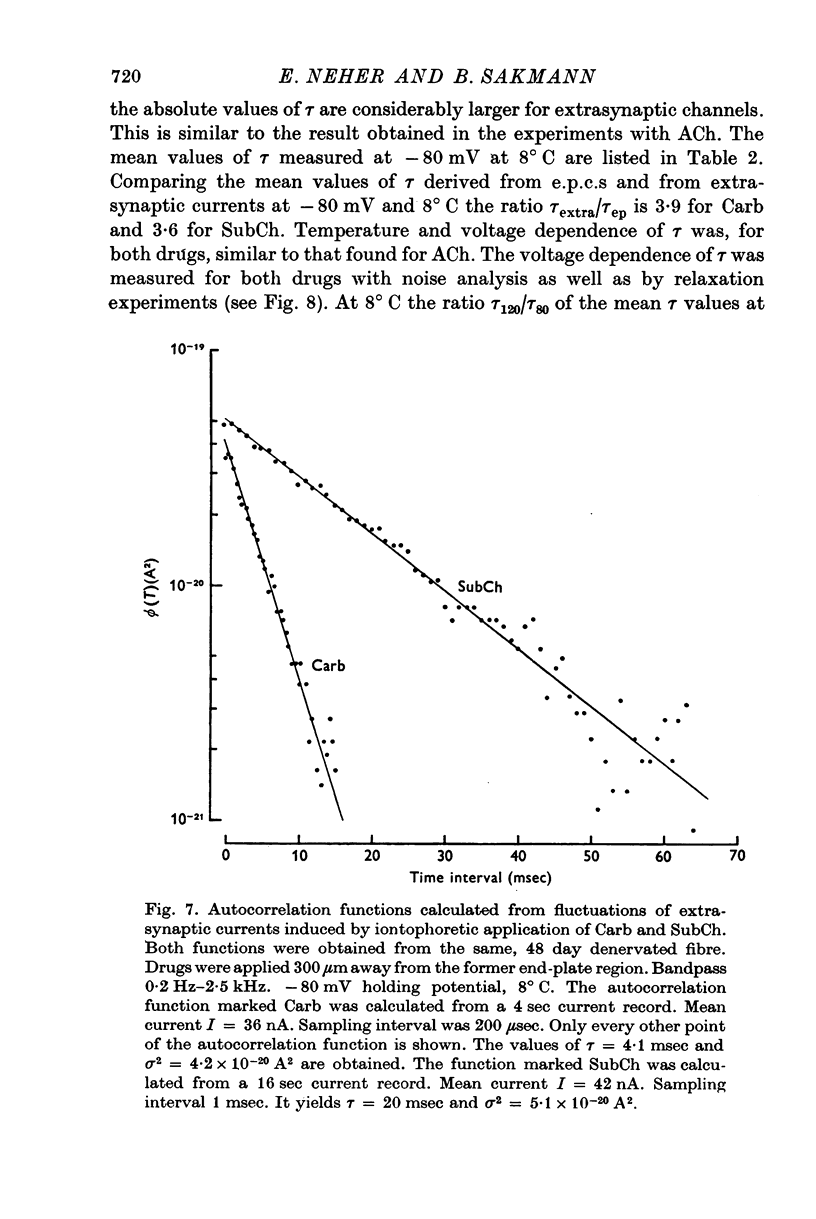
1. Voltage clamp currents were recorded during iontophoretic application of steady doses of acetylcholine (ACh), carbachol or suberyldicholine to hyperpersensitive extrasynaptic regions of chronically denervated frog muscle fibers. Autocorrelation functions of drug induced current fluctuations were calculated and estimates of conductance gamma and average open time tau of the extrasynaptic ion channels were derived. 2. The average open time of an extrajunctional channel induced by acetylcholine is tauACh = 11 +/- 1-6 msec (+/- S.E.) at -80 mV and 8 degrees C. Carbachol and suberyldicholine open channels of tauCarb = 3-9 +/- 0-4 msec and tauSubCh = 19 +/- 2-5 msec (+/- S.E.) duration under the same conditions. The average open time of the extrasynaptic channel produced by each drug is three to five times longer than the value found for junctional channels in normal fibres. 3. The average open time of the extrajunctional channel is dependent on temperature and membrane potential. Lowering the temperature or increasing the membrane potential increases the average open time of the channels induced by any one of the drugs. 4. The conductance of a single extrajunctional channel opened by the action of acetylcholine is estimated to be gammaextra = 15 +/- 1-8 pmho (+/- S.E.). This is somewhat lower than the value of gammaep = 23 +/- 2 pmho (+/- S.E.) found for the conductance of a single open channel in the junctional membrane of normal fibres. The extrasynaptic channels opened by the action of carbachol and suberyldicholine have similar conductances to those produced by ACh. 5. The autocorrelation function of drug-induced current fluctuations, recorded at the former end-plate region of chronically denervated fibres often shows both a fast and a slow time constant. They correspond in value to the time constant of the autocorrelation function obtained from end-plate currents in normal fibres and from extrasynaptic currents in denervated fibres respectively. This could indicate that two populations of channels exist at the former end-plate region of denervated muscle fibres.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. A study of desensitization using voltage clamp. Pflugers Arch. 1975 Oct 28;360(2):135–144. doi: 10.1007/BF00580536. [DOI] [PubMed] [Google Scholar]
- Adams P. R. Kinetics of agonist conductance changes during hyperolarization at frog endplates. Br J Pharmacol. 1975 Feb;53(2):308–310. doi: 10.1111/j.1476-5381.1975.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage dependence of agonist responses at voltage-clamped frog endplates. Pflugers Arch. 1976 Jan 30;361(2):145–151. doi: 10.1007/BF00583458. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Haim D., Dreyer F., Peper K. Acetylcholine receptor: modification of synaptic gating mechanism after treatment with a disulfide bond reducing agent. Pflugers Arch. 1975 Mar 22;355(1):19–26. doi: 10.1007/BF00584796. [DOI] [PubMed] [Google Scholar]
- Berg D. K., Hall Z. W. Fate of alpha-bungarotoxin bound to acetylcholine receptors of normal and denervated muscle. Science. 1974 Apr 26;184(4135):473–475. doi: 10.1126/science.184.4135.473. [DOI] [PubMed] [Google Scholar]
