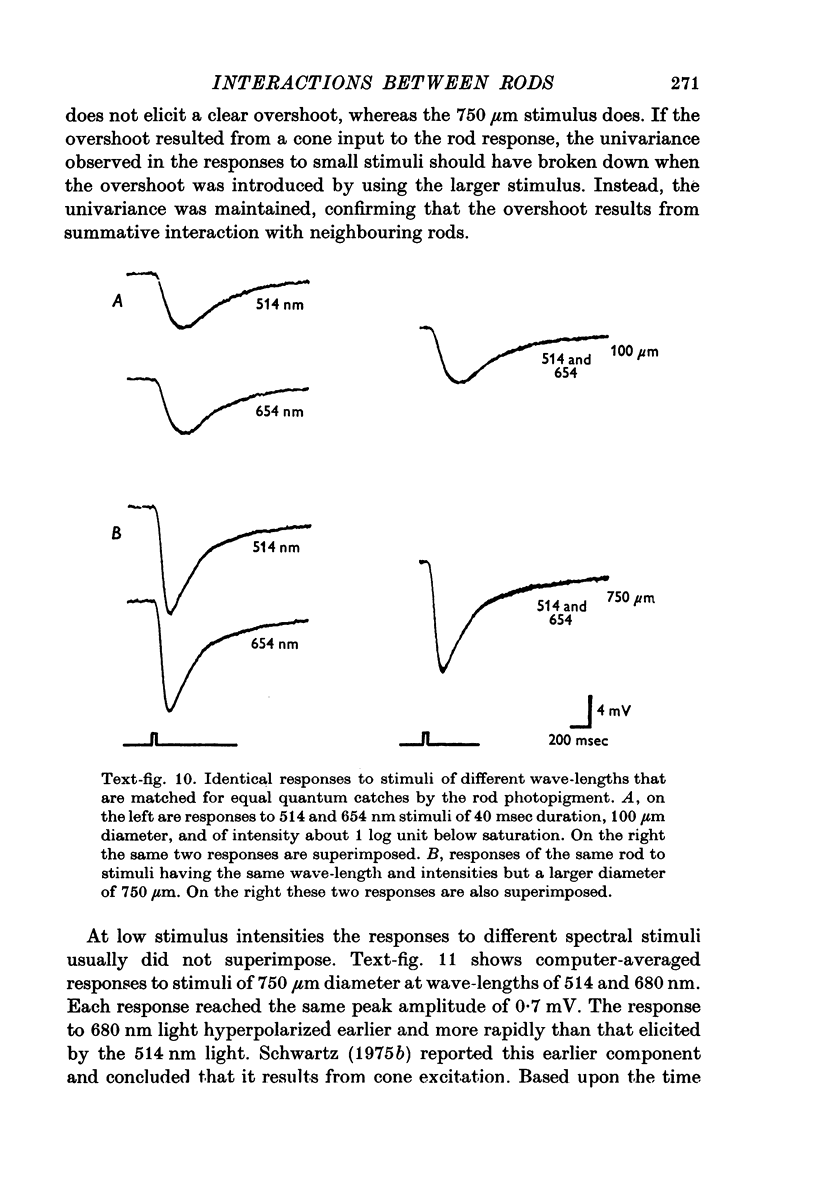
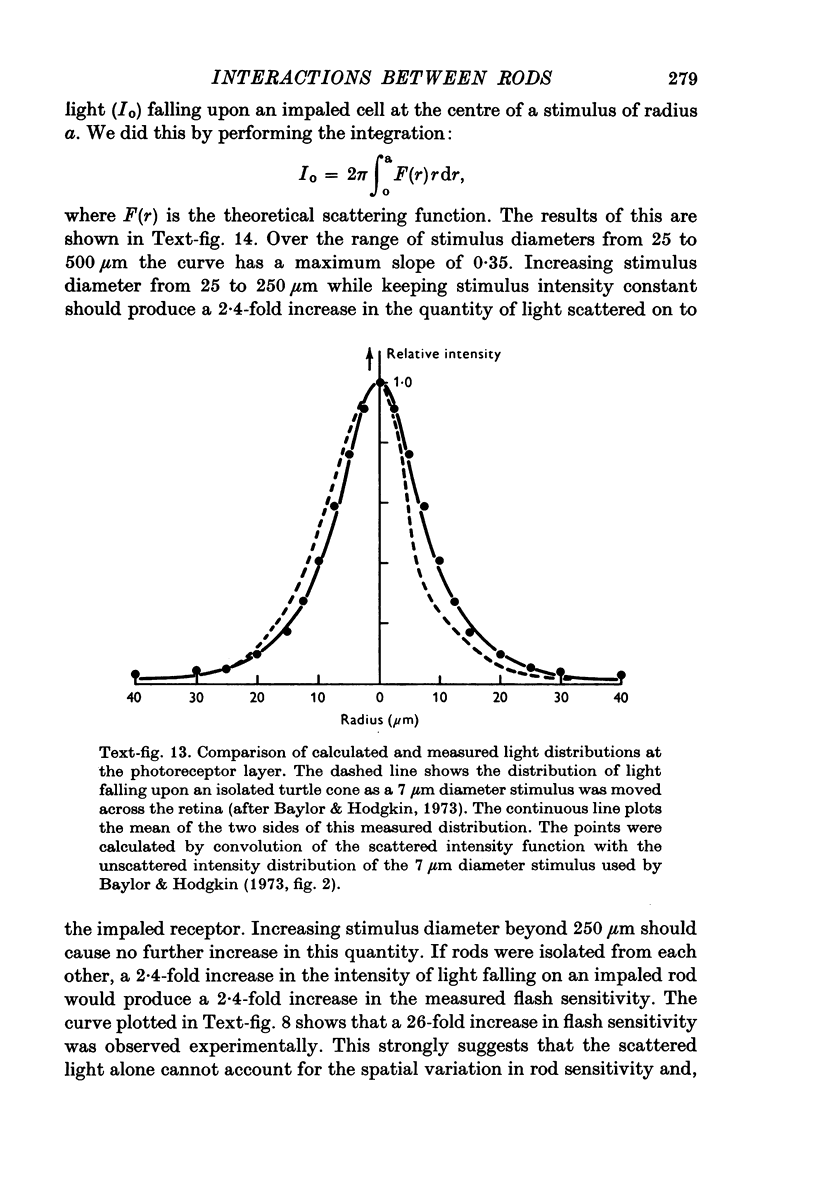
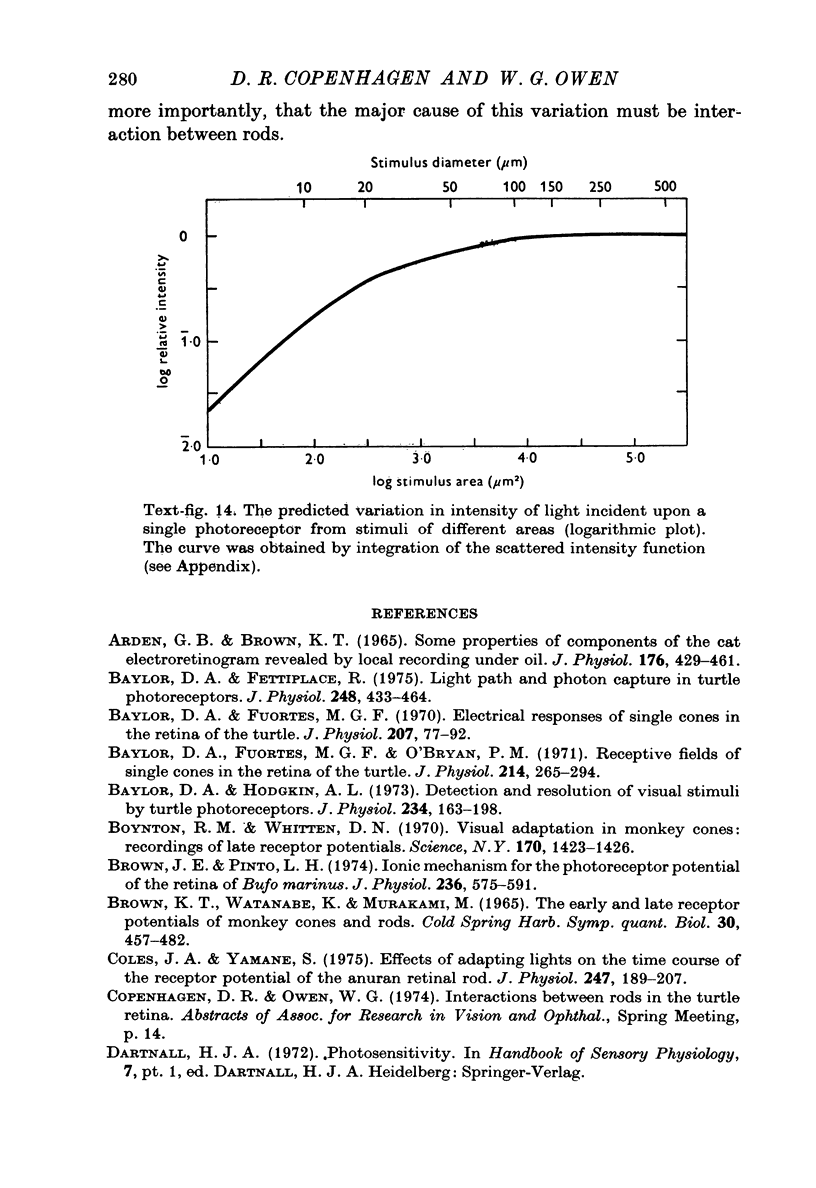
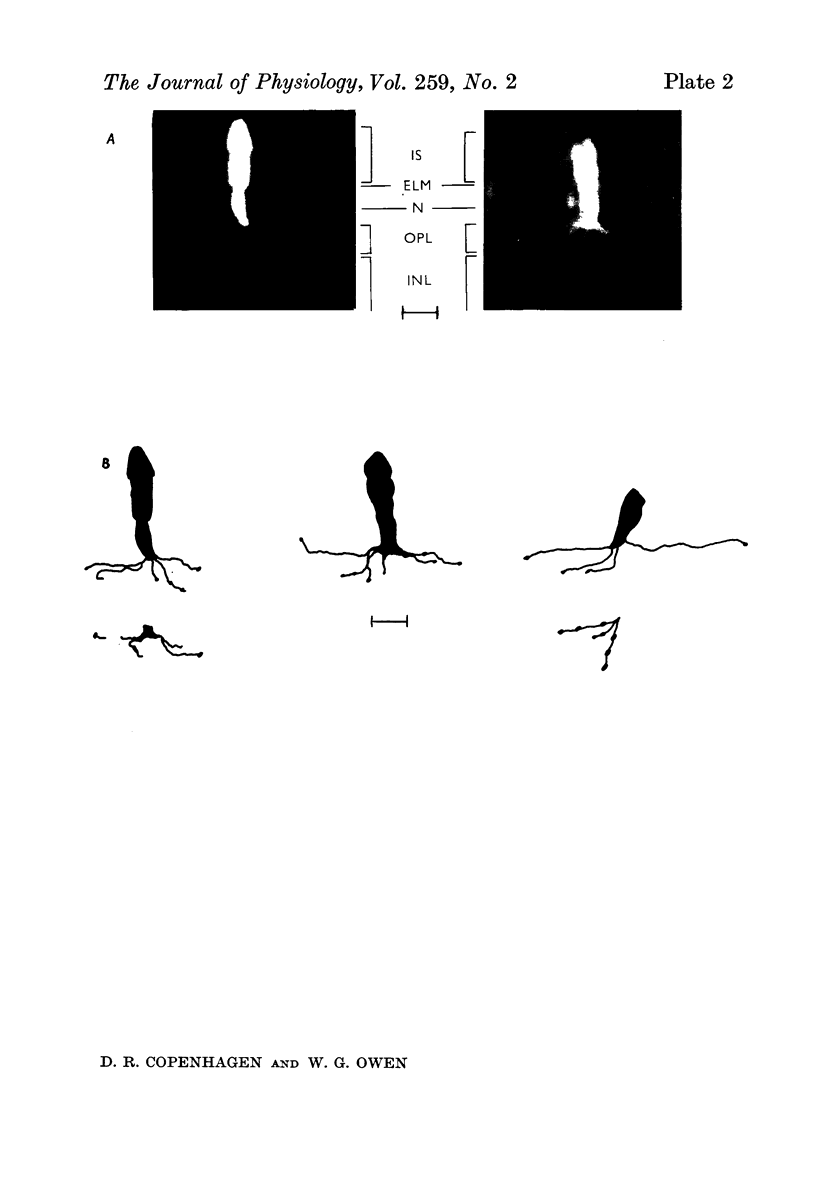
Abstract
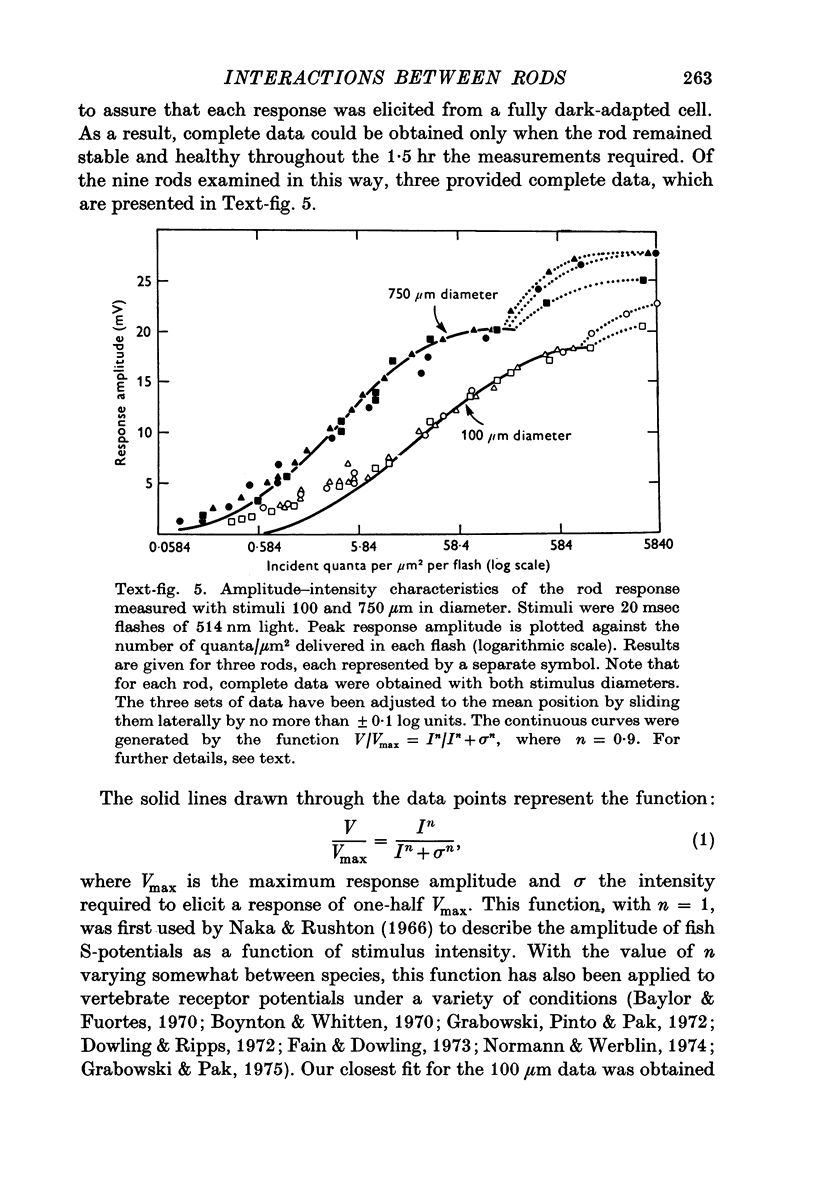
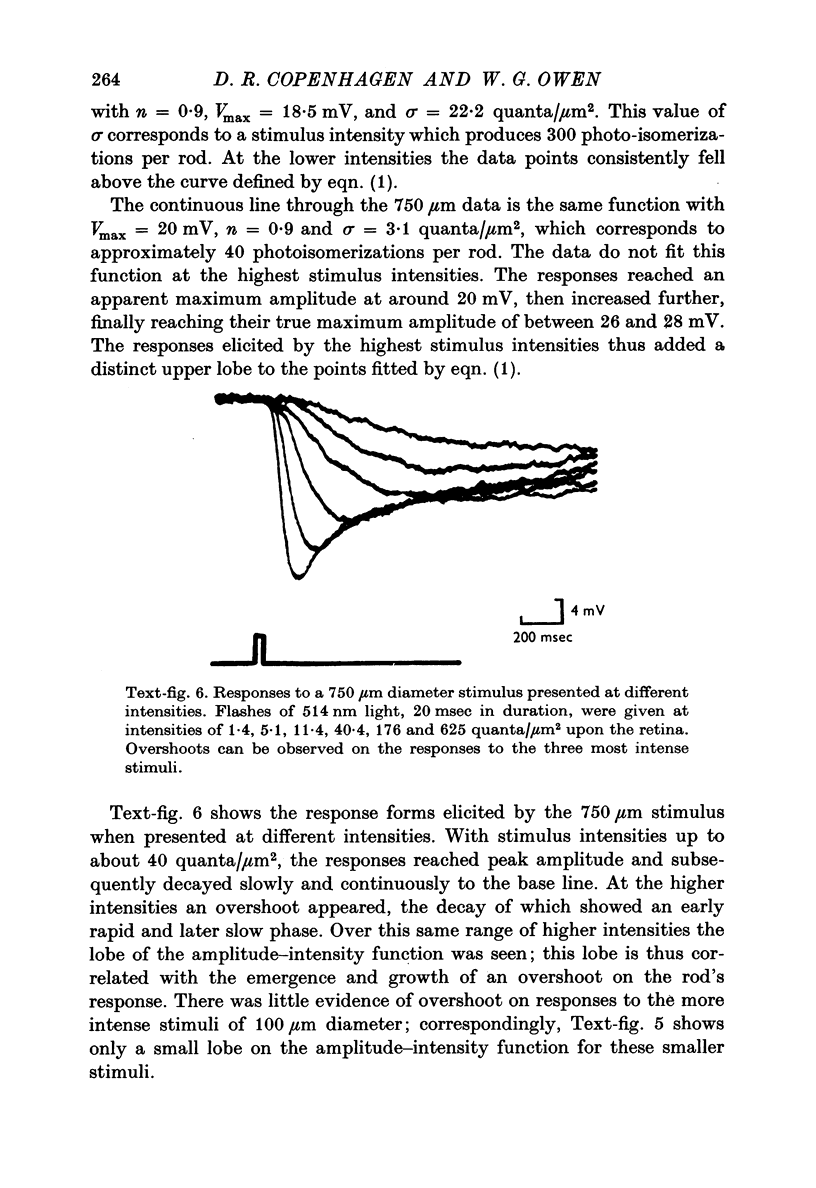
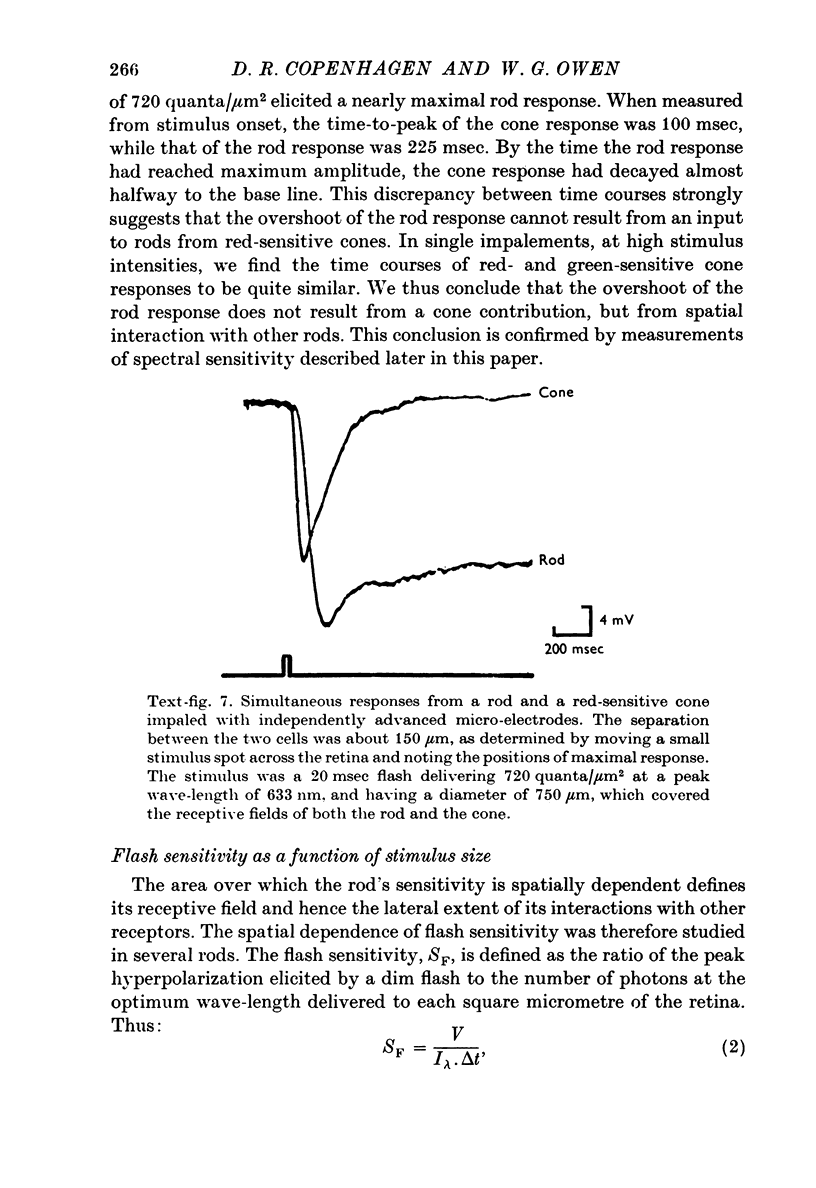
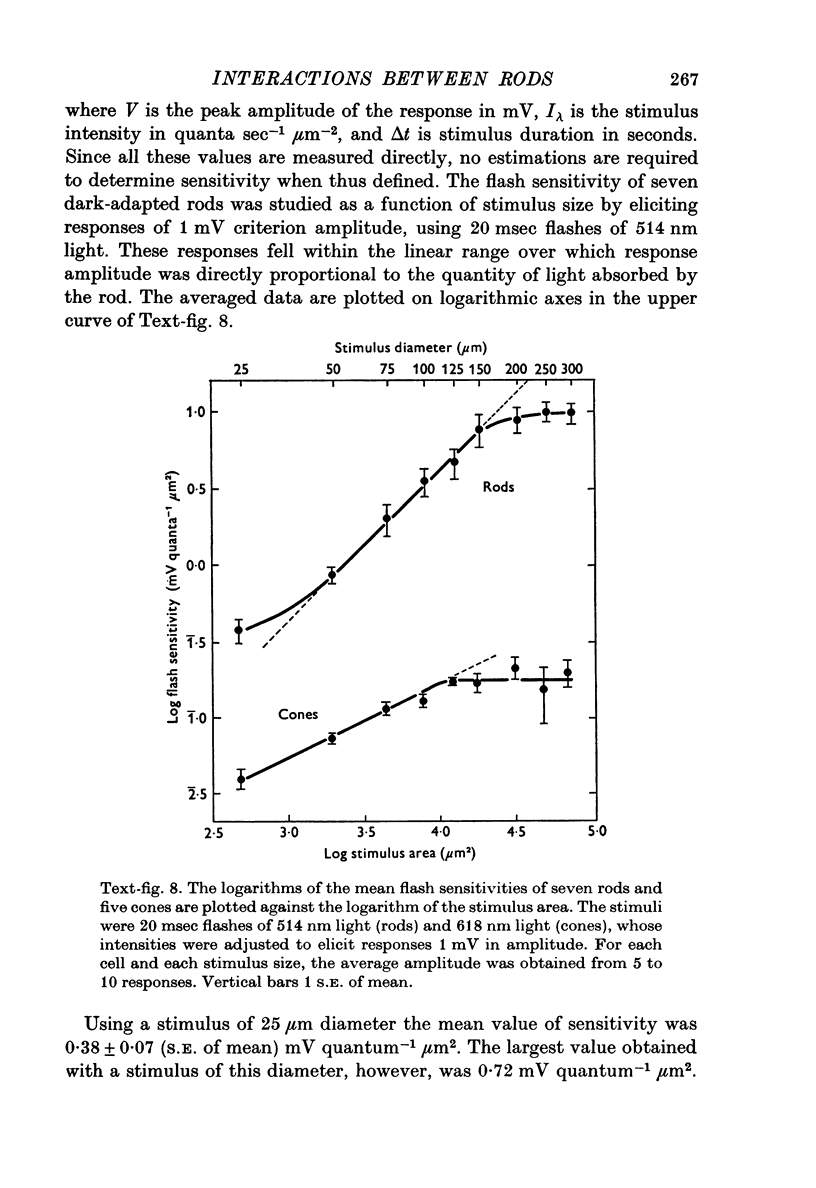
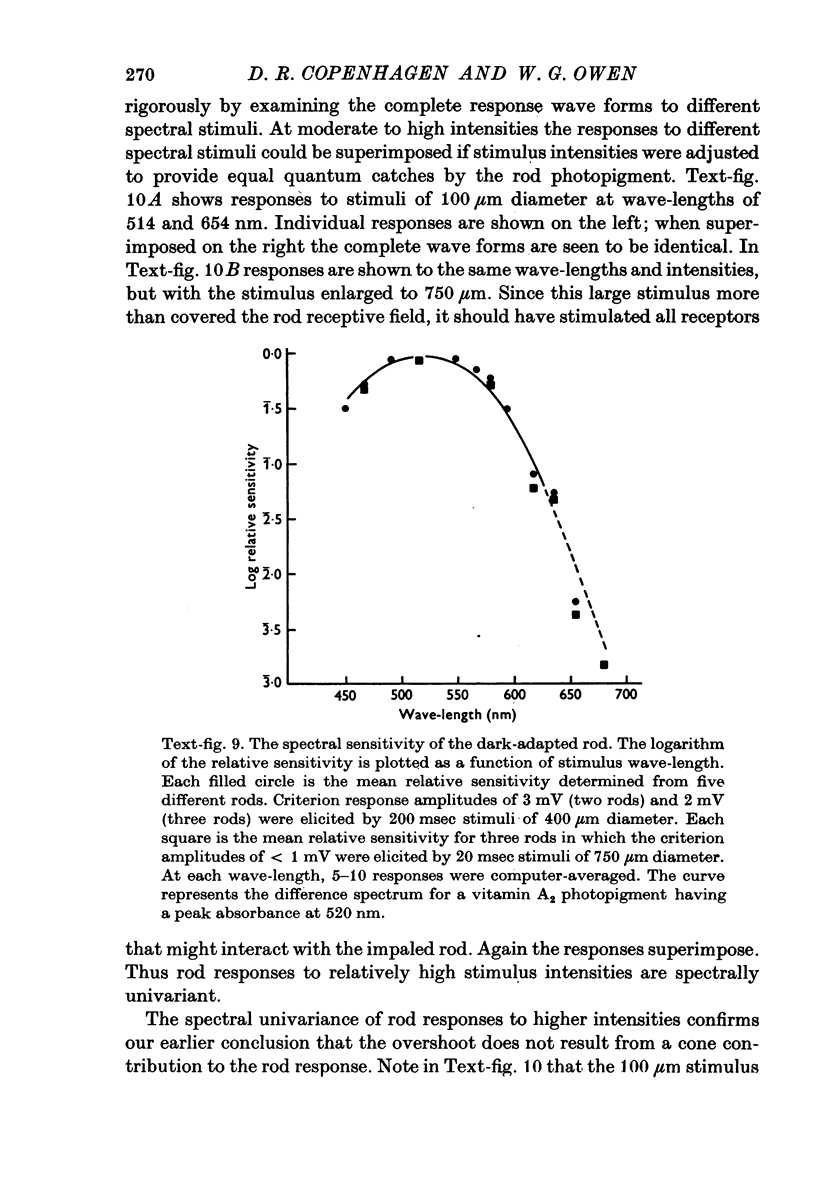
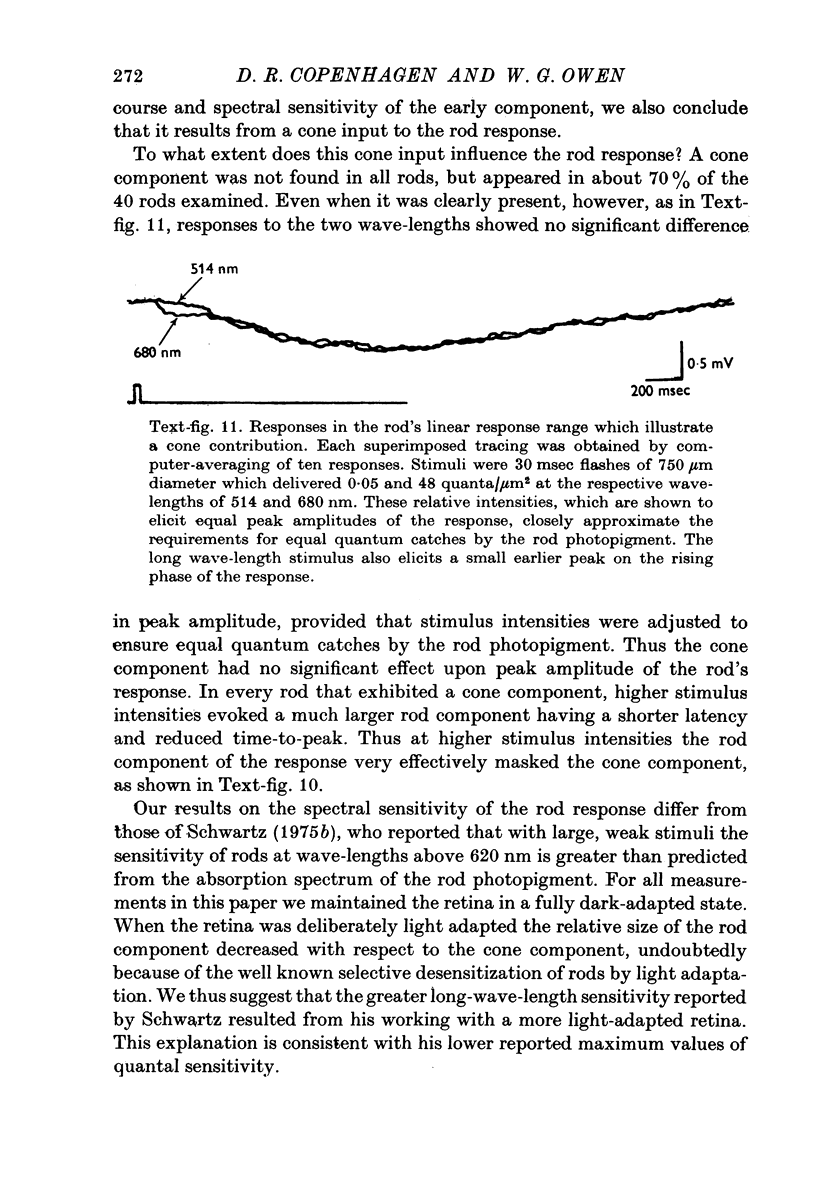
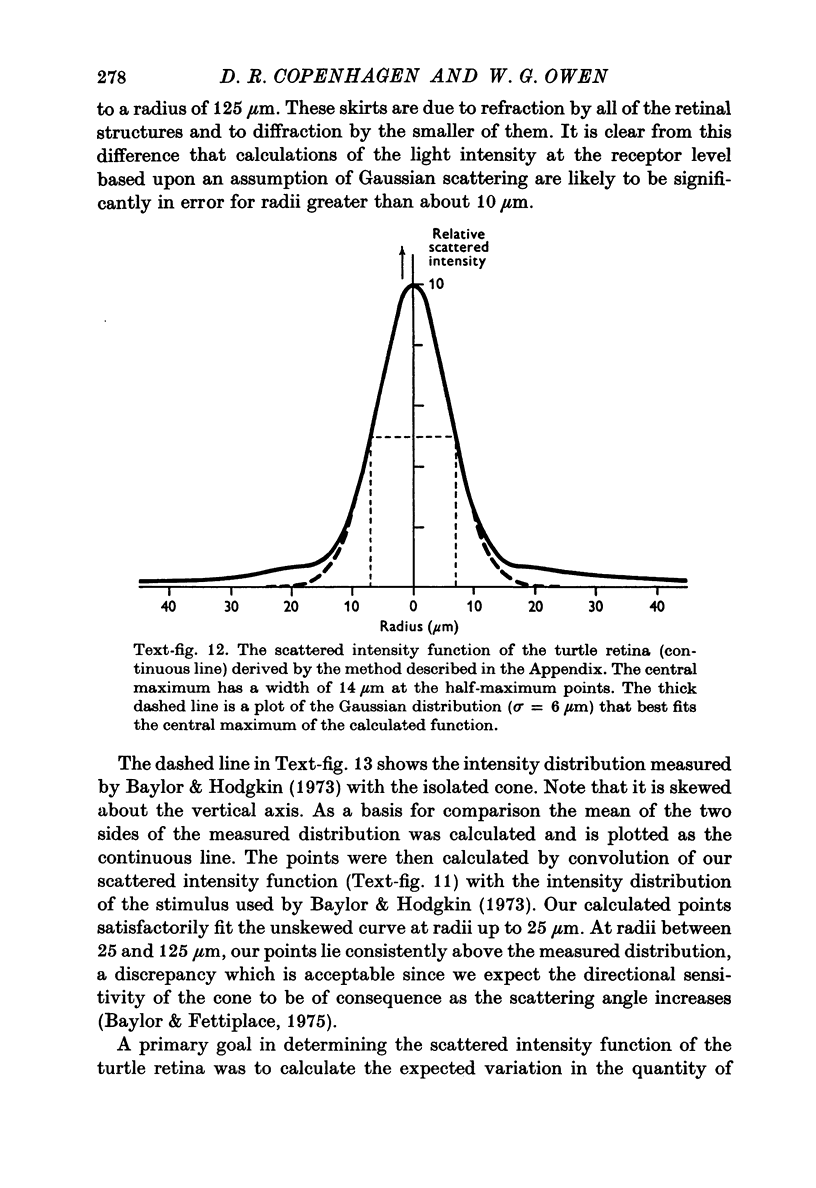
1. Intracellular recordings were made of the slow hyperpolarizing light responses of single rods in the retina of the snapping turtle. Physiological criteria used to identify rods were verified by intracellular injections of Procion Yellow. 2. The amplitudes of the responses elicited by fixed intensity flashes increased as the stimulus was enlarged to a diameter of 300 mum. Scattered light was found incapable of accounting for this effect, which must result from summative interaction of rods with neighbouring receptors. Effects of summative interaction were observed even at stimulus intensities that produced maximal responses. Enlarging the diameter of the higher intensity stimuli from 100 to 300 mum increased the peak response amplitude by almost 50%; it also produced a distinct initial peak of the response which we term overshoot. The amplitude of this overshoot was graded with stimulus size. 3. Complete intensity-response relationships were determined using stimulus diameters of 100 and 750 mum for each rod. With the smaller stimulus the intensity response range was 4-5 log units, and with the larger stimulus this was increased to 5-0 log units. For intensities below about 60 quanta/mum2 per flash (514 nm) the amplitudes elicited by the large stimulus followed a sigmoid-shaped curve. However, at higher intensities an additional lobe appeared on the intensity-response relationship. The appearance of this lobe correlated with the emergence of the overshoot on the response wave form. 4. Determinations of rod flash sensitivity (mV per quantum per mum2) showed that it increased with stimulus size up to a stimulus diameter of about 300 mum. With diameters between 50 and 150 mum, a linear relationship existed between the flash sensitivity and stimulus area. Absolute quantal sensitivities increased with stimulus area by a factor of 26, from a value of 28 muV per photoisomerization per rod with a stimulus 25 mum in diameter, to 720 muV per photoisomerization per rod with a stimulus 300 mum in diameter. 5. By comparison, red-sensitive cones showed increased sensitivity as a function of stimulus size only up to a stimulus diameter of 120 mum. Their over-all sensitivity was lower than that of rods and proved linear with stimulus diameter rather