- Beránek R., Vyskocil F. The action of tubocurarine and atropine on the normal and denervated rat diaphragm. J Physiol. 1967 Jan;188(1):53–66. doi: 10.1113/jphysiol.1967.sp008123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. P., Hall Z. W. Acetylcholine receptors in normal and denervated rat diaphragm muscle. II. Comparison of junctional and extrajunctional receptors. Biochemistry. 1975 May 20;14(10):2100–2106. doi: 10.1021/bi00681a009. [DOI] [PubMed] [Google Scholar]
- Chang C. C., Huang M. C. Turnover of junctional and extrajunctional acetylcholine receptors of the rat diaphragm. Nature. 1975 Feb 20;253(5493):643–644. doi: 10.1038/253643a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dionne V. E., Steinbach J. H., Stevens C. F. Conductance of channels opened by acetylcholine-like drugs in muscle end-plate. Nature. 1975 Jan 17;253(5488):204–206. doi: 10.1038/253204a0. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M. Acetylcholine receptors. Revised estimates of extrajunctional receptor density in denervated rat diaphragm. J Gen Physiol. 1974 Oct;64(4):468–472. doi: 10.1085/jgp.64.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Mallart A. Ionic permeability changes induced by some cholinergic agonists on normal and denervated frog muscles. J Physiol. 1971 Oct;218(1):101–116. doi: 10.1113/jphysiol.1971.sp009606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N., Schneider G. T. Effects of some aliphatic alcohols on the conductance change caused by a quantum of acetylcholine at the toad end-plate. J Physiol. 1975 Jan;244(2):409–429. doi: 10.1113/jphysiol.1975.sp010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The antagonism between tubocurarine and substances which depolarize the motor end-plate. J Physiol. 1960 Jul;152:309–324. doi: 10.1113/jphysiol.1960.sp006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J. THE LOCALIZATION OF CHOLINESTERASE ACTIVITY IN RAT CARDIAC MUSCLE BY ELECTRON MICROSCOPY. J Cell Biol. 1964 Nov;23:217–232. doi: 10.1083/jcb.23.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Further observations on acetylcholine noise. Nat New Biol. 1971 Jul 28;232(30):124–126. doi: 10.1038/newbio232124b0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Membrane noise produced by acetylcholine. Nature. 1970 Jun 6;226(5249):962–963. doi: 10.1038/226962a0. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The characteristics of 'end-plate noise' produced by different depolarizing drugs. J Physiol. 1973 May;230(3):707–717. doi: 10.1113/jphysiol.1973.sp010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Trautmann A. Ionic properties of the neuromuscular junction of the frog: effects of denervation and pH. J Physiol. 1973 Nov;234(3):553–567. doi: 10.1113/jphysiol.1973.sp010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. Inferences about membrane properties from electrical noise measurements. Biophys J. 1972 Aug;12(8):1028–1047. doi: 10.1016/S0006-3495(72)86141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]