than with stimulus area. 6. Simultaneous recordings were made from rod-cone pairs to determine whether the overshoot, and hence the lobe on the amplitude-intensity function, could result from a cone input to the rod response. The time course of the cone response proved much too rapid to fit the overshoot of the rod response. 7. The spectral sensitivity of the dark-adapted rod response closely followed the difference spectrum of the rod photopigment for wave-lengths greater than 450 nm. This was true throughout the intensity range of the response, including low intensities where response averaging was necessary. 8. At low response amplitudes (approximately 1 mV), about 70% of the 40 rods tested showed responses to long wave-length stimuli consisting of two components...
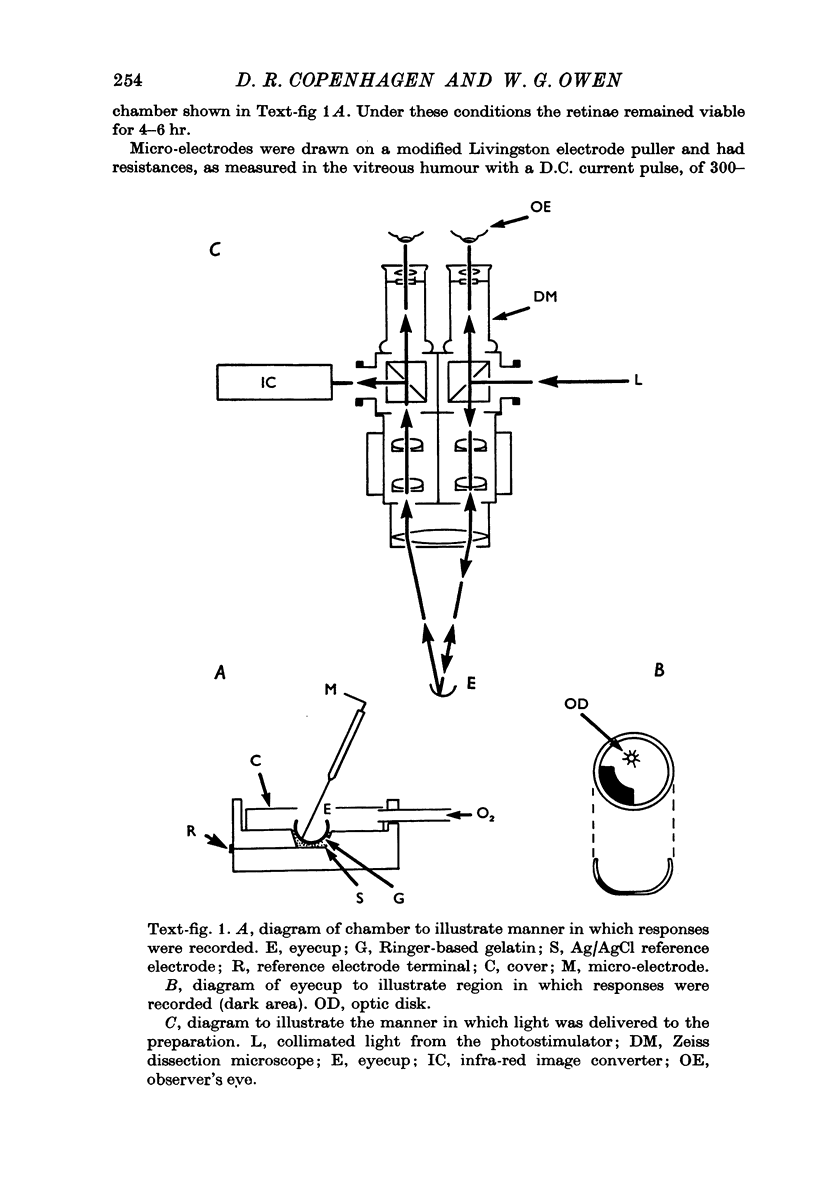
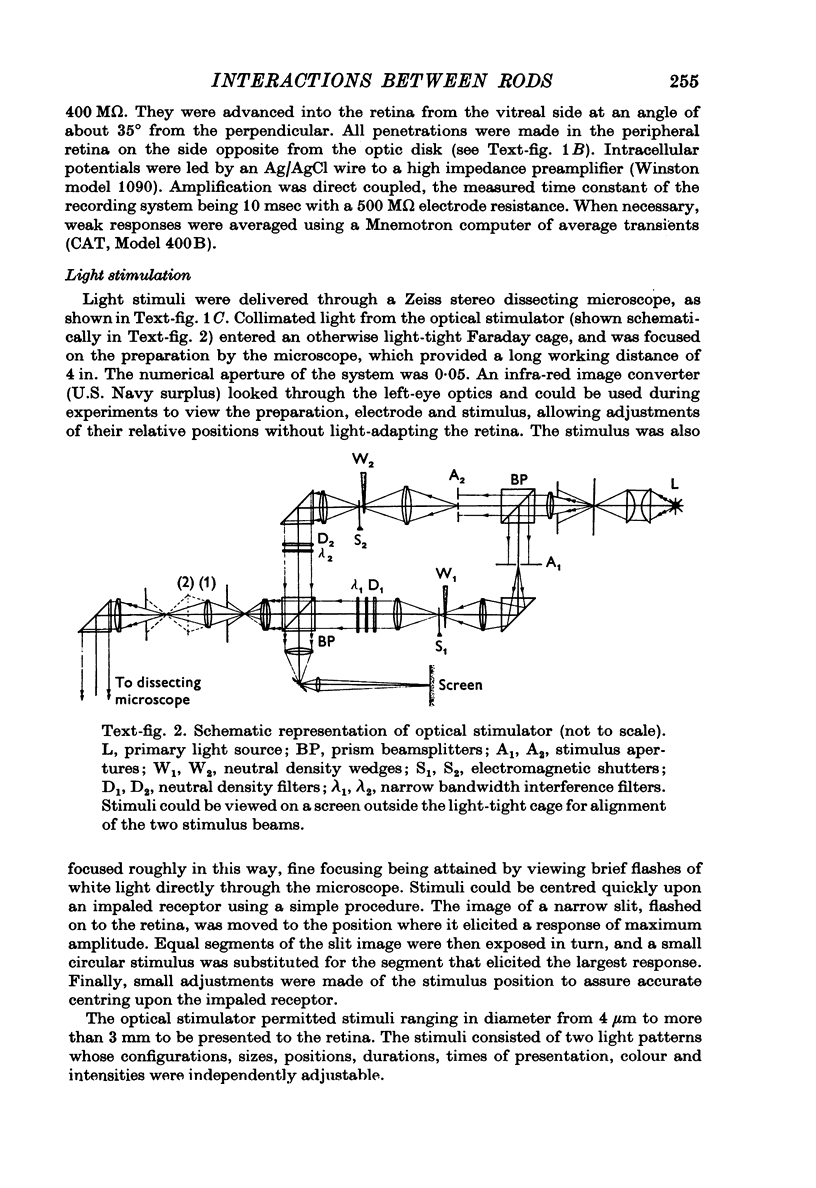
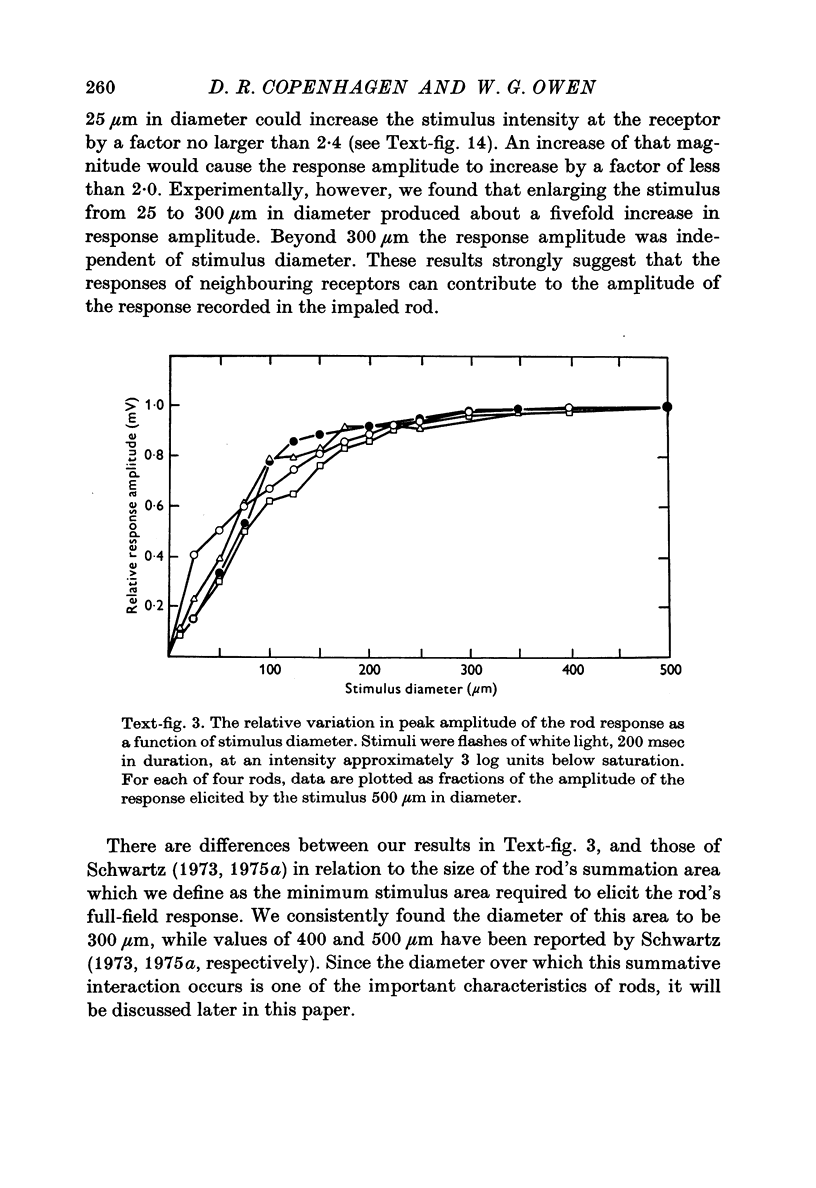
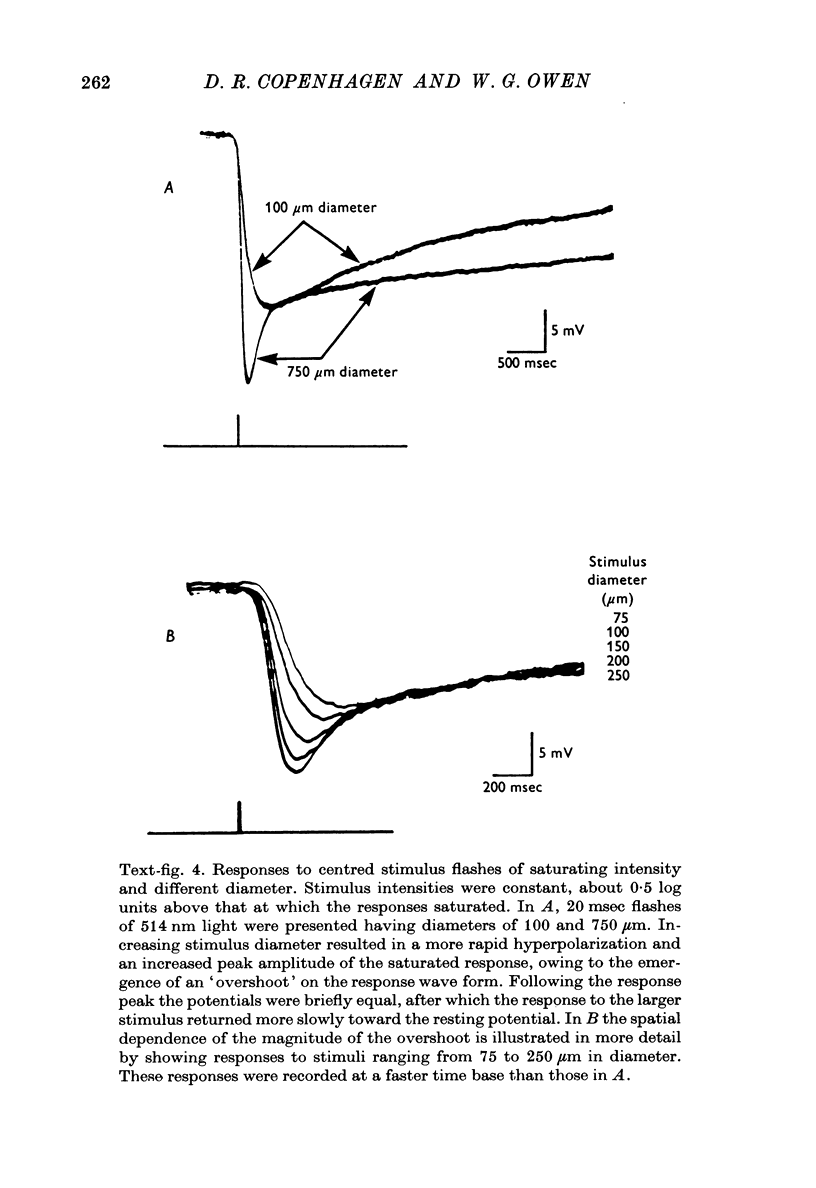
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARDEN G. B., BROWN K. T. SOME PROPERTIES OF COMPONENTS OF THE CAT ELECTRORETINOGRAM REVEALED BY LOCAL RECORDING UNDER OIL. J Physiol. 1965 Feb;176:429–461. doi: 10.1113/jphysiol.1965.sp007560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Light path and photon capture in turtle photoreceptors. J Physiol. 1975 Jun;248(2):433–464. doi: 10.1113/jphysiol.1975.sp010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Brown K. T., Watanabe K., Murakami M. The early and late receptor potentials of monkey cones and rods. Cold Spring Harb Symp Quant Biol. 1965;30:457–482. doi: 10.1101/sqb.1965.030.01.045. [DOI] [PubMed] [Google Scholar]
- Coles J. A., Yamane S. Effects of adapting lights on the time course of the receptor potential of the anuran retinal rod. J Physiol. 1975 May;247(1):189–207. doi: 10.1113/jphysiol.1975.sp010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Adaptation in skate photoreceptors. J Gen Physiol. 1972 Dec;60(6):698–719. doi: 10.1085/jgp.60.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Dowling J. E. Intracellular recordings from single rods and cones in the mudpuppy retina. Science. 1973 Jun 15;180(4091):1178–1181. doi: 10.1126/science.180.4091.1178. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Quantum sensitivity of rods in the toad retina. Science. 1975 Mar 7;187(4179):838–841. doi: 10.1126/science.1114328. [DOI] [PubMed] [Google Scholar]
- Grabowski S. R., Pak W. L. Intracellular recordings of rod responses during dark-adaptation. J Physiol. 1975 May;247(2):363–391. doi: 10.1113/jphysiol.1975.sp010936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski S. R., Pinto L. H., Pak W. L. Adaptation in retinal rods of axolotl: intracellular recordings. Science. 1972 Jun 16;176(4040):1240–1243. doi: 10.1126/science.176.4040.1240. [DOI] [PubMed] [Google Scholar]
- Munz F. W., Schwanzara S. A. A nomogram for retinene-2-based visual pigments. Vision Res. 1967 Mar;7(3):111–120. doi: 10.1016/0042-6989(67)90078-8. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from luminosity units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):587–599. doi: 10.1113/jphysiol.1966.sp008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'BRIEN B. Vision and resolution in the central retina. J Opt Soc Am. 1951 Dec;41(12):882–894. doi: 10.1364/josa.41.000882. [DOI] [PubMed] [Google Scholar]
- PALAY S. L. Synapses in the central nervous system. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):193–202. doi: 10.1083/jcb.2.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIDMAN R. L. The structure and concentration of solids in photoreceptor cells studied by refractometry and interference microscopy. J Biophys Biochem Cytol. 1957 Jan 25;3(1):15–30. doi: 10.1083/jcb.3.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJOSTRAND F. S. Ultrastructure of retinal rod synapses of the guinea pig eye as revealed by three-dimensional reconstructions from serial sections. J Ultrastruct Res. 1958 Nov;2(1):122–170. doi: 10.1016/s0022-5320(58)90050-9. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Cones excite rods in the retina of the turtle. J Physiol. 1975 Apr;246(3):639–651. doi: 10.1113/jphysiol.1975.sp010908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Rod-rod interaction in the retina of the turtle. J Physiol. 1975 Apr;246(3):617–638. doi: 10.1113/jphysiol.1975.sp010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Shakib M., Ripps H. Interreceptoral junctions in the teleost retina. Invest Ophthalmol. 1974 Dec;13(12):996–1009. [PubMed] [Google Scholar]





